Artificial Crab Burrows Facilitate Desalting of Rooted Mangrove Sediment in a Microcosm Study
Abstract
:1. Introduction
2. Experimental Section
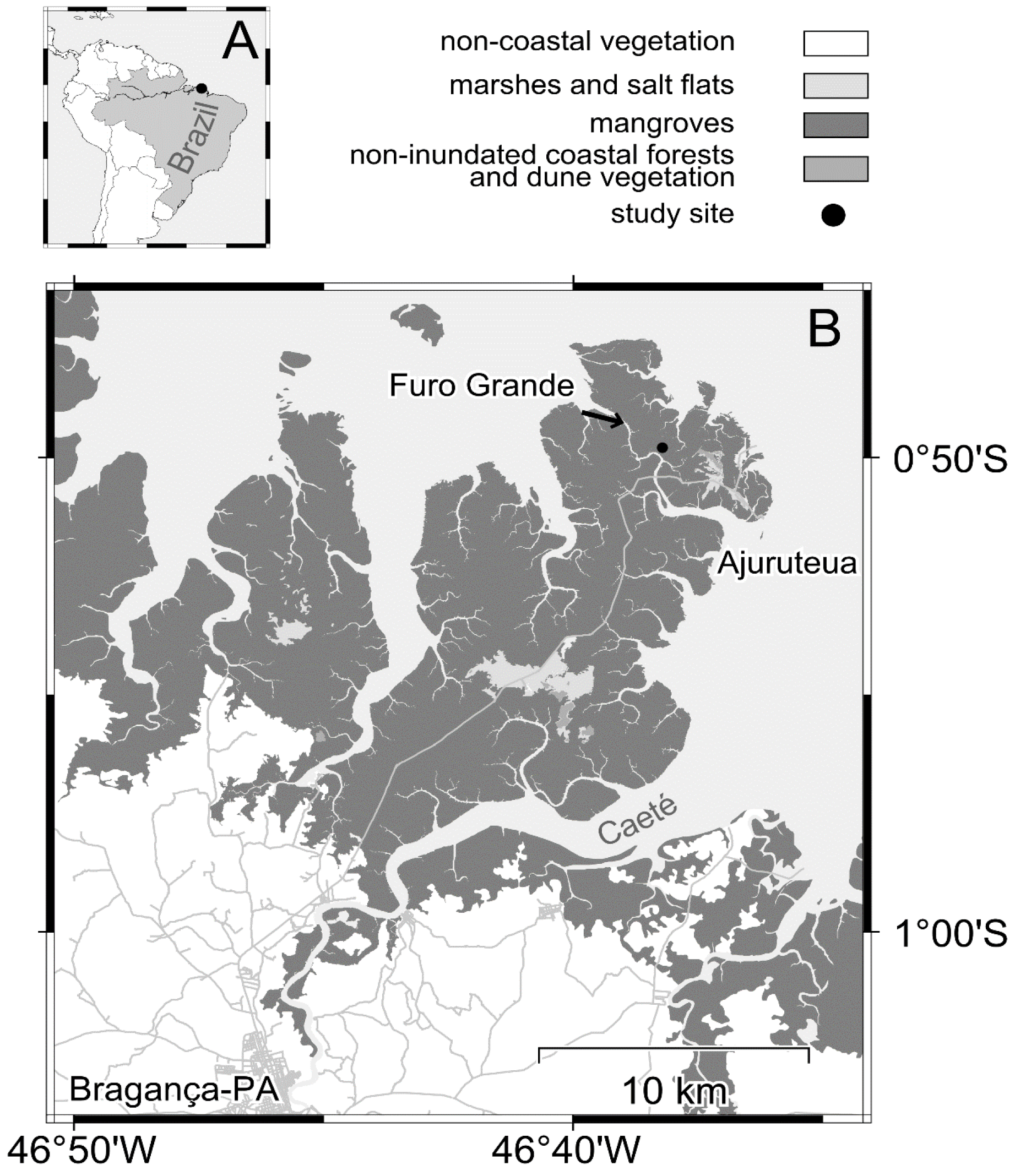
2.1. Study Area
2.2. Microcosm Experiment
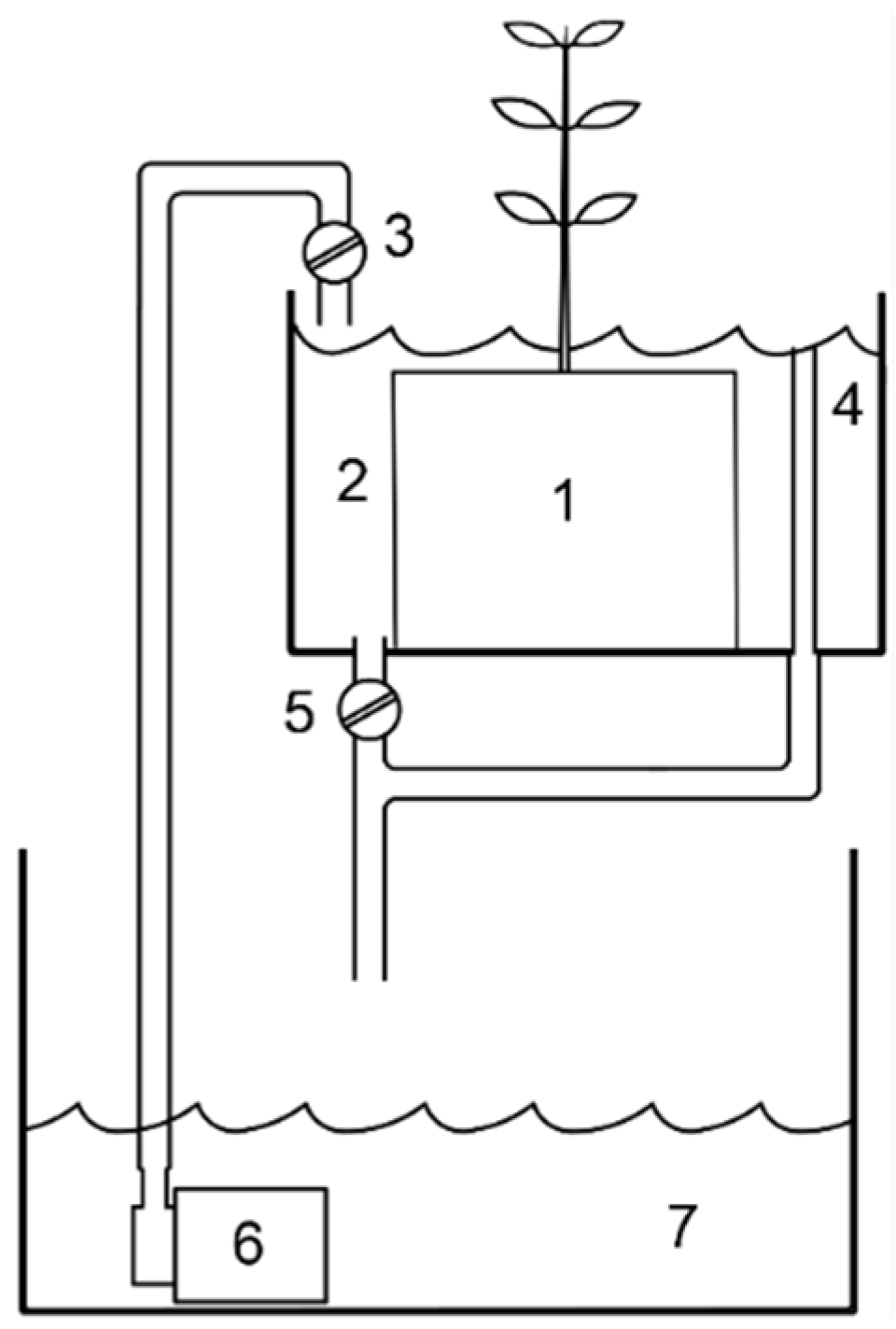
2.3. Microcosm Sampling
2.4. Field Sampling
2.5. Statistical Analyses
3. Results
3.1. Microcosm
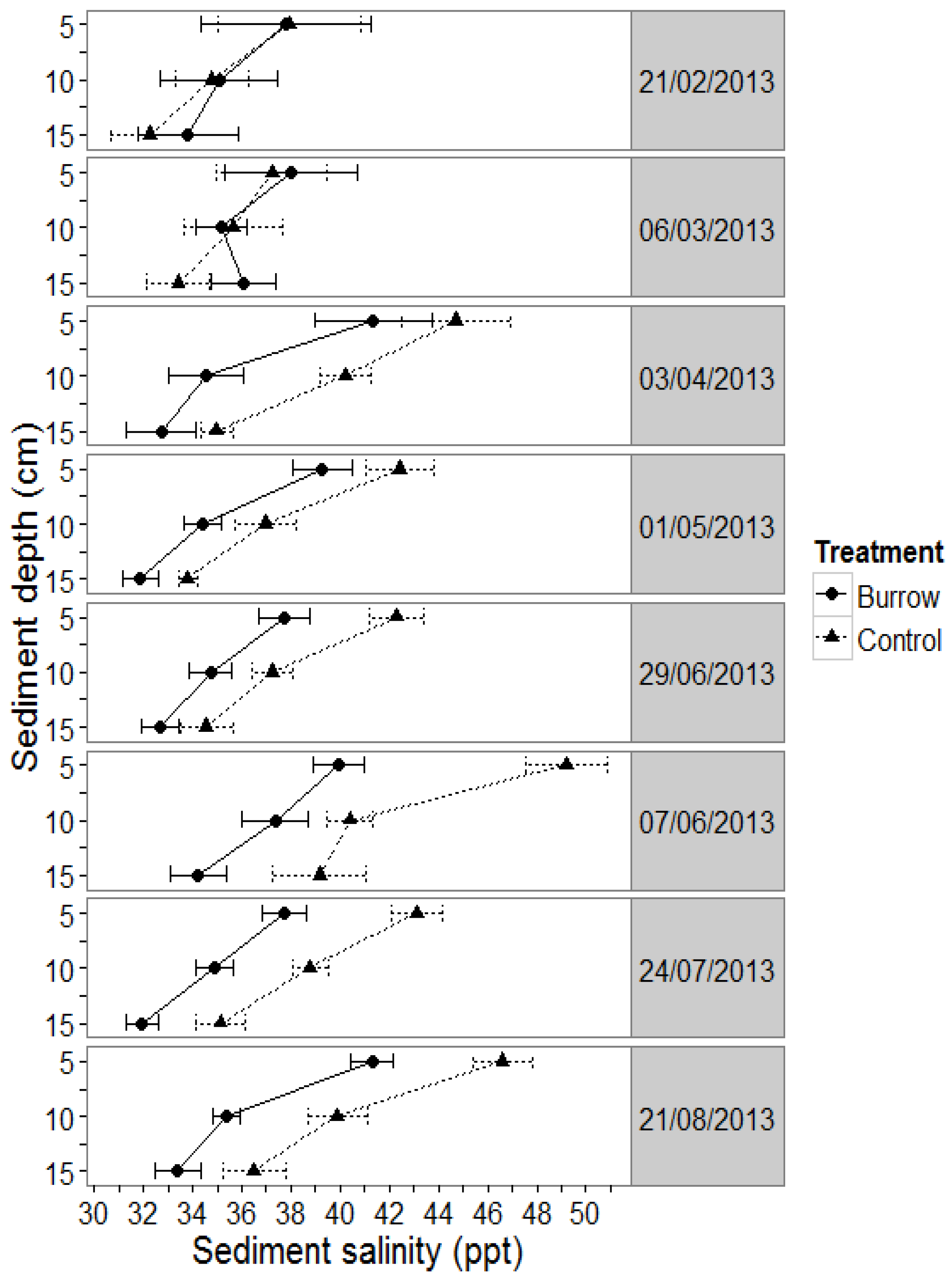
3.1.1. Sediment Salinity
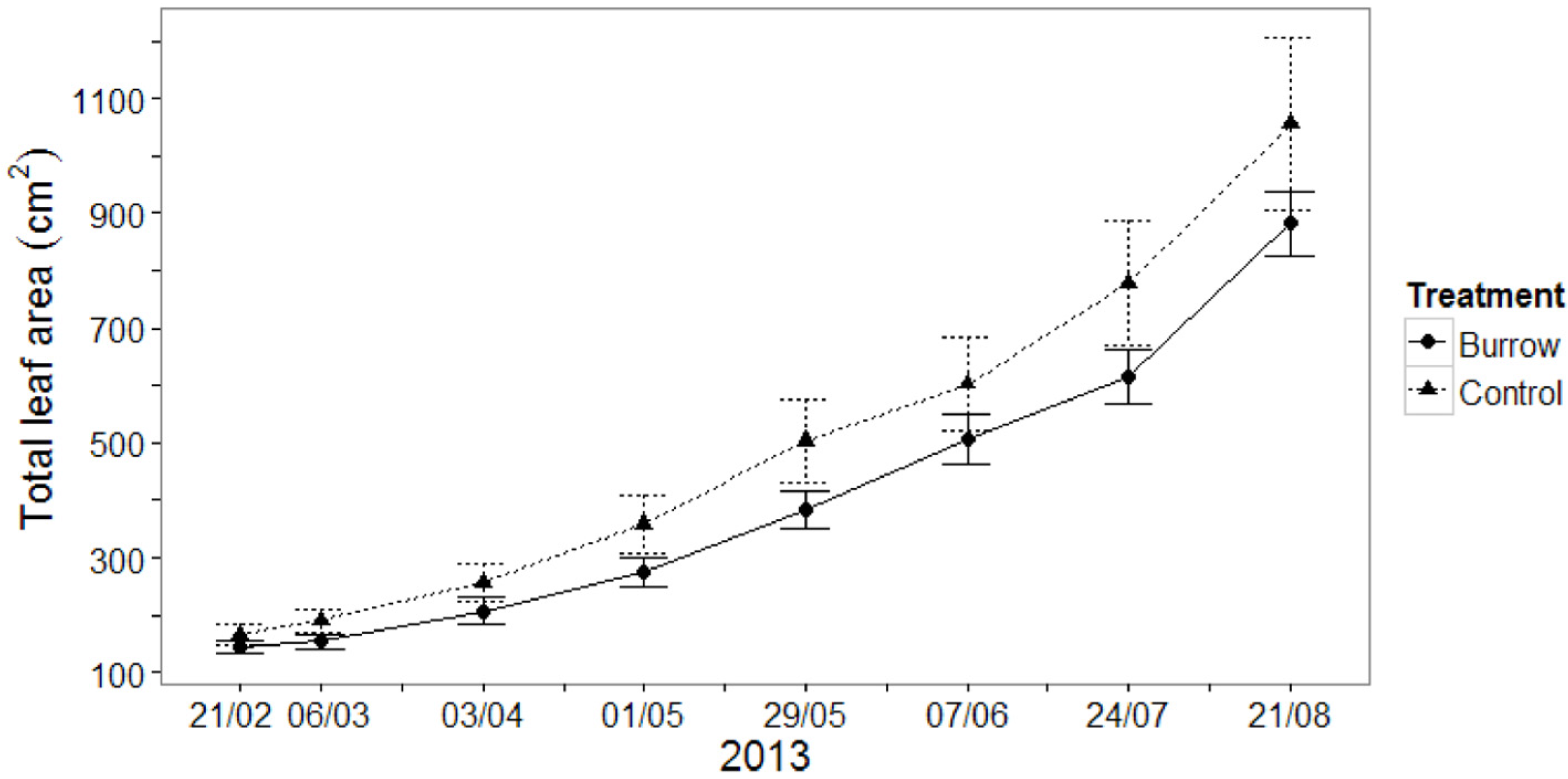
3.1.2. Seedling Growth

| Plant Part Analyses | ||||
|---|---|---|---|---|
| (a) | Regression Parameter | R2 | n | |
| Roots DB = −0.51 + TLA × 0.01 | 0.91 | 48 | ||
| Stem/branches DB = 3.86 + TLA × 0.02 | 0.88 | 48 | ||
| * Leaves DB = 0 + TLA × 0.02 | 0.99 | 48 | ||
| (b) | Plant Part | t-Value | Df | p-Value |
| roots | 1.9 | 13 | 0.07 | |
| stem/branches | −0.4 | 12 | 0.72 | |
| leaves | −0.2 | 16 | 0.84 | |
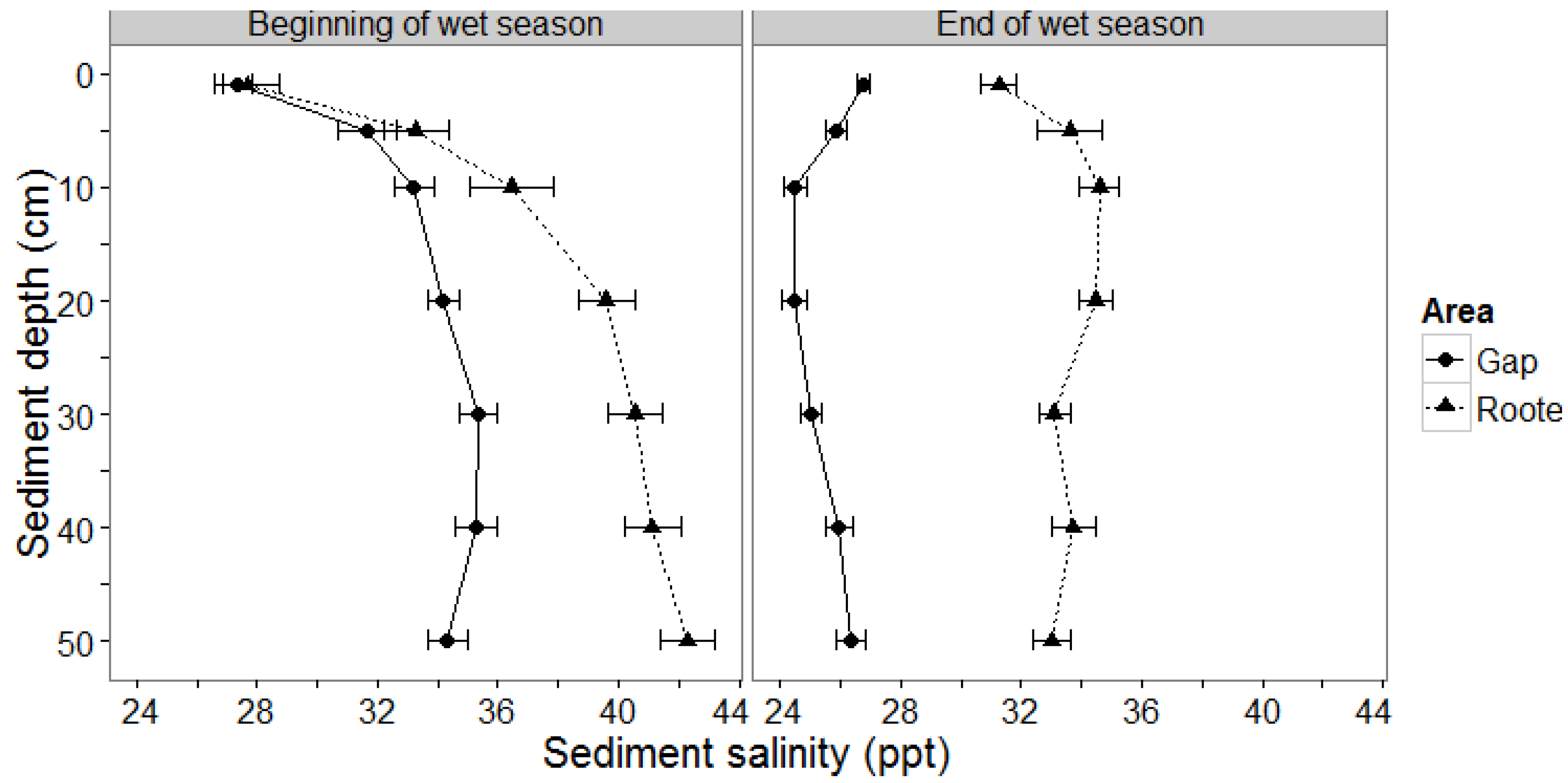
3.2. Field Experiment
4. Discussion
4.1. Microcosm Experiment
4.2. Mangrove Sediment Salinity
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
Appendix A1
Appendix A2
Appendix A3
Appendix A4
Appendix A5
References
- Ball, M.C. Ecophysiology of mangroves. Trees 1988, 2, 129–142. [Google Scholar] [CrossRef]
- Gill, A.M.; Tomlinson, P.B. Studies on the growth of red mangrove (Rhizophora mangle L.) 4. The adult root system. Biotropica 1977, 9, 145–155. [Google Scholar] [CrossRef]
