Anabolic Actions of the Regenerative Agent Enamel Matrix Derivative (EMD) in Oral Periosteal Fibroblasts and MG 63 Osteoblasts, Modulation by Nicotine and Glutathione in a Redox Environment
Abstract
:1. Introduction
1.1. Regenerative Capacity of Enamel Matrix Derivative
1.2. Anabolic and Antioxidant Actions of Androgens
1.3. Oxidative Actions of Nicotine
1.4. Periosteal Fibroblasts and Osteoblasts
- (1)
- The mechanism of action of enamel matrix derivative in periosteal fibroblasts and MG63 osteoblasts.
- (2)
- The modulating outcome of nicotine and glutathione on effects mediated by regenerative agents.
2. Experimental Section
2.1. Materials
2.2. Periosteal Fibroblasts and MG63 Osteoblasts
2.3. Experiments
2.3.1. Establishing Optimal Concentrations of EMD, Nicotine and Glutathione
2.3.2. Diagnostic Preparation of Multiwells with Optimal Inhibitory Concentrations of Nicotine (N200) and Optimal Effective Concentrations of Glutathione (G3) and EMD (E30).
2.3.3. Analysis of Metabolites in Cell Culture Eluates from the above Experiments
2.4. Characterization of Steroid Metabolites by gas Chromatography—Mass Spectrometry
2.5. Statistical Methodology
3. Results and Discussion
3.1. Results
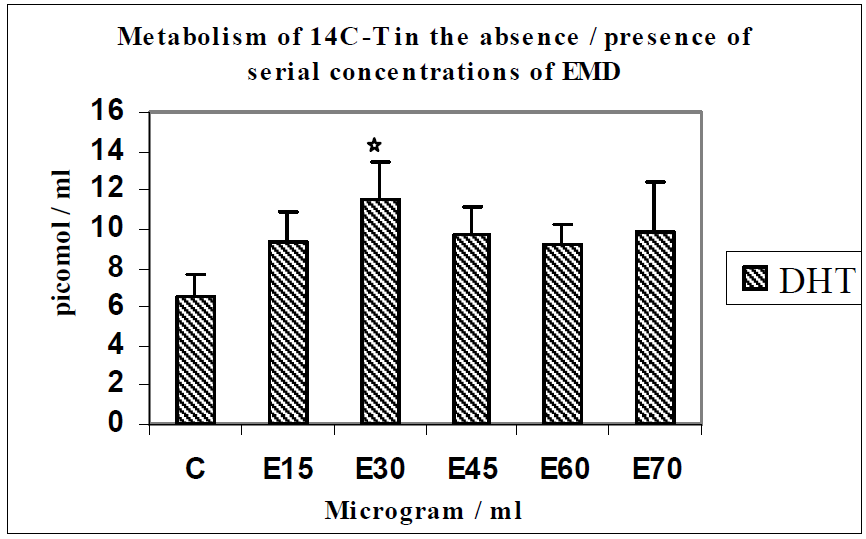
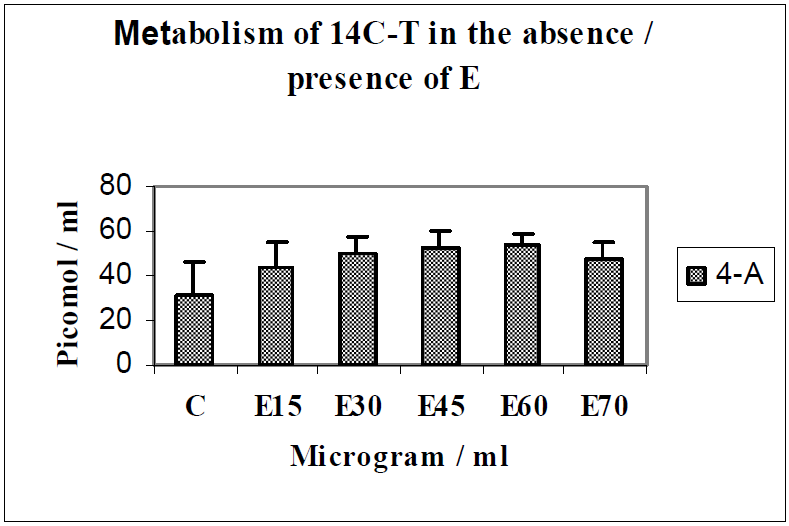
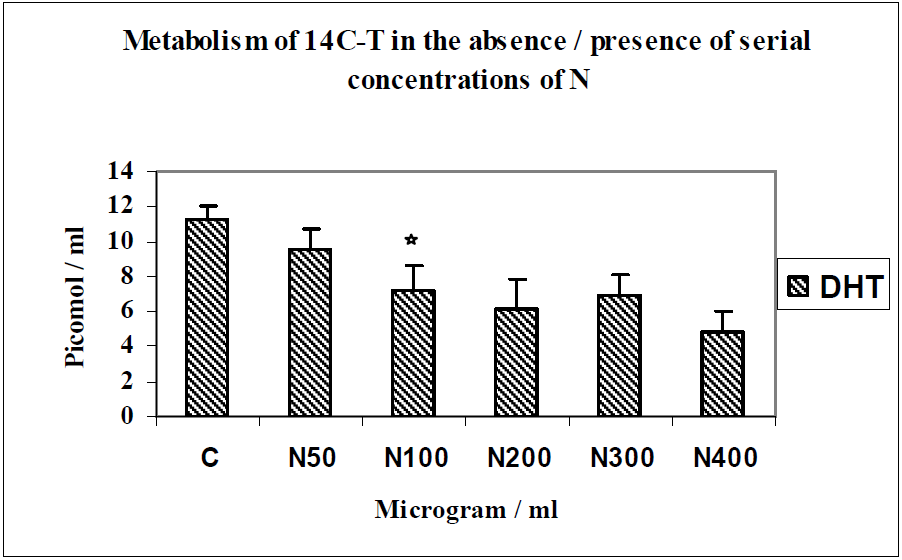
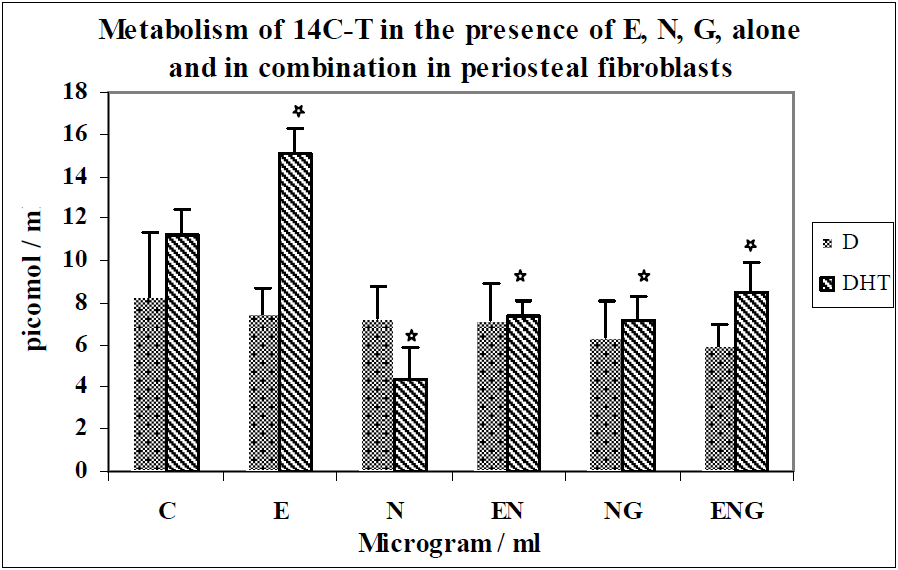
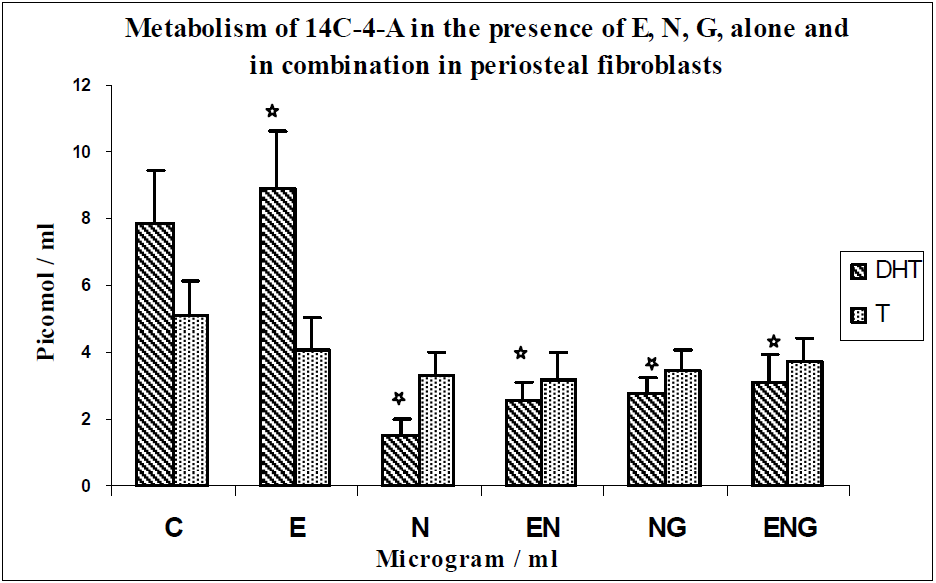
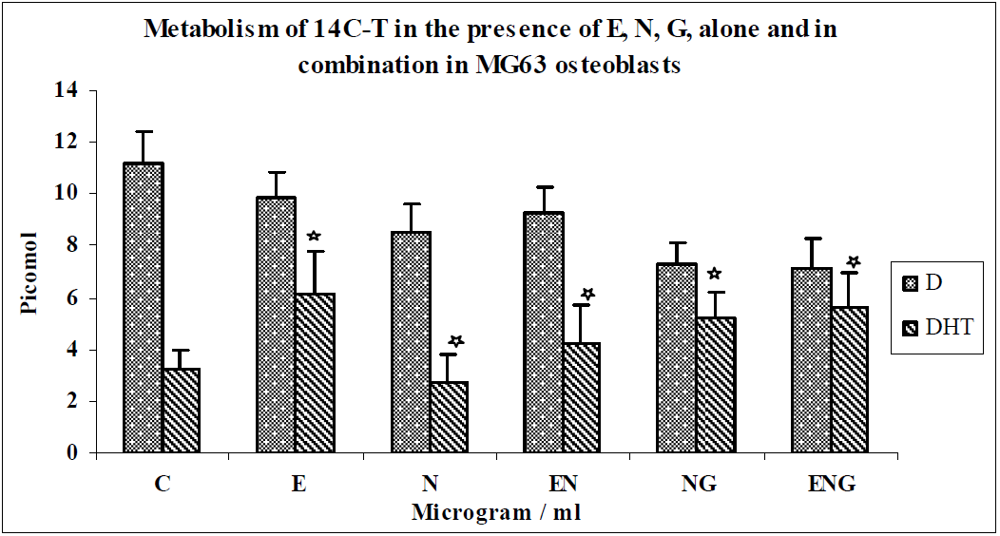
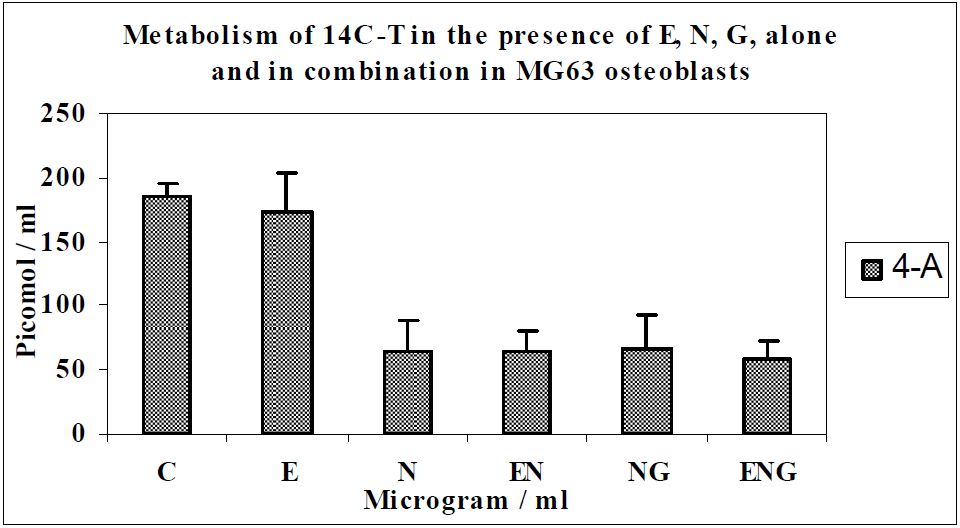

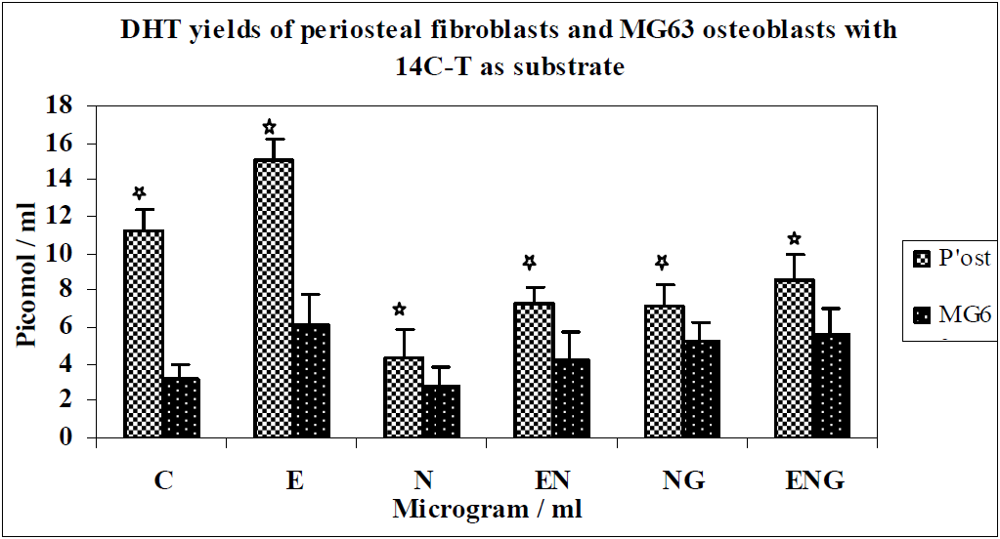

3.2. Discussion
4. Conclusions
Acknowledgments
Disclaimer
References
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 87–105. [Google Scholar] [CrossRef]
- Fujita, T.; Yamamoto, S.; Ota, M.; Shibukawa, Y.; Yamada, S. Coverage of gingival recession defects using guided tissue regeneration with or without adjunctive enamel matrix derivative in a dog model. Int. J. Periodontics Restor. Den. 2011, 31, 247–253. [Google Scholar]
- Okuda, K.; Miyazaki, A.; Momose, M.; Murata, M.; Nomura, T.; Kubota, T.; Wolff, L.F.; Yoshie, H. Levels of tissue inhibitor of metalloproteinases-1 and matrix metalloproteinases-1 and -8 in gingival crevicular fluid following treatment with enamel matrix derivative (EMDOGAIN). J. Periodontal Res. 2001, 36, 309–316. [Google Scholar]
