A Review of Molecular-Level Mechanism of Membrane Degradation in the Polymer Electrolyte Fuel Cell
Abstract
:1. Introduction
2. Membrane Degradation in PFSA
2.1. Mechanical Degradation
2.2. Thermal Degradation
2.3. Chemical Degradation
3. Chemical Degradation Mechanism by Experiments
3.1. PFSA Main Chain
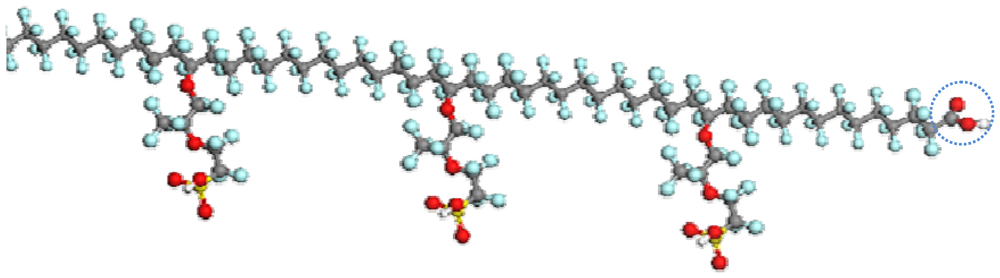
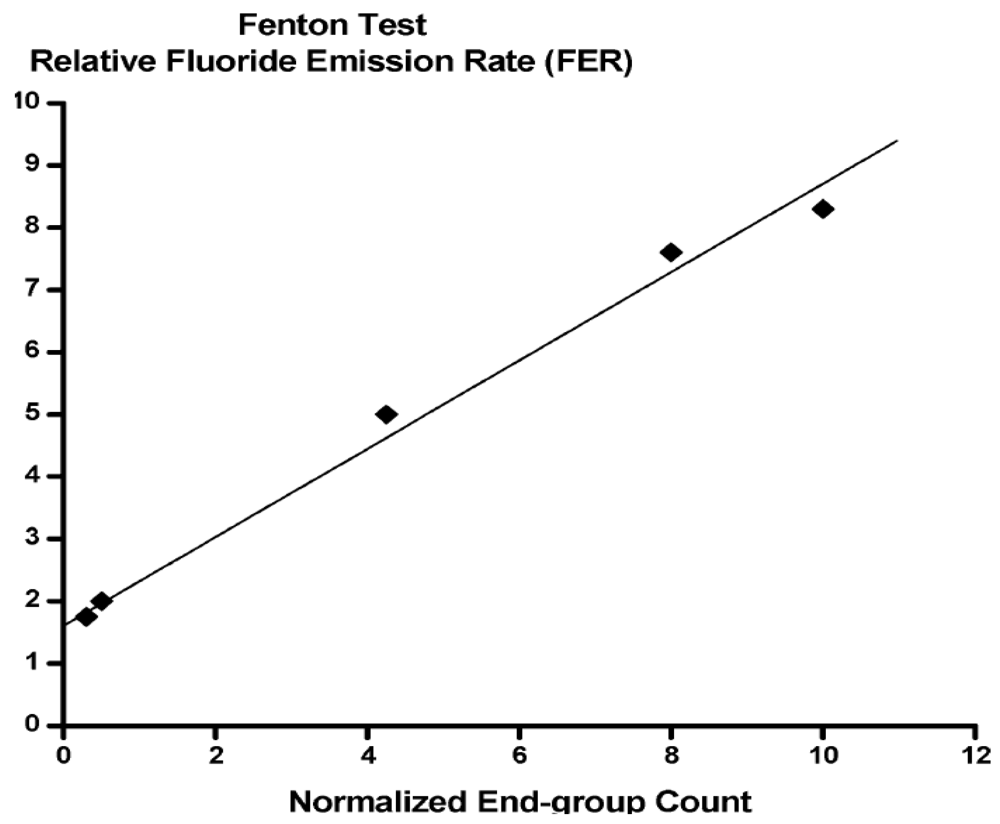
3.2. PFSA Side Chain
| Model compounds | Temperature (°C) | Time (h) | Recovery (%) |
|---|---|---|---|
 | 100 | 6 | 100 |
| 100 | 24 | 100 | |
 | 100 | 6 | 84 |
| 100 | 24 | 80 |
4. Chemical Degradation Mechanism Studied by Theoretical Methods
4.1. Theoretical Approaches for PFSA Membrane
4.2. Bond Dissociation Analysis
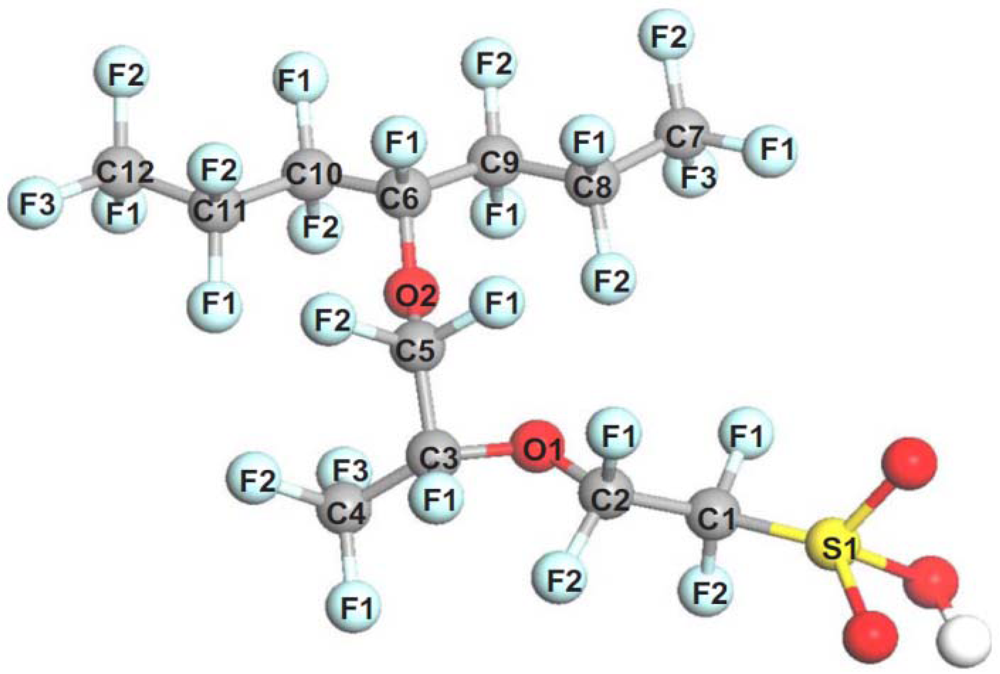
| Bond | Neutral | Ionized |
|---|---|---|
| S1–C1 | 257.3 | 349.8 |
| C1–C2 | 356.5 | 411.4 |
| C2–O1 | 342.7 | 383.4 |
| O1–C3 | 337.6 | 299.1 |
| C3–C4 | 332.8 | 338.1 |
| C3–C5 | 332.4 | 349.6 |
| C5–O2 | 306.2 | 328.4 |
| O2–C6 | 290.3 | 222.0 |