- Passioura, J.B.; Ball, M.C.; Knight, J.H. Mangroves may salinize the soil and in so doing limit their transpiration rate. Funct. Ecol. 1992, 6, 476–481. [Google Scholar] [CrossRef]
- Alongi, D.M.; Tirendi, F.; Dixon, P.; Trott, L.A.; Brunskill, G.J. Mineralization of organic matter in intertidal sediments of a tropical semi-enclosed delta. Estuar. Coast. Shelf Sci. 1999, 48, 451–467. [Google Scholar] [CrossRef]
- Holmer, M.; Andersen, F.Ø.; Holmboe, N.; Kristensen, E.; Thongtham, N. Transformation and exchange processes in the Bangrong mangrove forest-seagrass bed system, Thailand. Seasonal and spatial variations in benthic metabolism and sulfur biogeochemistry. Aquat. Microb. Ecol. 1999, 20, 203–212. [Google Scholar] [CrossRef]
- Schwendenmann, L.; Riecke, R.; Lara, R.J. Solute dynamics in a North Brazilian mangrove: The influence of sediment permeability and freshwater input. Wetl. Ecol. Manag. 2006, 14, 463–475. [Google Scholar] [CrossRef]
- Hollins, S.E.; Ridd, P.V.; Read, W.W. Measurement of the diffusion coefficient for salt in salt flat and mangrove soils. Wetl. Ecol. Manag. 2000, 8, 257–262. [Google Scholar] [CrossRef]
