Wavelength and Polarization Affect Phototaxis of the Asian Citrus Psyllid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
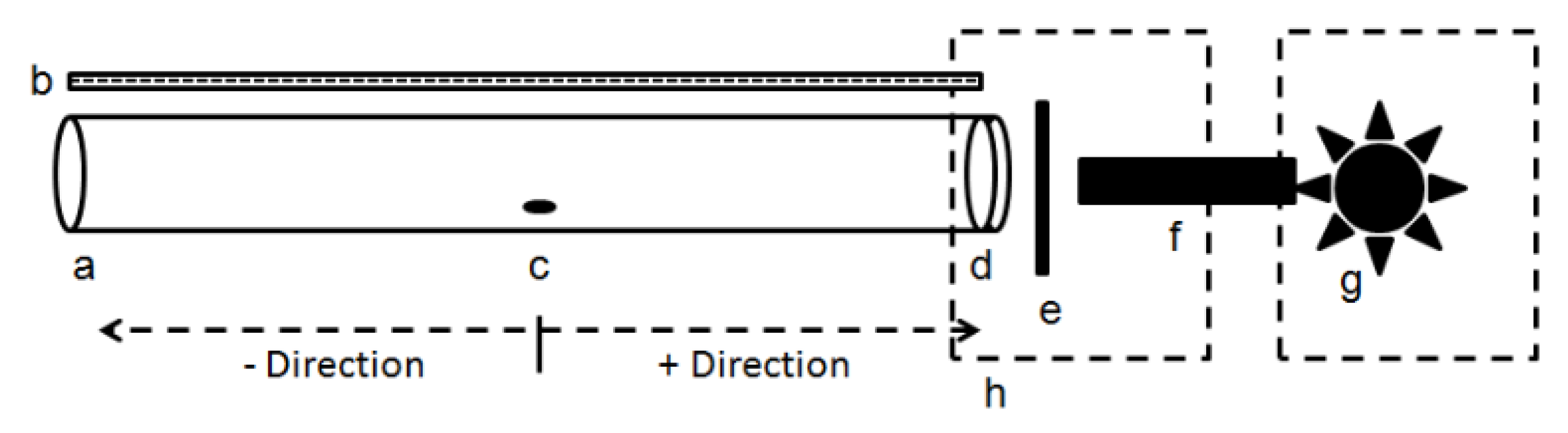
2.2. Bioassays
2.3. Effect of Wavelength
2.4. Effect of Polarization
2.5. Effect of Age
2.6. Statistical Analysis
3. Results
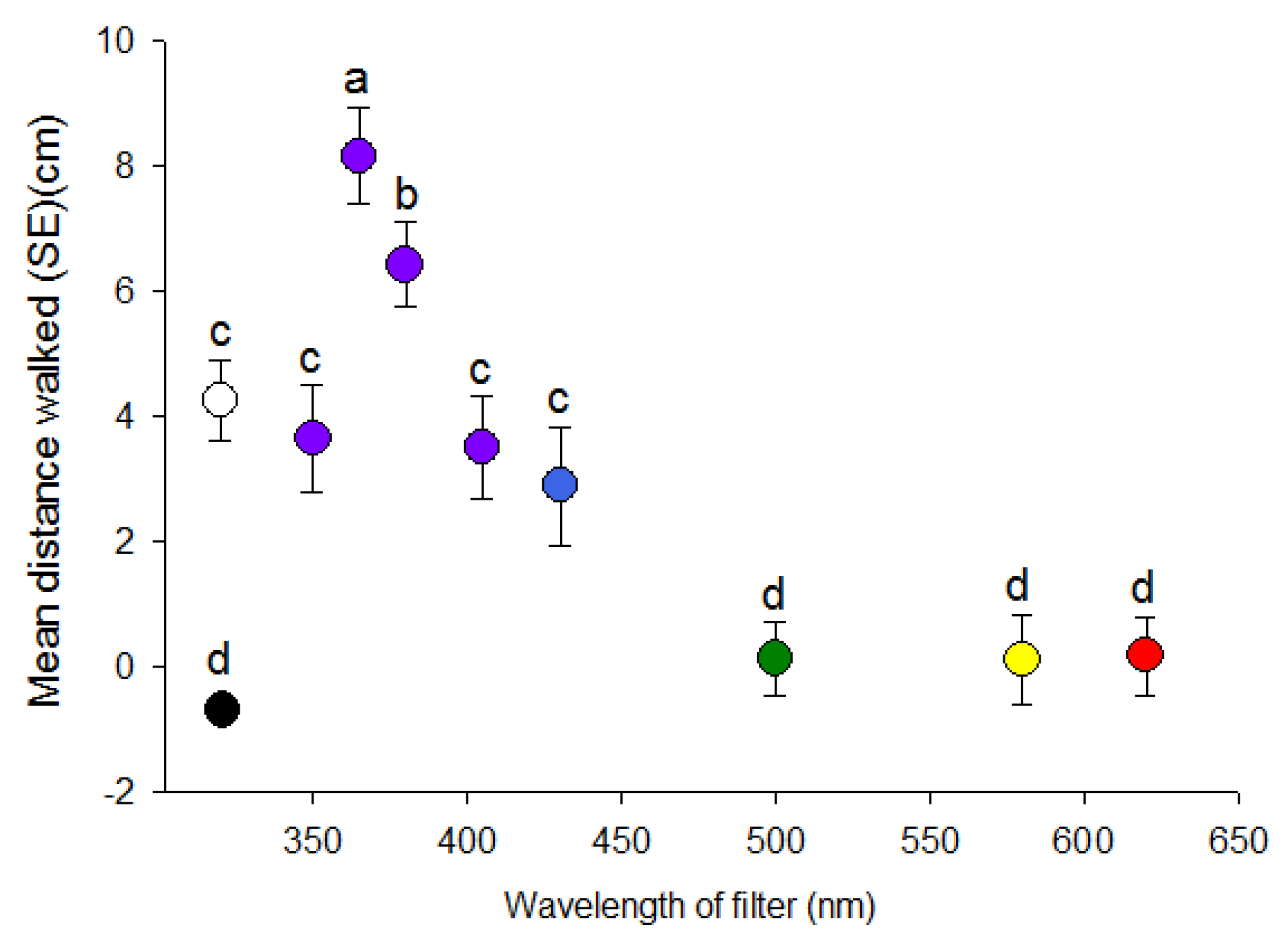
3.1. Effect of Wavelength
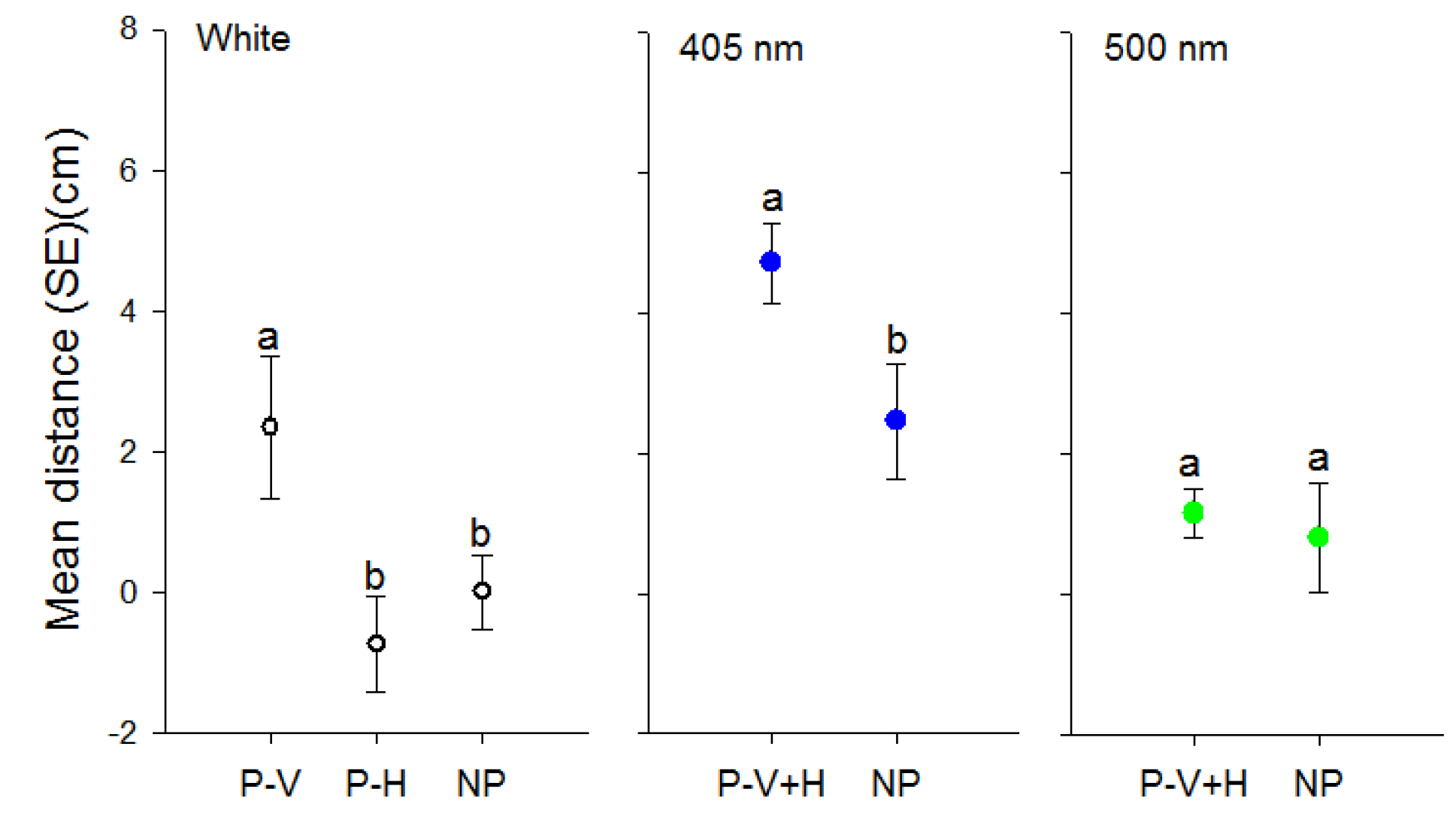
3.2. Effect of Polarization
3.3 Effect of Age
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grafton-Caldwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the Huanglongbing pathogens. Ann. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Hodges, A.W.; Spreen, T.H. Economic Impacts of Citrus Greening (HLB) in Florida; 2006/07-2010/11, EDIS Document FE903; IFAS: Gainesville, FL, USA, 2012. [Google Scholar]
- Qureshi, J.A.; Stansly, P.A. Rate, placement and timing of aldicarb applications to control Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), in oranges. Pest Manag. Sci. 2008, 64, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, J.A.; Stansly, P.A. Dormant season foliar sprays of broad-spectrum insecticides: An effective component of integrated management for Diaphorina citri (Hemiptera: Psyllidae) in citrus orchards. Crop Prot. 2010, 29, 860–866. [Google Scholar] [CrossRef]
- Rogers, M.E.; Stansly, P.A.; Stelinski, L.L. Florida Citrus Pest Management Guide: Asian Citrus Psyllid and Citrus Leafminer. Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, Electronic Data Information Source (University of Florida) ENY734/ IN686. 2016. Available online: http://edis.ifas.ufl.edu/in686 (accessed on 17 August 2017).