4.3. Degradation by Chemical Reaction of PFSA Polymer and OH Radical
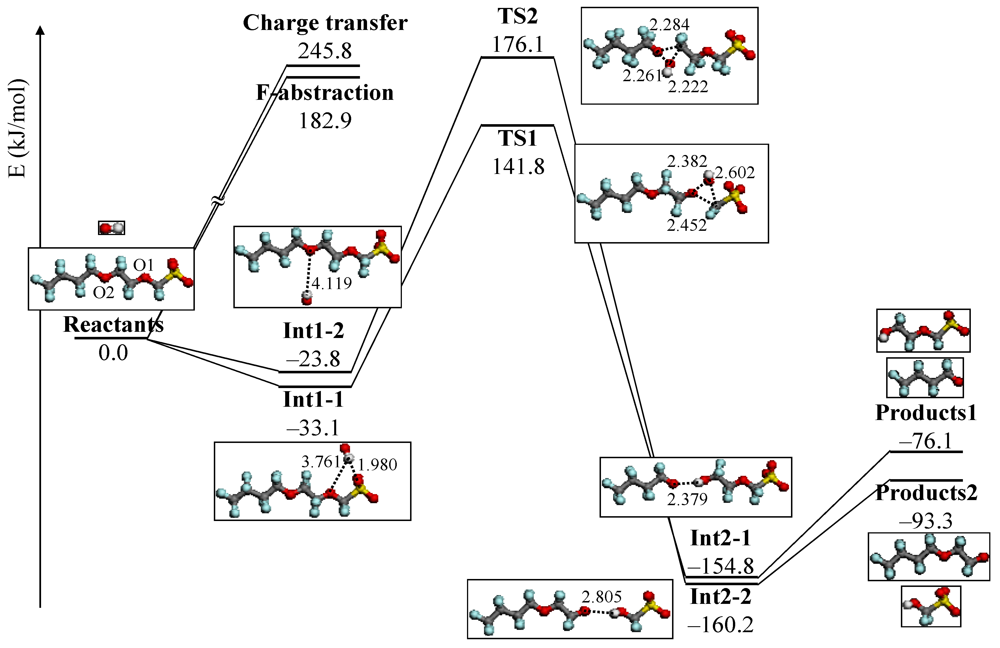
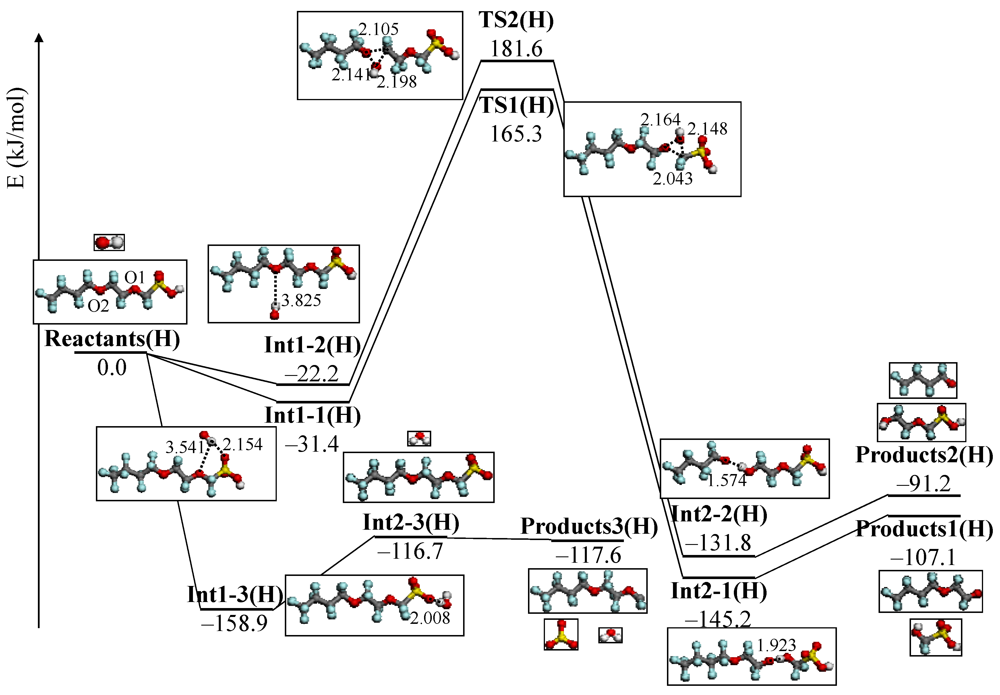
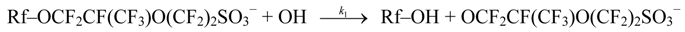

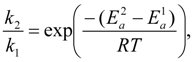
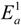 and
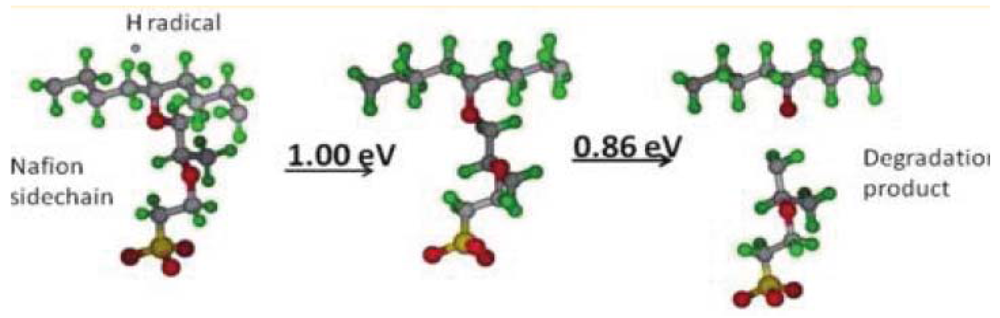
and 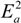 are activation energies of reactions (4) and (5), respectively. Because k2/k1 is estimated as 4.0 × 103, reaction (5) has a large advantage. We clearly demonstrated the degradation mechanism of the ether group in the Nafion® side chain by the OH radical from theoretical analysis as well as from the previous results for Nafion® model compounds.
are activation energies of reactions (4) and (5), respectively. Because k2/k1 is estimated as 4.0 × 103, reaction (5) has a large advantage. We clearly demonstrated the degradation mechanism of the ether group in the Nafion® side chain by the OH radical from theoretical analysis as well as from the previous results for Nafion® model compounds. 