- Stieglitz, T.C.; Clark, J.F.; Hancock, G.J. The mangrove pump: The tidal flushing of animal burrows in a tropical mangrove forest determined from radionuclide budgets. Geochim. Cosmochim. Acta 2013, 102, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Wolanski, E.; Gardiner, R. Flushing of salt from mangrove swamps. Mar. Freshw. Res. 1981, 32, 681–683. [Google Scholar] [CrossRef]
- Heron, S.F.; Ridd, P.V. The tidal flushing of multiple-loop animal burrows. Estuar. Coast. Shelf Sci. 2008, 78, 135–144. [Google Scholar] [CrossRef]
- Heron, S.F.; Ridd, P.V. The effect of water density variations on the tidal flushing of animal burrows. Estuar. Coast. Shelf Sci. 2003, 58, 137–145. [Google Scholar] [CrossRef]
- Stieglitz, T.C.; Ridd, P.V.; Müller, P. Passive irrigation and functional morphology of crustacean burrows in a tropical mangrove swamp. Hydrobiologia 2000, 421, 69–76. [Google Scholar] [CrossRef]
- Colbert, S.L.; Berelson, W.M.; Hammond, D.E. Radon-222 budget in Catalina Harbor, California: 2. Flow dynamics and residence time in a tidal beach. Limnol. Oceanogr. 2008, 53, 659–665. [Google Scholar] [CrossRef]
- Li, X.; Hu, B.X.; Burnett, W.C.; Santos, I.R.; Chanton, J.P. Submarine ground water discharge driven by tidal pumping in a heterogeneous aquifer. Ground Water 2009, 47, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Susilo, A.; Ridd, P.V.; Thomas, S. Comparison between tidally driven groundwater flow and flushing of animal burrows in tropical mangrove swamps. Wetl. Ecol. Manag. 2005, 13, 377–388. [Google Scholar] [CrossRef]