- Belasque, J.; Bassanezi, R.B.; Yamamoto, P.T.; Ayres, A.J.; Tachibana, A.; Violante, A.R.; Tank, A.; Di Giorgi, F.; Tersi, F.E.A.; Menezes, G.M.; et al. Lessons from huonglongbing management in Sao Paulo State, Brazil. J. Plant Pathol. 2010, 92, 285–302. [Google Scholar]
- Stansly, P.A.; Arevalo, H.A.; Qureshi, J.A.; Jones, M.M.; Hendricks, K.; Roberts, P.D.; Roka, F.M. Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by huanglongbing. Pest Manag. Sci. 2013, 70, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Monzo, C.; Arevalo, H.A.; Jones, M.M.; Vanaclocha, P.; Croxton, S.D.; Qureshi, J.A.; Stansly, P.A. Sampling methods for detection and monitoring of the Asian citrus psyllid (Hemiptera: Psyllidae). Environ. Entomol. 2015, 44, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G.; Sétamou, M.; Mizell III, R.F. A comparison of sticky traps for monitoring Asian citrus psyllid (Diaphorina citri Kuwayama). Crop Protect. 2010, 29, 1341–1346. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Roles of olfactory cues, visual cues, and mating status in orientation of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) to four different host plants. Environ. Entomol. 2009, 38, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Sétamou, M. Responses of the Asian citrus psyllid to volatiles emitted by the flushing shoots of its Rutaceous host plants. Environ. Entomol. 2010, 39, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.E.; Galindo, C.; Patt, J.M.; Luque-Williams, M. Evaluation of color and scent attractants used to trap and detect Asian citrus psyllid (Hemiptera: Liviidae) in urban environments. Fla. Entomol. 2013, 96, 1406–1416. [Google Scholar] [CrossRef]
- Sétamou, M.; Sanchez, A.; Saldaña, R.R.; Patt, J.M.; Summy, J. Visual responses of adult Asian citrus psyllid (Hemiptera: Liviidae) to colored sticky traps on citrus trees. J. Insect Behav. 2014. [Google Scholar] [CrossRef]
- Paris, T.M.; Croxton, S.D.; Stansly, P.A.; Allan, S.A. Temporal response and attraction of Diaphorina citri to visual stimuli. Entomol. Exp. App. 2015, 155, 137–147. [Google Scholar] [CrossRef]
- Paris, T.M.; Udell, B.J.; Stansly, P.A.; Allan, S.A. ‘Psyllid purple’: Evidence of behavior based utilization by the Asian citrus psyllid a combination of short and long wavelengths. PLOS One 2017. under review. [Google Scholar]
- Allan, S.A. Spectral sensitivity of the Asian citrus psyllid, Diaphorina citri. Grower Day International Research Conference HLB IV: Orlando, FL, USA, 2015; Available online: http://escholarship.org/uc/item/9jw2w985 (accessed on 17 August 2017).
- Croxton, S.D.; Stansly, P.A. Metalized polyethylene mulch to repel Asian citrus psyllid, slow spread of huanglongbing and daily activity and LED attraction of Diaphorina citri improve growth of new citrus plantings. Pest Manage. Sci. 2014, 70, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Menzel, R.; Backhaus, W. Colour vision in insects. In Vision and Visual Dysfunction; Gouras, P., Ed.; MacMillan: London, UK, 1991; pp. 262–293. [Google Scholar]
- Kelber, A. Invertebrate colour vision. In Invertebrate Vision; Warrant, E., Nilsson, D.-E., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 250–290. [Google Scholar]
- Döring, T.F.; Chittka, L. Visual ecology of aphids-a critical review on the role of colours in host finding. Arthropod-Plant Interac. 2007, 1, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Skorupski, P.; Chittka, L. Is colour cognitive? Optics Laser Technol. 2011, 43, 251–260. [Google Scholar] [CrossRef]