5. Summary
Acknowledgments
References
- Fuel Cell Commercialization Conference of Japan Home Page. Available online: http://www.fccj.jp (accessed on 28 June 2012).
- Staffell, I.; Green, R.J. Estimating future process for stationary fuel cells with empirically derived experience curves. Int. J. Hydrog. Energy 2009, 34, 5617–5628. [Google Scholar] [CrossRef]
- Vengatesan, S.; Fowler, M.W.; Yuan, X.Z.; Wang, H. Diagnosis of MEA degradation under accelerated relative humidity cycling. J. Power Sources 2011, 196, 5045–5052. [Google Scholar] [CrossRef]
- Lin, R.; Li, B.; Hou, Y.P.; Ma, J.M. Investigation of dynamic driving cycle effect on performance degradation and microstructure change of PEM fuel cell. Int. J. Hydrog. Energy 2009, 34, 2369–2376. [Google Scholar] [CrossRef]
- Shah, A.A.; Ralph, T.R.; Walsh, F.C. Modeling and simulation of the degradation of perfluorinated ion-exchange membranes in PEM fuel cells. J. Electrochem. Soc. 2009, 156, B465–B484. [Google Scholar] [CrossRef]
- Chen, C.; Fuller, T.F. XPS analysis of polymer membrane degradation in PEMFCs. J. Electrochem. Soc. 2009, 156, B1218–B1224. [Google Scholar] [CrossRef]
- Chen, C.; Fuller, T.F. The effect of humidity on the degradation of Nafion membrane. Polym. Degrad. Stab. 2009, 94, 1436–1447. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Marrony, M.; Barrera, R.; Kreuer, K.D.; Maier, J.; Müller, K. Chemical degradation of proton conducting perfluorosulfonic acid ionomer membranes studied by solid-state nuclear magnetic resonance spectroscopy. J. Power Sources 2009, 186, 334–338. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, X.; Wang, H.; Mérida, W.; Zhu, H.; Shen, J.; Wu, S.; Zhang, J. A review of accelerated stress tests of MEA durability in PEM fuel cells. Int. J. Hydrog. Energy 2009, 34, 388–404. [Google Scholar]
- Takizawa, S.; Nakazawa, A.; Inoue, M.; Umeda, M. Anodic Pt dissolution in concentrated trifluoromethanesulfonic acid. J. Power Sources 2010, 195, 5966–5970. [Google Scholar] [CrossRef]
- Yousfi-Steiner, N.; Moçotéguy, Ph.; Candusso, D.; Hissel, D. A review on polymer electrolyte membrane fuel cell catalyst degradation and starvation issues: Causes, consequences, and diagnostic for mitigation. J. Power Sources 2009, 194, 130–145. [Google Scholar] [CrossRef]
- Chung, C.G.; Kim, L.; Sung, Y.W.; Lee, J.; Chung, J.S. Degradation mechanism of electrocatalyst during long-term operation of PEMFC. Int. J. Hydrog. Energy 2009, 34, 8974–8981. [Google Scholar]
- Kim, L.; Chung, C.G.; Sung, Y.W.; Chung, J.S. Dissolution and migration of platinum after long-term operation of a polymer electrolyte fuel cell under various conditions. J. Power Sources 2008, 183, 524–532. [Google Scholar] [CrossRef]
- Mitsushima, S.; Koizumi, Y.; Uzuka, S.; Ota, K. Dissolution of platinum in acidic media. Electrochim. Acta 2008, 54, 455–460. [Google Scholar] [CrossRef]
- Borup, R.M.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; Zelenay, P.; More, K.; Stroh, K.; Zawodzinski, T.; Boncella, J.; McGrath, J.E.; Inaba, M.; Miyatake, K.; Hori, M.; Ota, K.; Ogumi, Z.; Miyata, S.; Mishikata, A.; Siroma, Z.; Uchimoto, Y.; Yasuda, K.; Kimijima, K.; Iwashita, N. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar]
- Shao-Horn, Y.; Sheng, W.C.; Chen, S.; Ferreira, P.J.; Holby, E.F.; Morgan, D. Instability of supported platinum nanoparticles in low-temperature fuel cells. Top. Catal. 2007, 46, 285–305. [Google Scholar]
- Yasuda, K.; Taniguchi, A.; Akita, T.; Ioroi, T.; Siroma, Z. Characteristics of a platinum black catalyst layer with regard to platinum dissolution phenomena in a membrane electrode assembly. J. Electrochem. Soc. 2006, 153, A1599–A1603. [Google Scholar] [CrossRef]
- Wang, X.; Kumar, R.; Myers, D.J. Effect of voltage on platinum dissolution. Electrochem. Solid-State Lett. 2006, 9, A225–A227. [Google Scholar] [CrossRef]
- Xie, J.; Wood, D.L., III; Wayne, D.M.; Zawodzinski, T.A.; Atanassov, P.; Borup, R.L. Durability of PEFCs at high humidity conditions. J. Electrochem. Soc. 2005, 152, A104–A113. [Google Scholar] [CrossRef]
- Antolini, E. Formation, microstructural characteristics and stability of carbon supported platinum catalysts for low temperature fuel cells. J. Mater. Sci. 2003, 38, 2995–3005. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Schmittinger, W.; Vahidi, A. A review of the main parameters influencing long-term performance and durability of PEM fuel cells. J. Power Sources 2008, 180, 1–14. [Google Scholar] [CrossRef]
- De Bruijn, F.A.; Dam, V.A.; Janssen, J.M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cell 2008, 1, 3–22. [Google Scholar]
- Hartnig, C.; Schmidt, T.J. Simulated start-stop as a rapid aging tool for polymer electrolyte fuel cell electrodes. J. Power Sources 2011, 196, 5564–5572. [Google Scholar] [CrossRef]
- Scholta, J.; Pawlik, J.; Chmielewski, N.; Jörissen, L. Longevity test results for reformate polymer electrolyte membrane fuel cell stacks. J. Power Sources 2011, 196, 5264–5271. [Google Scholar] [CrossRef]
- Ishigami, Y.; Takada, K.; Yano, H.; Inukai, J.; Uchida, M.; Nagumo, Y.; Hyakutake, T.; Nishide, H.; Watanabe, M. Corrosion of carbon supports at cathode during hydrogen/air replacement at anode studied by visualization of oxygen partial pressures in a PEFC—Start-up/shut-down simulation. J. Power Sources 2011, 196, 3003–3008. [Google Scholar]