- Adame, M.F.; Virdis, B.; Lovelock, C.E. Effect of geomorphological setting and rainfall on nutrient exchange in mangroves during tidal inundation. Mar. Freshw. Res. 2010, 61, 1197–1206. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Ikeda, S.; Toda, Y. Transport of nutrients and organic matter in a mangrove swamp. Estuar. Coast. Shelf Sci. 2009, 82, 233–242. [Google Scholar] [CrossRef]
- Bouillon, S.; Middelburg, J.J.; Dehairs, F.; Borges, A.V.; Abril, G.; Flindt, M.R.; Ulomi, S.; Kristensen, E. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences 2007, 4, 311–322. [Google Scholar] [CrossRef]
- Gleeson, J.; Santos, I.R.; Maher, D.T.; Golsby-Smith, L. Groundwater-surface water exchange in a mangrove tidal creek: Evidence from natural geochemical tracers and implications for nutrient budgets. Mar. Chem. 2013, 156, 27–37. [Google Scholar] [CrossRef]
- Lara, R.J.; Dittmar, T. Nutrient dynamics in a mangrove creek (North Brazil) during the dry season. Mangroves salt marshes 1999, 3, 185–195. [Google Scholar] [CrossRef]
- Maher, D.T.; Santos, I.R.; Golsby-Smith, L.; Gleeson, J.; Eyre, B.D. Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: The missing mangrove carbon sink? Limnol. Ocean. 2013, 58, 475–488. [Google Scholar]
