Adsorptive Removal of Carbamazepine and Diatrizoate in Iron Oxide Nanoparticles Amended Sand Column Mimicing Managed Aquifer Recharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Artificial Wastewater
2.2. Synthesis of Magnetite and MAA Coated Magnetite
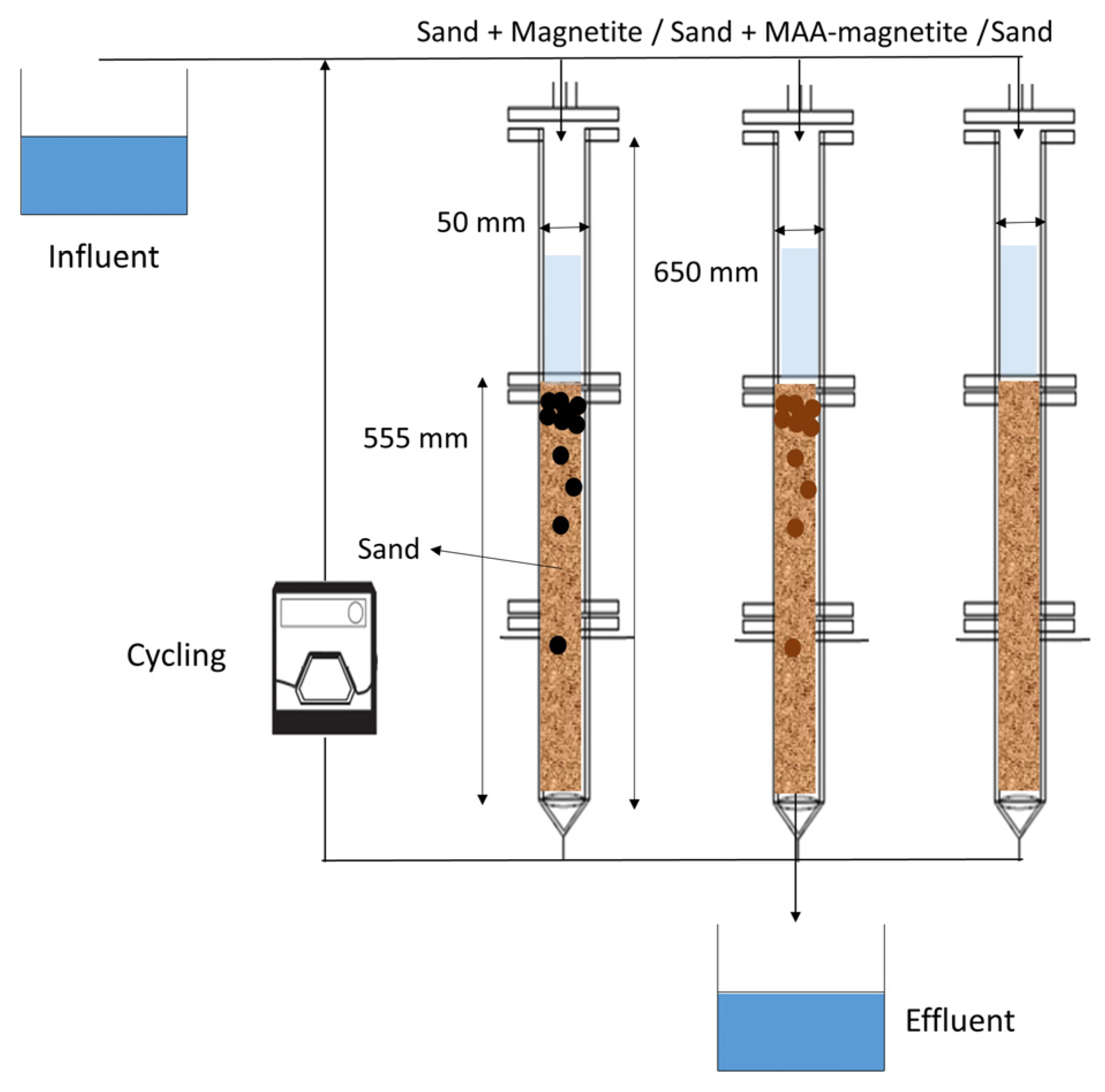
2.3. Column Experiment
2.4. Sample Pre-Processing and Analysis
2.4.1. Sample Pretreatment for CBZ and DTZ Analysis
2.4.2. HPLC Analysis of CBZ and DTZ
2.4.3. DOC Analysis
2.4.4. Metals Analysis
2.4.5. XPS Analysis
3. Results and Discussion
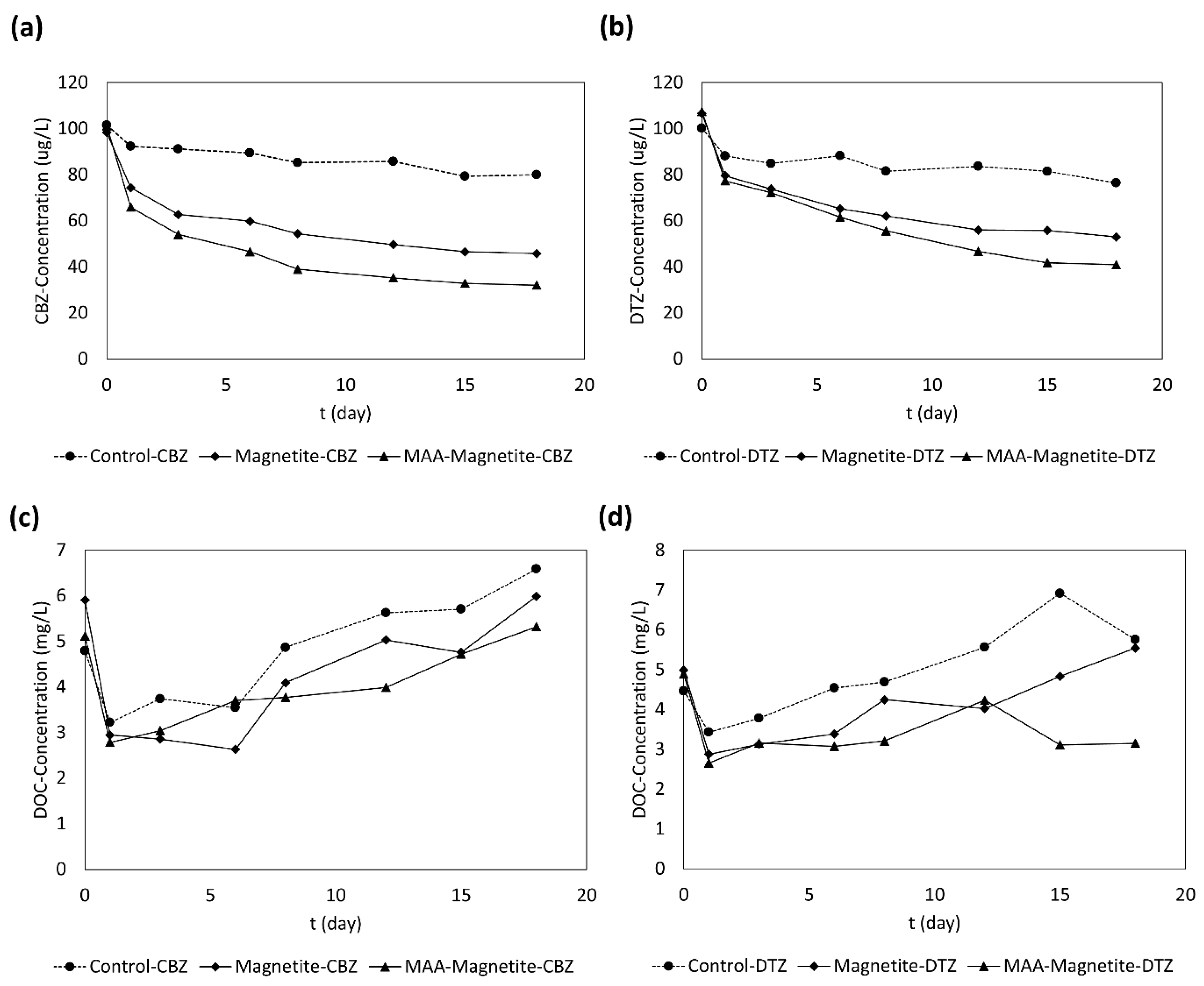
3.1. CBZ and DTZ Removal
3.2. DOC Trend in Sand Column Effluent
3.3. Metal Leaching Behavior
3.4. Characterization of the Magnetite before and after Column Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cunningham, V.L. Special Characteristics of Pharmaceuticals Related to Environmental Fate. In Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks; Springer: New York, NY, USA, 2004; p. 19. [Google Scholar]
- Lekkerkerker-Teunissen, K.; Chekol, E.T.; Maeng, S.K.; Ghebremichael, K.; Houtman, C.J.; Verliefde, A.R.D.; Verberk, J.Q.J.C.; Amy, G.L.; Van Dijk, J.C. Pharmaceutical removal during managed aquifer recharge with pretreatment by advanced oxidation. Water Sci. Technol. Water Supply 2012, 12, 755–767. [Google Scholar] [CrossRef]
- Li, D.; Alidina, M.; Ouf, M.; Sharp, J.O.; Saikaly, P.; Drewes, J.E. Microbial community evolution during simulated managed aquifer recharge in response to different biodegradable dissolved organic carbon (BDOC) concentrations. Water Res. 2013, 47, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Kazner, C.; Wintgens, T.; Dillo, P.J. Water Reclamation Technologies for Safe. Managed Aquifer Recharge; IWA Publishing: London, UK, 2012. [Google Scholar]
- Dillon, P. Future management of aquifer recharge. Hydrogeol. J. 2005, 13, 313–316. [Google Scholar] [CrossRef]