- Cronin, T.W.; Johnsen, S.; Marshall, N.J.; Warrant, E.J. Visual Ecology; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Hempel de Ibarra, N.; Vorobyev, M.; Menzel, R. Mechanisms, functions and ecology of colour vision in the honeybee. J. Comp. Physiol. A 2014, 200, 411–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, G.; Scherer, C. Experiments on wavelength specific behavior of Pieris brassicae L. during drumming and egg-laying. J. Comp. Physiol. 1982, 149, 325–353. [Google Scholar] [CrossRef]
- Arikawa, K. The eyes and vision of butterflies. J. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Coombe, P.E. Wavelength specific behavior of the whitefly Trialeurodes vaporariorum (Homoptera: Aleyrodidae). J. Comp. Physiol. 1981, 144, 83–90. [Google Scholar] [CrossRef]
- Scherer, C.H.; Kolb, G. Behavioural experiments on the visual processing of color stimuli in Pieris brassicae L. (Lepidoptera). J. Comp. Physiol. 1987, 129, 645–656. [Google Scholar] [CrossRef]
- Kelber, A. Innate preferences for flower features in the hawkmoth Macroglossum stellararum. J. Exp. Biol. 1997, 200, 827–836. [Google Scholar] [PubMed]
- Wehner, R.; Labhart, T. Polarization vision. In Invertebrate Vision; Warrant, E., Nilsson, D.-E., Eds.; Cambridge University Press: New York, NY, USA, 2006; pp. 291–348. [Google Scholar]
- Herzmann, D.; Labhart, T. Spectral sensitivity and absolute threshold of polarization vision in crickets: A behavioral study. J. Comp. Physiol. A 1989, 165, 315–319. [Google Scholar] [CrossRef]
- Labhart, T.; Meyer, E.P.; Schenker, L. Specialized ommatidia for polarization vision in the compound eye of cockchafers, Melolontha melolontha (Coleoptera: Scarabaeidae). Cell Tissue Res. 1992, 268, 289–429. [Google Scholar] [CrossRef]
- Labhart, T. Can invertebrates see the e-vector of polarization as a separate modality of light? J. Exp. Biol. 2016, 219, 3844–3856. [Google Scholar] [CrossRef] [PubMed]
- Rossel, S.; Wehner, R.; Lindauer, M. E-Vector orientation in bees. J. Comp. Physiol. 1978, 125, 1–12. [Google Scholar] [CrossRef]
- Rossel, S.; Wehner, R. Celestial orientation in bees: The use of spectral cues. J. Comp. Physiol. 1984, 155, 605–613. [Google Scholar] [CrossRef]
- Fent, K. Polarized skylight orientation in the desert ant Cataglyphis. J. Comp. Physiol. A 1986, 158, 145–150. [Google Scholar] [CrossRef]
- Mappes, M.; Homberg, U. Behavioral analysis of polarization vision in tethered flying insects. J. Comp. Physiol. A 2004, 190, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dacke, M.; Nilsson, D.-E.; Warrant, E.J.; Blest, A.D.; Land, M.F.; O’Carroll, D.C. Built-in polarizers form part of a compass organ in spiders. Nature 1999, 401, 470–473. [Google Scholar] [CrossRef]
- Dacke, M.; Byrne, M.; Smolka, J.; Warrant, E.; Baird, E. Dung beetles ignore landmarks for straight-line orientation. J. Comp. Physiol. A 2013, 199, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Schwind, R. The plunge reaction of the backswimmer Notonecta. J. Comp. Physiol. A 1984, 155, 319–321. [Google Scholar] [CrossRef]
- Kelber, A. Why ‘false’ colours are seen by butterflies. Nature 1999, 402, 251. [Google Scholar] [CrossRef] [PubMed]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Relationships between adult abdominal color and reproductive potential in Diaphorina citri (Hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2009, 102, 476–483. [Google Scholar] [CrossRef]
- Coombe, P.E. Visual behavior of the greenhouse whitefly, Trialeurodes vaporariorum. Physiol. Entomol. 1982, 7, 243–251. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical computing: Vienna, Austria Version 3.01. Available online: http://www.R-project.org/ (assessed on 17 August 2017).