- Mazda, Y.; Ikeda, Y. Behavior of the groundwater in a riverine-type mangrove forest. Wetl. Ecol. Manag. 2006, 14, 477–488. [Google Scholar] [CrossRef]
- Xin, P.; Jin, G.; Li, L.; Barry, D.A. Effects of crab burrows on pore water flows in salt marshes. Adv. Water Resour. 2009, 32, 439–449. [Google Scholar] [CrossRef]
- Heron, S.F.; Ridd, P.V. The use of computational fluid dynamics in predicting the tidal flushing of animal burrows. Estuar. Coast. Shelf Sci. 2001, 52, 411–421. [Google Scholar] [CrossRef]
- Ridd, P.V. Flow through animal burrows in mangrove creeks. Estuar. Coast. Shelf Sci. 1996, 43, 617–625. [Google Scholar] [CrossRef]
- Cannicci, S.; Burrows, D.; Fratini, S.; Smith, T.J., III; Offenberg, J.; Dahdouh-Guebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- Katz, L.C. Effects of burrowing by the fiddler crab, Uca pugnax (Smith). Estuar. Coast. Mar. Sci. 1980, 11, 233–237. [Google Scholar] [CrossRef]
- Kristensen, E. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 2000, 426, 1–24. [Google Scholar] [CrossRef]
- Qureshi, N.A.; Saher, N.U. Burrow morphology of three species of fiddler crab (Uca) along the coast of Pakistan. Belg. J. Zool. 2012, 142, 114–126. [Google Scholar]
- Thongtham, N.; Kristensen, E. Physical and chemical characteristics of mangrove crab (Neoepisesarma versicolor) burrows in the Bangrong mangrove forest, Phuket, Thailand; with emphasis on behavioural response to. Vie Milieu 2003, 53, 141–151. [Google Scholar]