- Pozio, A.; Cemmi, A.; Mura, F.; Masci, A.; Serra, E.; Silva, R.F. Long-term durability study of perfluoropolymer membranes in low humidification conditions. J. Solid State Electrochem. 2011, 15, 1209–1216. [Google Scholar] [CrossRef]
- Nakamura, S.; Kashiwa, E.; Sasou, H.; Hariyama, S.; Aoki, T.; Ogami, Y.; Nishikawa, H. Measurement of leak current generation distribution in PEFC and its application to load fluctuation testing under low humidification. Elec. Eng. Jpn. 2011, 174, 1–9. [Google Scholar]
- Zhang, J.; Song, C.; Zhang, J. Accelerated lifetime testing for proton exchange membrane fuel cells using extremely high temperature and unusually high load. J. Fuel Cell Sci. Technol. 2011, 8, 051006:1–051006:5. [Google Scholar]
- Bajpai, H.; Khandelwal, M.; Kumbur, E.C.; Mench, M.M. A computational model for assessing impact of interfacial morphology on polymer electrolyte fuel cell performance. J. Power Sources 2010, 195, 4196–4205. [Google Scholar]
- Kim, J.H.; Jo, Y.Y.; Cho, E.A.; Jang, J.H.; Kim, H.J.; Lim, T.H.; Oh, I.H.; Ko, J.J.; Son, I.J. Effects of cathode inlet relative humidity on PEMFC durability during startup-shutdown cycling. J. Electrochem. Soc. 2010, 157, B633–B642. [Google Scholar]
- Pinton, E.; Fourneron, Y.; Rosini, S.; Antoni, L. Experimental and theoretical investigations on a proton exchange membrane fuel cell starting up at subzero temperatures. J. Power Sources 2009, 186, 80–88. [Google Scholar] [CrossRef]
- Chen, J.; Zhai, M.; Asano, M.; Huang, L.; Maekawa, Y. Long-term performance of polyetheretherketone-based polymer electrolyte membrane in fuel cells at 95 °C. J. Mater. Sci. 2009, 44, 3674–3681. [Google Scholar] [CrossRef]
- Marrony, M.; Barrera, R.; Quenet, S.; Ginocchio, S.; Montelatici, L.; Aslanides, A. Durability study and lifetime prediction of baseline proton exchange membrane fuel cell under severe operating conditions. J. Power Sources 2008, 182, 469–475. [Google Scholar] [CrossRef]
- Oberholzer, P.; Boillat, P.; Siegrist, R.; Perego, R.; Kästner, A.; Lehmann, E.; Scherer, G.G.; Wokaun, A. Cold-start of a PEFC visualized with high resolution dynamics in-plane neutron imaging. J. Electrochem. Soc. 2012, 159, B235–B245. [Google Scholar]
- Hickner, M.A.; Siegel, N.P.; Chen, K.S.; Hussey, D.S.; Jacobson, D.L. Observations of transient flooding in a proton exchange membrane fuel cell using time-resolved neutron radiography. J. Electrochem. Soc. 2010, 157, B32–B38. [Google Scholar] [CrossRef]
- Turhan, A.; Heller, K.; Brenizer, J.S.; Mench, M.M. Passive control of liquid water storage and distribution in a PEFC through flow-field design. J. Power Sources 2008, 180, 773–783. [Google Scholar] [CrossRef]
- Yu, J.; Matsuura, T.; Yoshikawa, Y.; Islam, M.N.; Hori, M. In situ analysis of performance degradation of a PEMFC under nonsaturated humidification. Electrochem. Solid-State Lett. 2005, 8, A156–A158. [Google Scholar] [CrossRef]
- Serincan, M.F.; Pasaogullari, U. Mechanical behavior of the membrane during the polymer electrolyte fuel cell operation. J. Power Sources 2011, 196, 1303–1313. [Google Scholar] [CrossRef]
- He, S.; Mench, M.M. One-dimentional transient model for frost heave in polymer electrolyte fuel cells. J. Electrochem. Soc. 2006, 153, A1724–A1731. [Google Scholar] [CrossRef]
- Park, G.G.; Lim, S.J.; Park, J.S.; Yim, S.D.; Park, S.H.; Yang, T.H.; Yoon, Y.G.; Kim, C.S. Analysis on the freeze/thaw cycled polymer electrolyte fuel cell. Curr. Appl. Phys. 2010, 10, 562–565. [Google Scholar]
- Yang, X.G.; Tabuchi, Y.; Kagami, F.; Wang, C.Y. Durability of membrane electrode assemblies under polymer electrolyte fuel cell cold-start cycling. J. Electrochem. Soc. 2008, 155, B752–B761. [Google Scholar] [CrossRef]
- Kim, S.; Mench, M.M. Physical degradation of membrane electrode assemblies undergoing freeze/thaw cycling: Micro-structure effects. J. Power Sources 2007, 174, 206–220. [Google Scholar] [CrossRef]
- Carbone, A.; Saccá, A.; Busacca, C.; Frontera, P.; Antonucci, P.L.; Passalacqua, E. Nafion electro-spun reinforced membranes for polymer electrolyte fuel cell. J. Nanosci. NanoTechnol. 2011, 11, 8768–8774. [Google Scholar]
- Zhang, Z.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; Liu, Z.S.; Holdcroft, S. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Collier, A.; Wang, H.; Yuan, X.Z.; Zhang, J.; Wilkinson, D.P. Degradation of polymer electrolyte membranes. Int. J. Hydrog. Energy 2006, 31, 1838–1854. [Google Scholar] [CrossRef]
- Savadogo, O. Emerging membranes for electrochemical systems: Part II. High temperature composite membranes for polymer electrolyte fuel cell (PEFC) applications. J. Power Sources 2004, 127, 135–161. [Google Scholar] [CrossRef]
- Yang, C.; Srinivasan, S.; Bocarsly, A.B.; Tulyani, S.; Benziger, J.B. A comparison of physical properties and fuel cell performance of Nafion and zirconium phosphate/Nafion composite membranes. J. Membr. Sci. 2004, 237, 145–161. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, L.; Mukerjee, S.; Ofer, D.; Nair, B. An investigation of proton conduction in select PEM’s and reaction layer interfaces-designed for elevated temperature operation. J. Membr. Sci. 2003, 219, 123–136. [Google Scholar] [CrossRef]
- Okanishi, T.; Tsuji, Y.; Sakiyama, Y.; Matsuno, S.; Bae, B.; Miyatake, K.; Uchida, M.; Watanabe, M. Effect of PEFC operating conditions on the durability of sulfonated poly(arylene ether sulfone ketone) multiblock membranes. Electrochim. Acta 2011, 56, 8989–8996. [Google Scholar]