- Parkar, M.; Tonetti, M. Gene expression profiles of periodontal ligament cells treated with enamel matrix proteins in vitro: Analysis using cDNA arrays. J. Periodontol. 2004, 75, 1539–1546. [Google Scholar] [CrossRef]
- Myhre, A.E.; Lyngstadaas, S.P.; Dahle, M.K.; Stuestøl, J.F.; Foster, S.J.; Thiemermann, C.; Lilleaasen, P.; Wang, J.E.; Aasen, A.O. Anti-inflammatory properties of enamel matrix derivative in human blood. J. Periodontal Res. 2006, 41, 208–213. [Google Scholar] [CrossRef]
- Sato, S.; Kitagawa, M.; Sakamoto, K.; Iizuka, S.; Kudo, Y.; Ogawa, I; Miyauchi, M.; Chu, E.Y.; Foster, B.L.; Somerman, M.J.; Takata, T. Enamel matrix derivative exhibits anti-inflammatory properties in monocytes. J. Periodontol. 2008, 79, 535–540. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Deschner, B.; Winter, J.; Bourauel, C.; Jäger, A.; Jepsen, S.; Deschner, J. Anti-inflammatory effects of EMD in the presence of biomechanical loading and interleukin-1β in vitro. Clin. Oral Investig. 2012, 16, 275–283. [Google Scholar] [CrossRef]
- Schwartz, Z.; Carnes, D.L., Jr; Pulliam, R.; Lohmann, C.H.; Sylvia, V.L.; Liu, Y.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Porcine fetal enamel matrix derivative stimulates proliferation but not differentiation of pre-osteoblastic 2T9 cells, inhibits proliferation and stimulates differentiation of osteoblast-like MG63 cells, and increases proliferation and differentiation of normal human osteoblast NHOst cells. J. Periodontol. 2000, 71, 1287–1296. [Google Scholar] [CrossRef]
- Keila, S.; Nemcovsky, C.E.; Moses, O.; Artzi, Z.; Weinreb, M. In vitro effects of enamel matrix proteins on rat bone marrow cells and gingival fibroblasts. J. Dent. Res. 2004, 83, 134–138. [Google Scholar] [CrossRef]
- Hagewald, S.; Pischon, N.; Jawor, P.; Bernimoulin, J.P.; Zimmermann, B. Effects of enamel matrix derivative on proliferation and differentiation of primary osteoblasts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 243–249. [Google Scholar] [CrossRef]
- Reseland, J.E.; Reppe, S.; Larsen, A.M.; Berner, H.S.; Reinholt, F.P.; Gautvik, K.M.; Slaby, I.; Lyngstadaas, S.P. The effect of enamel matrix derivative on gene expression in osteoblasts. Eur. J. Oral Sci. 2006, 114, 205–211, 254–256, 381–382. [Google Scholar] [CrossRef]