- Wenninger, E.J.; Hall, D.G.; Mankin, R.W. Vibrational communication between the sexes in Diaphorina citri (Hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2009, 102, 547–555. [Google Scholar] [CrossRef]
- Barta, A.; Horváth, G. Why is it advantageous for animals to detect celestial polarization in the ultraviolet? Skylight polarization under clouds and canopies is strongest in the UV. J. Theor. Biol. 2004, 226, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sétamou, M.; Sanchez, A.; Patt, J.M.; Nelson, S.D.; Jifon, J.; Louzada, E.S. Diurnal patterns of light activity and effects of light on host finding behavior of the Asian citrus psyllid. J. Insect Behav. 2012, 25, 264–276. [Google Scholar] [CrossRef]
- Horváth, G.; Varju, D. Polarized Light in Animal Vision; Springer: New York, NY, USA, 2004. [Google Scholar]
- Kelber, A. Invertebrate colour vision. In Invertebrate Vision; Warrant, E., Nilsson, D.-E., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 250–290. [Google Scholar]
- Wehner, R. Polarized-light navigation by insects. Sci. Amer. 1976, 235, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Rossel, S.; Wehner, R. The bee’s map of the e-vector pattern in the sky. Proc. Natl. Acad. Sci. 1982, 79, 4451–4455. [Google Scholar] [CrossRef] [PubMed]
- Pourreza, A.; Lee, W.S.; Ehsani, R.; Schueller, J.K.; Raveh, E. An optimum method for real-time in-field detection of huanglongbing disease using a vision sensor. Comput. Electron Agric. 2015, 110, 221–232. [Google Scholar] [CrossRef]
- Wehner, R.; Labhart, T. Polarization vision. In Invertebrate Vision; Warrant, E., Nilsson, D.-E., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 291–348. [Google Scholar]
- Kinoshita, M.; Arikawa, K. Color and polarization vision in foraging Papilio. J. Comp. Physiol. A 2014, 200, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, A.; Kristoffersen, L.; Anderbrant, O. Physiological state of female and light intensity affect the host-plant selection of carrot psyllid, Trioza apicalis (Hemiptera: Triozidae). Eur. J. Entomol. 2008, 105, 227–232. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Hall, D.G. Daily timing of mating and age at reproductive maturity in Diaphorina citri (Hemiptera: Psyllidae). Fla. Entomol. 2007, 90, 715–722. [Google Scholar] [CrossRef]
- Skelley, L.H.; Hoy, M.A. A synchronous rearing method for the Asian citrus psyllid and its parasitoids in quarantine. Bio. Control 2001, 29, 14–23. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Hall, D.G. Daily and seasonal patterns in abdominal color in Diaphorina citri (Hemiptera: Psyllidae). Ann. Entomol. Soc. Amer. 2008, 102, 476–483. [Google Scholar] [CrossRef]
- Tiwari, S.; Nabil Killiny, N.; Mann, R.S.; Wenninger, E.J.; Stelinski, L.L. Abdominal color of the Asian citrus psyllid, Diaphorina citri, is associated with susceptibility to various insecticides. Pest Manag. Sci. 2013, 69, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Martini, X.; Hoyte, A.; Stelinski, L.L. Abdominal color of the Asian citrus psyllid (Hemiptera: Liviidae) is associated with flight capabilities. Ann. Entomol. Soc. Am. 2014, 107, 842–847. [Google Scholar] [CrossRef]


| Wavelength (nm) | % Positive Phototaxis | p Value | Light Intensity (W/cm2/sec) |
|---|---|---|---|
| 350 | 85.7 | <0.001 | 0.04 |
| 365 | 94.3 | <0.001 | 0.06 |
| 380 | 80.0 | <0.01 | 0.07 |
| 405 | 74.3 | <0.01 | 0.08 |
| 430 | 60.0 | 0.233 | 0.09 |
| 500 | 45.7 | 0.612 | 0.12 |
| 550 | 54.3 | 0.612 | 0.13 |
| 580 | 48.6 | 0.866 | 0.12 |
| 620 | 45.7 | 0.612 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paris, T.M.; Allan, S.A.; Udell, B.J.; Stansly, P.A. Wavelength and Polarization Affect Phototaxis of the Asian Citrus Psyllid. Insects 2017, 8, 88. https://doi.org/10.3390/insects8030088
Paris TM, Allan SA, Udell BJ, Stansly PA. Wavelength and Polarization Affect Phototaxis of the Asian Citrus Psyllid. Insects. 2017; 8(3):88. https://doi.org/10.3390/insects8030088
Chicago/Turabian StyleParis, Thomson M., Sandra A. Allan, Bradley J. Udell, and Philip A. Stansly. 2017. "Wavelength and Polarization Affect Phototaxis of the Asian Citrus Psyllid" Insects 8, no. 3: 88. https://doi.org/10.3390/insects8030088