- Schories, D.; Barletta-Bergan, A.; Barletta, M.; Krumme, U.; Mehlig, U.; Rademaker, V. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetl. Ecol. Manag. 2003, 11, 243–255. [Google Scholar] [CrossRef]
- Diele, K.; Koch, V.; Saint-Paul, U. Population structure, catch composition and CPUE of the artisanally harvested mangrove crab Ucides cordatus (Ocypodidae) in the Caeté estuary, North Brazil: Indications for overfishing? Aquat. Living Resour. 2005, 18, 169–178. [Google Scholar] [CrossRef]
- Tavares, M. True Crabs. In The Living Marine Resources of the Western Central Atlantic; Carpenter, K.E., Ed.; Food and Agriculture Organization (FAO): Rome, Italy, 2003; pp. 327–352. [Google Scholar]
- Piou, C.; Berger, U.; Feller, I.C. Spatial structure of a leaf-removing crab population in a mangrove of North-Brazil. Wetl. Ecol. Manag. 2009, 17, 93–106. [Google Scholar] [CrossRef]
- Tamooh, F.; Huxham, M.; Karachi, M.; Mencuccini, M.; Kairo, J.G.; Kirui, B. Below-ground root yield and distribution in natural and replanted mangrove forests at Gazi bay, Kenya. For. Ecol. Manag. 2008, 256, 1290–1297. [Google Scholar] [CrossRef]