- Okanishi, T.; Tsuji, Y.; Sakiyama, Y.; Matsuno, S.; Bae, B.; Miyatake, K.; Uchida, M.; Watanabe, M. Effect of PEFC operating conditions on the durability of sulfonated polyimide membranes. Electrochim. Acta 2011, 58, 589–598. [Google Scholar] [CrossRef]
- Kabasawa, A.; Saito, J.; Miyatake, K.; Uchida, H.; Watanabe, M. Effects of the decomposition products of sulfonated polyimide and Nafion membranes on the degradation and recovery of electrode performance in PEFCs. Electrochim. Acta 2009, 54, 2754–2760. [Google Scholar]
- Kabasawa, A.; Saito, J.; Yano, H.; Miyatake, K.; Uchida, H.; Watanabe, M. Durability of a novel sulfonated polyimide membrane in polymer electrolyte fuel cell operation. Electrochim. Acta 2009, 54, 1076–1082. [Google Scholar]
- Kerres, J. Blend concepts for fuel cell membranes. Polym. Membr. Fuel Cells 2009, 10, 185–210. [Google Scholar]
- Kang, M.S.; Lee, M.J. Anhydrous solid proton conductors based on perfluorosulfonic ionomer with polymeric solvent for polymer electrolyte fuel cell. Electrochem. Comm. 2009, 11, 457–460. [Google Scholar] [CrossRef]
- Lee, H.S.; Badami, A.S.; Roy, A.; McGrath, J.E. Segmented sulfonated poly(arylene ether sulfone)-b-polyimide copolymers for proton exchange membrane fuel cell. I. Copolymer synthesis and fundamental properties. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4879–4890. [Google Scholar]
- Yamaki, T.; Tsukada, J.; Asano, M.; Katakai, R.; Yoshida, M. Preparation of highly stable ion exchange membranes by radiation-induced graft copolymerization of stylene and bis(vinyl phenyl)ethane into crosslinked polytetrafluoroethylene films. J. Fuel Cell Sci. Technol. 2007, 4, 56–64. [Google Scholar] [CrossRef]
- Meyer, G.; Gebel, G.; Gonon, L.; Capron, P.; Marscaq, D.; Marestin, C.; Mercier, R. Degradation of sulfonated polyimide membranes in fuel cell conditions. J. Power Sources 2006, 157, 293–301. [Google Scholar] [CrossRef]
- Aoki, M.; Chikashige, Y.; Miyatake, K.; Uchida, H.; Watanabe, M. Durability of novel sulfonated poly(arylene ether) membrane in PEFC operation. Electrochem. Comm. 2006, 8, 1412–1416. [Google Scholar] [CrossRef]
- Gubler, L.; Koppenol, W.H. Kinetic simulation of the chemical stabilization mechanism in fuel cell membranes using cerium and manganese redox couples. J. Electrochem. Soc. 2012, 159, B211–B218. [Google Scholar] [CrossRef]
- Gubler, L.; Dockheer, S.M.; Koppenol, W.H. Radical (HO·, H. and HOO·) formation and ionomer degradation in polymer electrolyte fuel cells. J. Electrochem. Soc. 2011, 158, B755–B769. [Google Scholar]
- Nosaka, Y.; Ohtaka, K.; Ohguri, N.; Nosaka, A.Y. Detection of OH radicals generated in polymer electrolyte fuel cells. J. Electrochem. Soc. 2011, 158, B430–B433. [Google Scholar]
- Ohguri, N.; Nosaka, A.Y.; Nosaka, Y. Detection of OH radicals as the effect of Pt particles in the membrane of polymer electrolyte fuel cells. J. Power Sources 2010, 195, 4647–4652. [Google Scholar] [CrossRef]
- Gummalla, M.; Atrazhev, V.V.; Condit, D.; Cipollini, N.; Madden, T.; Kuzminyh, N.Y.; Weiss, D.; Burlatsky, S.F. Degradation of polymer-electrolyte membranes in fuel cells II. Theoretical model. J. Electrochem. Soc. 2010, 157, B1542–B1548. [Google Scholar]
- Ohguri, N.; Nosaka, A.Y.; Nosaka, Y. Detection of OH radicals formed at PEFC electrodes by means of a fluorescence probe. Electrochem. Solid-State Lett. 2009, 12, B94–B96. [Google Scholar] [CrossRef]
- Franco, A.A.; Guinard, M.; Barthe, B.; Lemaire, O. Impact of carbon monoxide on PEFC catalyst carbon support degradation under current-cycled operating conditions. Electrochim. Acta 2009, 54, 5267–5279. [Google Scholar]
- Inaba, M.; Sugishita, M.; Wada, J.; Matsuzawa, K.; Yamada, H.; Tasaka, A. Impacts of air bleeding on membrane degradation in polymer electrolyte fuel cells. J. Power Sources 2008, 178, 699–705. [Google Scholar] [CrossRef]
- Kabasawa, A.; Uchida, H.; Watanabe, M. Influence of decomposition products from perfluorosulfonic acid membrane on fuel cell performance. Electrochem. Solid-State Lett. 2008, 11, B190–B192. [Google Scholar] [CrossRef]
- Hommura, S.; Kawahara, K.; Shimohira, T.; Teraoka, Y. Development of a method for clarifying the perfluorosulfonated membrane degradation mechanism in a fuel cell environment. J. Electrochem. Soc. 2008, 155, A29–A33. [Google Scholar]
- Mittal, V.O.; Kunz, H.R.; Fenton, J.M. Membrane degradation mechanisms in PEMFCs. J. Electrochem. Soc. 2007, 154, B652–B656. [Google Scholar]
- Kinumoto, T.; Inaba, M.; Nakayama, Y.; Ogata, K.; Umebayashi, R.; Tasaka, A.; Iriyama, Y.; Abe, T.; Ogumi, Z. Durability of perfluorinated ionomer membrane against hydrogen peroxide. J. Power Sources 2006, 158, 1222–1228. [Google Scholar] [CrossRef]
- Aoki, M.; Uchida, H.; Watanabe, M. Decomposition mechanism of perfluorosulfonic acid electrolyte in polymer electrolyte fuel cells. Electrochem. Comm. 2006, 8, 1509–1513. [Google Scholar]
- Inaba, M.; Kinumoto, T.; Kiriake, M.; Umebayashi, R.; Tasaka, A.; Ogumi, Z. Gas crossover and membrane degradation in polymer electrolyte fuel cells. Electrochim. Acta 2006, 51, 5746–5753. [Google Scholar] [CrossRef]
- Endoh, E. Highly durable MEA for PEMFC under high temperature and low humidity. ECS Trans. 2006, 3, 9–18. [Google Scholar] [CrossRef]
- Aoki, M.; Uchida, M.; Watanabe, M. Novel evaluation method for degradation rate of polymer electrolytes in fuel cells. Electrochem. Comm. 2005, 7, 1434–1438. [Google Scholar] [CrossRef]