- Davey, R.A.; Morris, H.A. Effects of estradiol and dihydrotestosterone on osteoblast gene expression in osteopenic ovariectomized rats. J. Bone Miner. Metab. 2005, 23, 212–218. [Google Scholar]
- Basaria, S.; Wahlstrom, J.T.; Dobs, A.S. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J. Clin. Endocrinol. Metab. 2001, 86, 5108–5117. [Google Scholar] [CrossRef]
- Soory, M. Hormone mediation of immune responses in the progression of diabetes, rheumatoid arthritis and periodontal diseases. Curr. Drug Target. Immun. Endocr. Metab. Disord. 2002, 2, 13–25. [Google Scholar] [CrossRef]
- Ganesan, K.; Tiwari, M.; Balachandran, C.; Manohar, B.M.; Puvanakrishnan, R. Estrogen and testosterone attenuate extracellular matrix loss in collagen-induced arthritis in rats. Calcif. Tissue Int. 2008, 83, 354–364. [Google Scholar] [CrossRef]
- Fujita, T.; Kawata, T.; Tokimasa, C.; Tanne, K. Influence of oestrogen and androgen on modelling of the mandibular condylar bone in ovariectomized and orchiectomized growing mice. Arch. Oral Biol. 2001, 46, 57–65. [Google Scholar] [CrossRef]
- Lu, H.K.; Tseng, C.C.; Lee, Y.H.; Li, C.L.; Wang, L.F. Flutamide inhibits nifedipine- and interleukin-1 beta-induced collagen overproduction in gingival fibroblasts. J. Periodontal Res. 2010, 45, 451–457. [Google Scholar]
- Parkar, M.H.; Newman, H.N.; Olsen, I. Polymerase chain reaction analysis of oestrogen and androgen receptor expression in human gingival and periodontal tissue. Arch. Oral Biol. 1996, 41, 979–983. [Google Scholar]
- Wiren, K.M. Androgens and bone growth: Its Location, location, location. Curr. Opin. Pharmacol. 2005, 5, 626–632. [Google Scholar] [CrossRef]
- Hugoson, A.; Rolandsson, M. Periodontal disease in relation to smoking and the use of Swedish snus: Epidemiological studies covering 20 years (1983–2003). J. Clin. Periodontol. 2011, 38, 809–816. [Google Scholar] [CrossRef]
- Kumar, P.S.; Matthews, C.R.; Joshi, V.; de Jager, M.; Aspiras, M. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect. Immun. 2011, 79, 4730–4738. [Google Scholar] [CrossRef]
- Kinane, D.F.; Chestnutt, I.G. Smoking and periodontal disease. Crit. Rev. Oral Biol. Med. 2000, 11, 356–365. [Google Scholar] [CrossRef]