- Araújo, J.M.C., Jr.; Otero, X.L.; Marques, A.G.B.; Nóbrega, G.N.; Silva, J.R.F.; Ferreira, T.O. Selective geochemistry of iron in mangrove soils in a semiarid tropical climate: Effects of the burrowing activity of the crabs Ucides cordatus and Uca maracoani. Geo-Mar. Lett. 2012, 32, 289–300. [Google Scholar] [CrossRef]
- Bouillon, S.; Koedam, N.; Raman, A.V.; Dehairs, F. Primary producers sustaining macro-invertebrate communities in intertidal mangrove forests. Oecologia 2002, 130, 441–448. [Google Scholar] [Green Version]
- Dye, A.H.; Lasiak, T.A. Microbenthos, meiobenthos and fiddler crabs: Trophic interactions in a tropical mangrove sediment. Mar. Ecol. Prog. Ser. 1986, 32, 259–264. [Google Scholar] [CrossRef]
- France, R. Estimating the assimilation of mangrove detritus by fiddler crabs in Laguna Joyuda, Puerto Rico, using dual stable isotopes. J. Trop. Ecol. 1998, 14, 413–425. [Google Scholar] [CrossRef]
- Miller, D.C. The feeding mechanism of fiddler crabs, with ecological considerations of feeding adaptations. Zoologica 1961, 46, 89–101. [Google Scholar]
- Mehlig, U.; Menezes, M.P.M.; Reise, A.; Schories, D.; Medina, E. Mangrove Vegetation of the Caeté Estuary. In Mangrove Dynamics and Management in North Brazil; Saint-Paul, U., Schneider, H., Eds.; Ecological Studies; Springer: Berlin, Germany, 2010; Volume 211, pp. 71–107. [Google Scholar]
- INMET. Available online: http://www.inmet.gov.br (accessed on 15 January 2013).