- Healy, J.; Hayden, C.; Xie, T.; Olson, K.; Waldo, R.; Brundage, M.; Gasteiger, H.; Abbott, J. Aspects of the chemical degradation of PFSA ionomers used in PEM fuel cells. Fuel Cells 2005, 2, 302–308. [Google Scholar]
- Pozio, A.; Silva, R.F.; de Francesco, M.; Giorge, L. Nafion degradation in PEFCs from end plate iron contamination. Electrochim. Acta 2003, 48, 1543–1549. [Google Scholar] [CrossRef]
- Huang, C.; Tan, K.S.; Lin, J.; Tan, K.L. XRD and XPS analysis of the degradation of the polymer electrolyte in H2-O2 fuel cell. Chem. Phys. Lett. 2003, 371, 80–85. [Google Scholar] [CrossRef]
- Delaney, W.E.; Liu, W. Use of FTIR to analyze ex situ and in situ degradation of perfluorinated fuel cell ionomers. ECS Trans. 2007, 11, 1093–1104. [Google Scholar] [CrossRef]
- Endoh, E.; Hommura, S.; Terazono, S.; Wadjaja, H.; Anzai, J. Degradation mechanism of the PFSA membrane and influence of deposited Pt in the membrane. ECS Trans. 2007, 11, 1083–1091. [Google Scholar] [CrossRef]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced materials for improved PEMFC performance and life. J. Power Sources 2004, 131, 41–48. [Google Scholar] [CrossRef]
- Zhou, C.; Guerra, M.A.; Qiu, Z.M.; Zawodzinski, T.A., Jr.; Schiraldi, D.A. Chemical durability studies of perfluorinated sulfonic acid polymers and model compounds under mimic fuel cell conditions. Macromolecules 2007, 40, 8695–8707. [Google Scholar]
- Schiraldi, D.A. Perfluorinated polymer electrolyte membrane durability. J. Macromol. Sci. Part C Polym. Rev. 2006, 46, 315–327. [Google Scholar] [CrossRef]
- Xie, T.; Hayden, C.A. A kinetic model for the chemical degradation of perfluorinated sulfonic acid ionomers: Weak end group versus side chain cleavage. Polymer 2007, 48, 5497–5506. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Kreuer, K.D.; Maier, J.; Müller, K. Evaluating chemical degradation of proton conducting perfluorosulfonic acid ionomers in a Fenton test by solid-state 19F NMR spectroscopy. J. Power Sources 2011, 196, 2490–2497. [Google Scholar] [CrossRef]
- Ghassemzadeh, L.; Kreuer, K.D.; Maier, J.; Müller, K. Chemical degradation of Nafion membranes under mimic fuel cell conditions as investigated by solid-state NMR spectroscopy. J. Phys. Chem. C 2010, 114, 14635–14645. [Google Scholar]
- Ghassemzadeh, L.; Morrony, M.; Barrera, R.; Kreuer, K.D.; Maier, J.; Müller, K. Chemical degradation of proton conducting perfluorosulfonic acid ionomer membranes studied by solid-state nuclear magnetic resonance spectroscopy. J. Power Sources 2009, 186, 334–338. [Google Scholar] [CrossRef]
- Madden, T.; Weiss, D.; Cipollini, N.; Condit, D.; Gummalla, M.; Burlatsky, S.; Atrazhev, V. Degradation of polymer-electrolyte membranes in fuel cells I. Experimental. J. Electrochem. Soc. 2009, 156, B657–B662. [Google Scholar] [CrossRef]
- Fang, X.; Shen, P.K.; Song, S.; Stetgiopoulos, V.; Tsiakaras, P. Degradation of perfluorinated sulfonic acid films: An in situ infrared spectro-electrochemical study. Polym. Degrad. Stab. 2009, 94, 1707–1713. [Google Scholar] [CrossRef]
- Tang, H.; Peikang, S.; Jiang, S.P.; Wang, F.; Pan, M. A degradation study of Nafion proton exchange membrane of PEM fuel cells. J. Power Sources 2007, 170, 85–92. [Google Scholar] [CrossRef]
- Uchimoto, Y. (Ed.) Fundamental studies to identify the degradation mechanism of single cell of PEFCs. Project Annual Report for FYH20 (ID: 100014110). 2012. Available online: http://www.nedo.go.jp/ (accessed on 28 June 2012).
- Dreizler, A.M.; Roduner, E. Reaction kinetics of hydroxyl radicals with model compounds of fuel cell polymer membranes. Fuel Cells 2012, 12, 132–140. [Google Scholar] [CrossRef]
- Serincan, M.F.; Pasaogullari, U. Effect of gas diffusion layer anisotropy on mechanical stresses in a polymer electrolyte membrane. J. Power Sources 2011, 196, 1314–1320. [Google Scholar] [CrossRef]
- Shah, A.A.; Luo, K.H.; Ralph, T.R.; Walsh, F.C. Recent trends and developments in polymer electrolyte membrane fuel cell modelling. Electrochim. Acta 2011, 56, 3731–3757. [Google Scholar]
- Serincan, M.F.; Pasaogullari, U.; Molter, T. Modeling the cation transport in an operating polymer electrolyte fuel cell (PEFC). Int. J. Hydrog. Energy 2010, 35, 5539–5551. [Google Scholar] [CrossRef]
- Ramousse, J.; Adzakpa, K.P.; Dubé, Y.; Agbossou, K.; Fournier, M.; Poulin, A.; Dostie, M. Local voltage degradations (drying and flooding) analysis through 3D stack thermal modeling. J. Fuel Cell Sci. Technol. 2010, 7, 041006:1–041006:10. [Google Scholar]
- Franco, A.A.; Gerard, M. Multiscale model of carbon corrosion in a PEFC: Coupling with electrocatalysis and impact of performance degradation. J. Electrochem. Soc. 2008, 155, B367–B384. [Google Scholar] [CrossRef]
- Franco, A.A.; Tembely, M. Transient multiscale modeling of aging mechanisms in a PEFC cathode. J. Electrochem. Soc. 2007, 154, B712–B723. [Google Scholar] [CrossRef]
- Eikerling, M.; Kornyshev, A.; Kulikovsky, A. Can theory help to improve fuel cells? Fuel Cell Rev. 2005, 1, 15–25. [Google Scholar]
- Wang, C.Y. Fundamental models for fuel cell engineering. Chem. Rev. 2004, 104, 4727–4766. [Google Scholar] [CrossRef]
- Fowler, M.W.; Mann, R.F.; Amphlett, J.C.; Peppley, B.A.; Roberge, P.R. Incorporation of voltage degradation into a generalized steady state electrochemical model for a PEM fuel cell. J. Power Sources 2002, 106, 274–283. [Google Scholar] [CrossRef]
- Kurniawan, C.; Morita, S.; Kitagawa, K. Hydration structure of trifluoromethanesulfonate studied by quantum chemical calculations. Comput. Theor. Chem. 2012, 982, 30–33. [Google Scholar] [CrossRef]