- Palmer, R.M.; Wilson, R.F.; Hasan, A.S.; Scott, D.A. Mechanisms of action of environmental factors—Tobacco smoking. J. Clin. Periodontol. 2005, 32, 180–195. [Google Scholar] [CrossRef]
- Ojima, M.; Hanioka, T. Destructive effects of smoking on molecular and genetic factors of periodontal disease. Tob. Induc. Dis. 2010, 8, 4–11. [Google Scholar] [CrossRef]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J Dent. Res. 2012, 91, 142–149. [Google Scholar] [CrossRef]
- Matthews, J.B.; Chen, F.M.; Milward, M.R.; Wright, H.J.; Carter, K.; McDonagh, A.; Chapple, I.L. Effect of nicotine, cotinine and cigarette smoke extract on the neutrophil respiratory burst. J. Clin. Periodontol. 2011, 38, 208–218. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wu, L.A.; Wang, J.; Wen, L.Y.; Wang, X.J. Micro-computerized tomography analysis of alveolar bone loss in ligature- and nicotine-induced experimental periodontitis in rats. J. Periodontal Res. 2010, 45, 714–719. [Google Scholar] [CrossRef]
- Yanagita, M.; Kojima, Y.; Kawahara, T.; Kajikawa, T.; Oohara, H.; Takedachi, M.; Yamada, S.; Murakami, S. Suppressive effects of nicotine on the cytodifferentiation of murine periodontal ligament cells. Oral Dis. 2010, 16, 812–817. [Google Scholar] [CrossRef]
- Soory, M.; Suchak, A. Effects of alkaline phosphatase and its inhibitor levamisole on the modulation of androgen metabolism by nicotine and minocycline in human gingival and oral periosteal fibroblasts. Arch. Oral Biol. 2003, 48, 69–76. [Google Scholar] [CrossRef]
- Gallo, C.; Renzi, P.; Loizzo, S.; Loizzo, A.; Piacente, S.; Festa, M.; Caputo, M.; Tecce, M.F.; Capasso, A. Potential therapeutic effects of vitamin e and C on placental oxidative stress induced by nicotine: An in vitro evidence. Open Biochem. J. 2010, 4, 77–82. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hsieh, Y.S.; Lii, C.K.; Huang, F.M.; Tai, K.W.; Chou, M.Y. Induction of c-fos expression by nicotine in human periodontal ligament fibroblasts is related to cellular thiol levels. J. Periodontal Res. 2003, 38, 44–50. [Google Scholar] [CrossRef]
- Dey, S.K.; Roy, S. Role of reduced glutathione in the amelioration of nicotine-induced oxidative stress. Bull. Environ. Contam. Toxicol. 2010, 84, 385–389. [Google Scholar] [CrossRef]