- Brabo, L.B. Medidas de Trocas Gasosas de Plantas de Manguezal sob Diferentes Condições de Salinidade e Inundação em Estufa e Campo (Bragança-PA-Brasil); Universidade federal do Pará, Campus universitário de Bragança: Belem, Bragança, Brazil, 2004. [Google Scholar]
- Steubing, L.; Fangmeier, A. Pflanzenökologisches Praktikum: Gelände- und Laborpraktikum der terrestrischen Pflanzenökologie; Ulmer: Stuttgart, Germany, 1992. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models. In R Package Version; R Foundation for Statistical Computing: Vienna, Austria, 2012; pp. 1–102. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analyzing Ecological Data; Springer: New York, NY, USA, 2007. [Google Scholar]
- Nordhaus, I.; Diele, K.; Wolff, M. Activity patterns, feeding and burrowing behaviour of the crab Ucides cordatus (Ucididae) in a high intertidal mangrove forest in North Brazil. J. Exp. Mar. Bio. Ecol. 2009, 374, 104–112. [Google Scholar] [CrossRef]
- Biber, P.D. Measuring the effects of salinity stress in the red mangrove, Rhizophora mangle L. Afr. J. Agric. Res. 2006, 1, 1–4. [Google Scholar]
- Hoppe-Speer, S.C.L.; Adams, J.B.; Rajkaran, A.; Bailey, D. The response of the red mangrove Rhizophora mucronata Lam. to salinity and inundation in South Africa. Aquat. Bot. 2011, 95, 71–76. [Google Scholar] [CrossRef]
- Krauss, K.W.; Allen, J.A. Influences of salinity and shade on seedling photosynthesis and growth of two mangrove species, Rhizophora mangle and Bruguiera sexangula, introduced to Hawaii. Aquat. Bot. 2003, 77, 311–324. [Google Scholar] [CrossRef]
- Smith, S.M.; Snedaker, S.C. Salinity responses in two populations of viviparous Rhizophora mangle L. seedlings. Biotropica 1995, 27, 435–440. [Google Scholar] [CrossRef]
- Lin, G.; Sternberg, L.S.L. Effect of growth form, salinity, nutrient and sulfide on photosynthesis, carbon isotope discrimination and growth of red mangrove (Rhizophora mangle L.). Funct. Plant. Biol. 1992, 19, 509–517. [Google Scholar]
- Ball, M.C.; Cochrane, M.J.; Rawson, H.M. Growth and water use of the mangroves Rhizophora apiculata and R. stylosa in response to salinity and humidity under ambient and elevated concentrations of atmospheric CO2. Plant Cell Environ. 1997, 20, 1158–1166. [Google Scholar] [CrossRef]
- López-Hoffman, L.; Anten, N.P.R.; Martínez-Ramos, M.; Ackerly, D.D. Salinity and light interactively affect neotropical mangrove seedlings at the leaf and whole plant levels. Oecologia 2007, 150, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, biomass, and productivity of mangrove forests: A review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Smith, T.J., III. Effects of seed predators and light level on the distribution of Avicennia marina (Forsk.) Vierh in tropical, tidal forests. Estuar. Coast. Shelf Sci. 1987, 25, 43–51. [Google Scholar] [CrossRef]
- Stieglitz, T.C.; Ridd, P.V.; Hollins, S.E. A small sensor for detecting animal burrows and monitoring burrow water conductivity. Wetl. Ecol. Manag. 2000, 8, 1–7. [Google Scholar] [CrossRef]
- Marchand, C.; Baltzer, F.; Lallier-Vergès, E.; Albéric, P. Pore-water chemistry in mangrove sediments: Relationship with species composition and developmental stages (French Guiana). Mar. Geol. 2004, 208, 361–381. [Google Scholar] [CrossRef] [Green Version]
- Mehlig, U. Phenology of the red mangrove, Rhizophora mangle L., in the Caeté Estuary, Pará, equatorial Brazil. Aquat. Bot. 2006, 84, 158–164. [Google Scholar] [CrossRef]
- Menezes, M.; Berger, U.; Worbes, M. Annual growth rings and long-term growth patterns of mangrove trees from the Bragança peninsula, North Brazil. Wetl. Ecol. Manag. 2003, 11, 233–242. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pülmanns, N.; Nordhaus, I.; Diele, K.; Mehlig, U. Artificial Crab Burrows Facilitate Desalting of Rooted Mangrove Sediment in a Microcosm Study. J. Mar. Sci. Eng. 2015, 3, 539-559. https://doi.org/10.3390/jmse3030539
Pülmanns N, Nordhaus I, Diele K, Mehlig U. Artificial Crab Burrows Facilitate Desalting of Rooted Mangrove Sediment in a Microcosm Study. Journal of Marine Science and Engineering. 2015; 3(3):539-559. https://doi.org/10.3390/jmse3030539
Chicago/Turabian StylePülmanns, Nathalie, Inga Nordhaus, Karen Diele, and Ulf Mehlig. 2015. "Artificial Crab Burrows Facilitate Desalting of Rooted Mangrove Sediment in a Microcosm Study" Journal of Marine Science and Engineering 3, no. 3: 539-559. https://doi.org/10.3390/jmse3030539
APA StylePülmanns, N., Nordhaus, I., Diele, K., & Mehlig, U. (2015). Artificial Crab Burrows Facilitate Desalting of Rooted Mangrove Sediment in a Microcosm Study. Journal of Marine Science and Engineering, 3(3), 539-559. https://doi.org/10.3390/jmse3030539