- Ishimoto, T.; Ogura, T.; Koyama, M. Stability and hydration structure of model perfluorosulfonic acid compound systems, CF3SO3H(H2O)n (n = 1–4), and its isotopomer by the direct treatment of H/D nuclear quantum effects. Comput. Theor. Chem. 2011, 975, 92–98. [Google Scholar]
- Idupulapati, N.; Devanathan, R.; Dupuis, M. Ab initio study of hydration and proton dissociation in ionomer membranes. J. Phys. Chem. A 2010, 114, 6904–6912. [Google Scholar]
- Krishtal, A.; Senet, P.; Alsenoy, C.V. Influence of structure on the polarizability of hydrated methane sulfonic acid clusters. J. Chem. Theory Comput. 2008, 4, 2122–2129. [Google Scholar] [CrossRef]
- Vishnyakov, A.; Neimark, A.V. Specifics of solvation of sulfonated polyelectrolytes in water, dimethylmethylphosphonate, and their mixture: A molecular simulation study. J. Chem. Phys. 2008, 128, 164902:1–164902:11. [Google Scholar]
- Koyama, M.; Bada, K.; Sasaki, K.; Tsuboi, H.; Endou, A.; Kubo, M.; Del Carpio, C.A.; Broclawik, E.; Miyamoto, A. First-principles study on proton dissociation properties of fluorocarbon- and hydrocarbon-based membranes in low humidity conditions. J. Phys. Chem. B 2006, 110, 17872–17877. [Google Scholar]
- Urata, S.; Irisawa, J.; Takada, A.; Tsuzuki, S.; Shinoda, W.; Mikami, M. Intermolecular interaction between the pendant chain of perfluorinated ionomer and water. Phys. Chem. Chem. Phys. 2004, 6, 3325–3332. [Google Scholar]
- Paddison, S.J. The modeling of molecular structure and ion transport in sulfonic acid based ionomer membranes. J. New Mater. Electrochem. Syst. 2001, 4, 197–207. [Google Scholar]
- Phonyiem, M.; Chaiwongwattana, S.; Leo-ngam, C.; Sagarik, K. Proton transfer reactions and dynamics of sulfonic acid group in Nafion. Phys. Chem. Chem. Phys. 2011, 13, 10923–10939. [Google Scholar]
- Li, X.; Li, F.; Shi, Y.; Chen, Q.; Sun, H. Predicting water uptake in poly(perfluorosulfonic acids) using force field simulation methods. Phys. Chem. Chem. Phys. 2010, 12, 14543–14552. [Google Scholar]
- Ahadian, S.; Ranjbar, A.; Mizuseki, H.; Kawazoe, Y. A novel computational approach to study proton transfer in perfluorosulfonic acid membranes. Int. J. Hydrog. Energy 2010, 35, 3648–3655. [Google Scholar]
- Choe, Y.K.; Tsuchida, E.; Ikeshoji, T.; Ohira, A.; Kidena, K. An ab initio modeling study on a modeled hydrated polymer electrolyte membrane, sulfonated polyethersulfone (SPES). J. Phys. Chem. B 2010, 114, 2411–2421. [Google Scholar]
- Choe, Y.K.; Tsuchida, E.; Ikeshoji, T.; Yamakawa, S.; Hyodo, S. Nature of proton dynamics in a polymer electrolyte membrane, Nafion: A first-principles molecular dynamics study. Phys. Chem. Chem. Phys. 2009, 11, 3892–3899. [Google Scholar]
- Choe, Y.K.; Tsuchida, E.; Ikeshoji, T.; Yamakawa, S.; Hyodo, S. Nature of water transport and electro-osmosis in Nafion: Insights from first-principles molecular dynamics simulations under an electric field. J. Phys. Chem. B 2008, 112, 11586–11594. [Google Scholar]
- Wilhelm, M.; Jeske, M.; Marschall, R.; Cavalcanti, W.L.; Tölle, P.; Köhler, C.; Koch, D.; Frauenheim, T.; Grathwohl, G.; Caro, J.; Wark, M. New proton conducting hybrid membranes for HT-PEMFC systems based on polysiloxanes and SO3H-functionalized mesoporous Si-MCM-41 particles. J. Membr. Sci. 2008, 316, 164–175. [Google Scholar]
- Jinnouchi, R.; Okazaki, K. Molecular dynamics study of transport phenomena in perfluorosulfonate ionomer membranes for polymer electrolyte fuel cells. J. Electrochem. Soc. 2003, 150, E66–E73. [Google Scholar] [CrossRef]
- Jinnouchi, R.; Okazaki, K. New insight into microscale transport phenomena in PEFC by quantum MD. Microscale Thermophys. Eng. 2003, 7, 15–31. [Google Scholar] [CrossRef]
- Clark II, J.K.; Paddison, S.J. The effect of side chain connectivity and local hydration on proton transfer in 3 M perfluorosulfonic acid membranes. Solid State Ionics 2012, 213, 83–91. [Google Scholar] [CrossRef]
- Danilczuk, M.; Lin, L.; Schlick, S.; Hamrock, S.J.; Schaberg, M.S. Understanding the fingerprint region in the infra-red spectra of perfluorinated ionomer membranes and corresponding model compounds: Experiments and theoretical calculations. J. Power Sources 2011, 196, 8216–8224. [Google Scholar]
- Li, X.; Liao, S.; Piao, J.; Wang, X. Theoretical study on sulfonated and phosphonated poly[(aryloxy)phosphazene] as proton-conducting membranes for fuel cell applications. Eur. Polym. J. 2009, 45, 2391–2394. [Google Scholar] [CrossRef]
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101–119. [Google Scholar] [CrossRef]
- Narasimachary, S.P.; Roudgar, A.; Eikerling, M.H. Ab initio study of interfacial correlations in polymer electrolyte membranes for fuel cells at low hydration. Electrochim. Acta 2008, 53, 6920–6927. [Google Scholar] [CrossRef]
- Jagur-Grodzinski, J. Polymeric materials for fuel cells: Concise review of recent studies. Polym. Adv. Technol. 2007, 18, 785–799. [Google Scholar] [CrossRef]
- Eikerling, M.H.; Malek, K. Physical modeling of materials for PEFCs: A balancing act of water and complex morphologies. In Proton Exchange Membrane Fuel Cells: Materials Properties and Performance; Willkinson, D.P., Zhang, J., Hui, R., Fergus, J., Li, X., Eds.; CRC Press: Florida, FL, USA, 2009; pp. 343–426. [Google Scholar]
- Kraka, E.; Cremer, D. Characterization of CF bonds with multiple-bond character: Bond length, stretching force constants, and bond dissociation energies. Chem. Phys. Chem. 2009, 10, 686–698. [Google Scholar] [CrossRef]