- Suleyman, H.; Gumustekin, K.; Taysi, S.; Keles, S.; Oztasan, N.; Aktas, O.; Altinkaynak, K.; Timur, H.; Akcay, F.; Akar, S.; Dane, S.; Gul, M. Beneficial effects of Hippophae Rhamnoides L, on nicotine induced oxidative stress in rat blood compared with vitamin E. Biol. Pharm. Bull. 2002, 25, 1133–1136. [Google Scholar] [CrossRef]
- Neogy, S.; Das, S.; Mahapatra, S.K.; Mandal, N.; Roy, S. Amelioratory effect of andrographis paniculata nees on liver, kidney, heart, lung and spleen during nicotine induced oxidative stress. Environ. Toxicol. Pharmacol. 2008, 25, 321–328. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Sittinger, M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003, 9, S45–S64. [Google Scholar] [CrossRef]
- Zhang, X.; Awad, H.A.; O’Keefe, R.J.; Guldberg, R.E.; Schwarz, E.M. A perspective: Engineering periosteum for structural bone graft healing. Clin. Orthop. Relat. Res. 2008, 466, 1777–1787. [Google Scholar] [CrossRef]
- Arnsdorf, E.J.; Jones, L.M.; Carter, D.R.; Jacobs, C.R. The periosteum as a cellular source for functional tissue engineering. Tissue Eng. Part A 2009, 15, 2637–2642. [Google Scholar] [CrossRef]
- Sooriyamoorthy, M.; Gower, D.B. Phenytoin stimulation of testosterone metabolism in inflamed human gingival fibroblasts. Biochem. Soc. Trans. 1989, 17, 1020–1021. [Google Scholar]
- Ojanotko, A.; Nienstedt, W.; Harri, M.P. Metabolism of testosterone by human healthy and inflamed gingiva in vitro. Arch. Oral Biol. 1980, 25, 481–484. [Google Scholar] [CrossRef]
- Billiau, A.; Edy, V.G.; Heremans, H.; van Damme, J.; Desmyter, J.; Georgiades, J.A.; de Somer, P. Human interferon: Mass production in a newly established cell line, MG-63. Antimicrob. Agents Chem. 1977, 12, 11–15. [Google Scholar] [CrossRef]
- Pradel, W.; Mai, R.; Gedrange, T.; Lauer, G. Cell passage and composition of culture medium effects proliferation and differentiation of human osteoblast-like cells from facial bone. J. Physiol. Pharmacol. 2008, 59 (Suppl. 5), 47–58. [Google Scholar]
- Mauro, A.; Buscemi, M.; Gerbino, A. Immunohistochemical and transcriptional expression of matrix metalloproteinases in full-term human umbilical cord and human umbilical vein endothelial cells. J. Mol. Histol. 2010, 41, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Rahman, Z.A.; Soory, M. Antioxidant effects of glutathione and IGF in a hyperglycaemic cell culture model of fibroblasts: Some actions of advanced glycaemic end products (AGE) and nicotine. Endocrin. Metab. Imm. Dis. Drug Targ. 2006, 6, 279–286. [Google Scholar] [CrossRef]
- Ryu, Y.-M.; Hah, Y.-S.; Park, B.-W.; Kim, D.R.; Roh, G.S.; Kim, J.-R.; Kim, U.-K.; Rho, G.-J.; Maeng, G.-H.; Byun, J.-H. Osteogenic differentiation of human periosteal-derived cells in a three-dimensional collagen scaffold. Mol. Biol. Rep. 2011, 38, 2887–2894. [Google Scholar]
- Soory, M. Bacterial steroidogenesis by periodontal pathogens and the effect of bacterial enzymes on steroid conversions by human gingival fibroblasts in culture. J. Periodont. Res. 1995, 30, 124–131. [Google Scholar] [CrossRef]
- Soory, M.; Tilakaratne, A. Modulation of androgen metabolism by phenytoin, oestradiol and tamoxifen in human gingival fibroblasts. J. Clin. Periodontol. 2003, 30, 556–561. [Google Scholar] [CrossRef]
- Krum, S.A. Direct transcriptional targets of sex steroid hormones in bone. J. Cell. Biochem. 2011, 112, 401–408. [Google Scholar]
- Lee, S.H.; Heo, J.S.; Lee, M.Y.; Han, H.J. Effect of dihydrotestosterone on hydrogen peroxide-induced apoptosis of mouse embryonic stem cells. J. Cell. Phys. 2008, 216, 269–275. [Google Scholar] [CrossRef]
- Miron, R.J.; Hedbom, E.; Ruggiero, S.; Bosshardt, D.D.; Zhang, Y.; Mauth, C.; Gemperli, A.C.; Iizuka, T.; Buser, D.; Sculean, A. Premature osteoblast clustering by enamelmatrix proteins induces osteoblast differentiation through up-regulation of connexin 43 and N-cadherin. PLoS One 2011, 6, 1–11. [Google Scholar]
- Kémoun, P.; Gronthos, S.; Snead, M.L.; Rue, J.; Courtois, B.; Vaysse, F.; Salles, J.P.; Brunel, G. The role of cell surface markers and enamelmatrix derivatives on human periodontal ligament mesenchymal progenitor responses in vitro. Biomaterials 2011, 32, 7375–7388. [Google Scholar]
- Chambrone, L.; Pannuti, CM.; Tu, Y.K.; Chambrone, L.A. Evidence-based periodontal plastic surgery. II. An individual data meta-analysis for evaluating factors in achieving complete root coverage. J. Periodontol. 2011. [Google Scholar] [CrossRef]