- Bernardes, C.E.S.; da Piedade, M.E.M.; Amaral, L.M.P.F.; Ferreira, I.M.C.L.; da Silva, A.V.R.; Diogo, H.P.; Cabral, B.J.C. Energies of C–F, C–Cl, C–Br, and C–I bonds in 2-haloethanols, enthalpies of formation of XCH2CH2OH (X = F, Cl, Br, I) compounds and of the 2-hydroxyethyl radical. J. Phys. Chem. A 2007, 111, 1713–1720. [Google Scholar]
- Nam, P.C.; Nguyen, M.T.; Chandra, A.K. The C–H and α(C–X) bond dissociation enthalpies of toluene, C6H5–CH2X (X = F, Cl), and their substituted derivatives: A DFT study. J. Phys. Chem. A 2005, 109, 10342–10347. [Google Scholar]
- Izgorodina, E.I.; Coote, M.L.; Radom, L. Trends in R–X bond dissociation energies (R = Me, Et, i-Pr, t-Bu; X = H, CH3, OCH3, OH, F): A surprising shortcoming of density functional theory. J. Phys. Chem. A 2005, 109, 7558–7566. [Google Scholar]
- Coote, M.L.; Pross, A.; Radom, L. Variable trends in R–X bond dissociation energies (R = Me, Et, i-Pr, t-Bu. Org. Lett. 2003, 5, 4689–4692. [Google Scholar] [CrossRef]
- Blanksby, S.L.; Ellison, G.B. Bond dissociation energies of organic molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef]
- Zavitsas, A.A. The relation between bond lengths and dissociation energies of carbon–carbon bonds. J. Phys. Chem. A 2003, 107, 897–898. [Google Scholar]
- Matsunaga, N.; Rogers, D.W.; Zavitsas, A.A. Pauling’s electronegativity equation and a new corollary accurately predict bond dissociation enthalpies and enhance current understanding of the nature of the chemical bond. J. Org. Chem. 2003, 68, 3158–3172. [Google Scholar]
- Beyer, M. The mechanical strength of a covalent bond calculated by density functional theory. J. Chem. Phys. 2000, 112, 7307–7312. [Google Scholar] [CrossRef]
- Pratt, D.A.; Wright, J.S.; Ingold, K.U. Theoretical study of carbon–halogen bond dissociation enthalpies of substituted benzyl halides. How important are polar effects? J. Am. Chem. Soc. 1999, 121, 4877–4882. [Google Scholar]
- Coms, F.D. The chemistry of fuel cell membrane chemical degradation. ECS Trans. 2008, 16, 235–255. [Google Scholar] [CrossRef]
- Tokumasu, T.; Ogawa, I.; Koyama, M.; Ishimoto, T.; Miyamoto, A. A DFT study of bond dissociation trends of perfluorosulfonic acid membrane. J. Electrochem. Soc. 2011, 158, B175–B179. [Google Scholar] [CrossRef]
- Davi, K.J.; Chandra, A.K. Theoretical investigation of the gas-phase reactions of (CF3)2CHOCH3 with OH radical. Chem. Phys. Lett. 2011, 502, 23–28. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Y.; Sun, J.; Sun, H.; Su, Z.; Pan, X.; Wang, R. Theoretical investigation of the reactions of CF3CHFOCF3 with the OH radical and Cl atom. J. Phys. Chem. A 2010, 114, 417–424. [Google Scholar]
- El-Nahas, A.M.; Uchimaru, T.; Sugie, M.; Tokuhashi, K.; Sekiya, A. Hydrogen abstraction from dimethyl ether (DME) and dimethyl sulfide (DMS) by OH radical: A computational study. J. Mol. Struct. Theochem. 2005, 722, 9–19. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Li, Z.; Sun, C. Dual-level direct dynamics studies for the reactions of CH3OCH3 and CF3OCH3 with the OH radical. J. Chem. Phys. 2003, 118, 10986–10995. [Google Scholar] [CrossRef]
- Atadin, F.; Seluki, C.; Sari, L.; Aviyente, V. Theoretical study of hydrogen abstraction from dimethyl ether and methyl tert-butyl ether by hydroxyl radical. Phys. Chem. Chem. Phys. 2002, 4, 1797–1806. [Google Scholar]
- Good, D.A.; Francisco, J.S. Tropospheric oxidation mechanism of dimethyl ether and methyl formate. J. Phys. Chem. A 2000, 104, 1171–1185. [Google Scholar]
- Ishimoto, T.; Nagumo, R.; Ogura, T.; Ishihara, T.; Kim, B.; Miyamoto, A.; Koyama, A. Chemical degradation mechanism of model compound, CF3(CF2)3O(CF2)2OCF2SO3H, of PFSA polymer by attack of hydroxyl radical in PEMFCs. J. Electrochem. Soc. 2010, 157, B1305–B1309. [Google Scholar] [CrossRef]
- Ishimoto, T.; Ogura, T.; Koyama, M. Theoretical study on chemical degradation mechanism of Nafion side chain by the attack of OH radical in polymer electrolyte fuel cell. J. Electrochem. Soc. 2011, 35, 1–6. [Google Scholar]
- Uegaki, R.; Akiyama, Y.; Tojo, S.; Honda, Y.; Nishijima, S. Radical-induced degradation mechanism of perfluorinated polymer electrolyte membrane. J. Power Sources 2011, 196, 9856–9861. [Google Scholar] [CrossRef]
- Yu, T.H.; Sha, Y.; Liu, W.G.; Merinov, B.V.; Shirvanian, P.; Goddard, W.A., III. Mechanism for degradation of Nafion in PEM fuel cell from quantum mechanics calculations. J. Am. Chem. Soc. 2011, 133, 19857–19863. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ishimoto, T.; Koyama, M. A Review of Molecular-Level Mechanism of Membrane Degradation in the Polymer Electrolyte Fuel Cell. Membranes 2012, 2, 395-414. https://doi.org/10.3390/membranes2030395
Ishimoto T, Koyama M. A Review of Molecular-Level Mechanism of Membrane Degradation in the Polymer Electrolyte Fuel Cell. Membranes. 2012; 2(3):395-414. https://doi.org/10.3390/membranes2030395
Chicago/Turabian StyleIshimoto, Takayoshi, and Michihisa Koyama. 2012. "A Review of Molecular-Level Mechanism of Membrane Degradation in the Polymer Electrolyte Fuel Cell" Membranes 2, no. 3: 395-414. https://doi.org/10.3390/membranes2030395
APA StyleIshimoto, T., & Koyama, M. (2012). A Review of Molecular-Level Mechanism of Membrane Degradation in the Polymer Electrolyte Fuel Cell. Membranes, 2(3), 395-414. https://doi.org/10.3390/membranes2030395