- Soory, M. Healing in periodontal bone defects: A role for promoters? In Wound Healing: Process, Phases and Promoting; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; Chapter 1; pp. 1–24. [Google Scholar]
- Van der Pauw, M.T.; van den Bos, T.; Everts, V.; Beertsen, W. Enamel matrix-derived protein stimulates attachment of periodontal ligament fibroblasts and enhances alkaline phosphatase activity and transforming growth factor beta1 release of periodontal ligament and gingival fibroblasts. J. Periodontol. 2000, 71, 31–43. [Google Scholar] [CrossRef]
- AboElsaad, N.S.; Soory, M.; Gadalla, L.M.; Ragab, L.I.; Dunne, S.; Zalata, K.R.; Louca, C. Effect of soft laser and bioactive glass on bone regeneration in the treatment of bone defects (An experimental study). Lasers Med. Sci. 2009, 24, 527–533. [Google Scholar] [CrossRef]
- AboElsaad, N.S.; Soory, M.; Gadalla, L.M.; Ragab, L.I.; Dunne, S.; Zalata, K.R.; Louca, C. Effect of soft laser and bioactive glass on bone regeneration in the treatment of infrabony defects (A clinical study). Lasers Med. Sci. 2009, 24, 387–395. [Google Scholar] [CrossRef]
- Soory, M. Periodontal regenerative materials and their applications: Goodness of fit? Rec. Patents Endocr. Metab. Immune Drug Discov. 2008, 2, 35–44. [Google Scholar] [CrossRef]
- Helen, A.; Krishnakumar, K.; Vijayammal, P.L.; Augusti, K.T. Antioxidant effect of onion oil (Allium cepa. Linn) on the damages induced by nicotine in rats as compared to alpha-tocopherol. Toxicol Lett. 2000, 116, 61–68. [Google Scholar] [CrossRef]
- Walker, A.; Uduppa, K.B.; Chowdhury, P. Mitogenic and functional responses by nicotine and hydrogen peroxide in AR42J cells: A comparative study. Tob. Ind. Dis. 2008, 4, 5–12. [Google Scholar] [CrossRef]
- Dickinson, D.A.; Forman, H.J. Glutathione in defense and signaling: Lessons from a small thiol. Ann. NY Acad. Sci. 2002, 973, 488–504. [Google Scholar] [CrossRef]
- Fraternale, A.; Paoletti, M.F.; Casabianca, A.; Oiry, J.; Clayette, P.; Vogel, J.U.; Cinatl, J., Jr.; Palamara, A.T.; Sgarbanti, R.; Garaci, E.; Millo, E.; Benatti, U.; Magnani, M. Antiviral and immunomodulatory properties of new pro-glutathione (GSH) molecules. Curr. Med. Chem. 2006, 13, 1749–1755. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hsieh, Y.S.; Lii, C.K.; Huang, F.M.; Tai, K.W.; Chou, M.Y. Induction of c-fos expression by nicotine in human periodontal ligament fibroblasts is related to cellular thiol levels. J. Periodont. Res. 2003, 38, 44–50. [Google Scholar] [CrossRef]
- Soory, M.; Tilakaratne, A. The effect of minocycline on the metabolism of androgens by human oral periosteal fibroblasts and its inhibition by finasteride. Arch. Oral Biol. 2000, 45, 347–354. [Google Scholar] [CrossRef]
- Pang, S.T.; Dillner, K.; Wu, X.; Pousette, A.; Norstedt, G.; Flores-Morales, A. Gene expression profiling of androgen deficiency predicts a pathway of prostate apoptosis that involves genes related to oxidative stress. Endocrinology 2002, 43, 4897–4906. [Google Scholar]
- Demirbag, R.; Yilmaz, R.; Erel, O. The association of total antioxidant capacity with sex hormones. Scand. Cardiovasc. J. 2005, 39, 172–176. [Google Scholar] [CrossRef]
- Nokhbehsaim, M.; Winter, J.; Rath, B.; Jäger, A.; Jepsen, S.; Deschner, J. Effects of enamel matrix derivative on periodontal wound healing in an inflammatory environment in vitro. J. Clin. Periodontol. 2011, 38, 479–490. [Google Scholar] [CrossRef]
- Hughes, F.J.; Turner, W.; Belibasakis, G.; Martuscelli, G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontology 2006, 41, 48–72. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Qattan, T.; Soory, M. Anabolic Actions of the Regenerative Agent Enamel Matrix Derivative (EMD) in Oral Periosteal Fibroblasts and MG 63 Osteoblasts, Modulation by Nicotine and Glutathione in a Redox Environment. J. Funct. Biomater. 2012, 3, 143-162. https://doi.org/10.3390/jfb3010143
Al-Qattan T, Soory M. Anabolic Actions of the Regenerative Agent Enamel Matrix Derivative (EMD) in Oral Periosteal Fibroblasts and MG 63 Osteoblasts, Modulation by Nicotine and Glutathione in a Redox Environment. Journal of Functional Biomaterials. 2012; 3(1):143-162. https://doi.org/10.3390/jfb3010143
Chicago/Turabian StyleAl-Qattan, Tareq, and Mena Soory. 2012. "Anabolic Actions of the Regenerative Agent Enamel Matrix Derivative (EMD) in Oral Periosteal Fibroblasts and MG 63 Osteoblasts, Modulation by Nicotine and Glutathione in a Redox Environment" Journal of Functional Biomaterials 3, no. 1: 143-162. https://doi.org/10.3390/jfb3010143