- Quanrud, D.M.; Arnold, R.G.; Wilson, L.G.; Gordon, H.J.; Graham, D.W.; Amy, G.L. Fate of Organics During Column Studies of Soil Aquifer Treatment. J. Environ. Eng. 1996, 122, 314–321. [Google Scholar] [CrossRef]
- Maeng, S.K.; Sharma, S.K.; Lekkerkerker-Teunissen, K.; Amy, G.L. Occurrence and fate of bulk organic matter and pharmaceutically active compounds in managed aquifer recharge: A review. Water Res. 2011, 45, 3015–3033. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Moog, G. Carboxyl group (– CO 2 H ) functionalized ferrimagnetic iron oxide nanoparticles for potential bio-applications. J. Mater. Chem. 2004, 14, 2781–2786. [Google Scholar] [CrossRef]
- Yoon, S.U.; Mahanty, B.; Kim, C.-G.G. Preparation of superparamagnetic iron oxide nanoparticles and evaluation of their adsorption capacity toward carbamazepine and diatrizoate. Desalin. Water Treat. 2016, 57, 7789–7800. [Google Scholar] [CrossRef]
- Yoon, S.U.; Mahanty, B.; Ha, H.M.; Kim, C.G. Phenol adsorption on surface-functionalized iron oxide nanoparticles: Modeling of the kinetics, isotherm, and mechanism. J. Nanopart. Res. 2016, 18. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Kreuzinger, N. Carbamazepine as a possible anthropogenic marker in the aquatic environment: Investigations on the behaviour of Carbamazepine in wastewater treatment and during groundwater infiltration. Water Res. 2004, 38, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Arye, G.; Dror, I.; Berkowitz, B. Fate and transport of carbamazepine in soil aquifer treatment (SAT) infiltration basin soils. Chemosphere 2011, 82, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Badruddoza, A.Z.M.; Hidajat, K.; Uddin, M.S. Adsorptive removal of emerging contaminants from water using superparamagnetic Fe3O4 nanoparticles bearing aminated β-cyclodextrin. J. Environ. Chem. Eng. 2013, 1, 122–130. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Liu, C. Preparation and use of magnetic poly(glycidyl methacrylate) resin in drinking water treatment. J. Appl. Polym. Sci. 2013, 130, 106–112. [Google Scholar] [CrossRef]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem. Eng. J. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Chefetz, B.; Mualem, T.; Ben-Ari, J. Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere 2008, 73, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Scheytt, T.; Mersmann, P.; Rejman-Rasinski, E.; These, A. Tracing pharmaceuticals in the unsaturated zone. J. Soils Sediments 2007, 7, 75–84. [Google Scholar] [CrossRef]
- Elliott, D.W.; Zhang, W.X. Field assessment of nanoscale bimetallic particles for groundwater treatment. Environ. Sci. Technol. 2001, 35, 4922–4926. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Fan, D.; Johnson, R.L.; Tratnyek, P.G.; Nurmi, J.T.; Wu, Y.; Williams, K.H. Methods for characterizing the fate and effects of nano zerovalent iron during groundwater remediation. J. Contam. Hydrol. 2015, 181, 17–35. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Grafe, M.; Eick, M.J.; Grossl, P.R.; Saunders, A.M. Adsorption of Arsenate and Arsenite on Ferrihydrite in the Presence and Absence of of Dissolved Organic Carbon. J. Environ. Qual. 2002, 31, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Metzler, D.R.; Dwyer, B.P. Removal of As, Mn, Mo, Se, U, V and Zn from groundwater by zero-valent iron in a passive treatment cell: Reaction progress modeling. J. Contam. Hydrol. 2002, 56, 99–116. [Google Scholar] [CrossRef]
- Kalbitz, K.; Wennrich, R. Mobilization of heavy metals and arsenic in polluted wetland soils and its dependence on on dissolved organic matter. Sci. Total Environ. 1998, 209, 27–39. [Google Scholar] [CrossRef]
- Weng, L.; Temminghoff, E.J.M.; Lofts, S.; Tipping, E.; Van Riemsdijk, W.H. Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ. Sci. Technol. 2002, 36, 4804–4810. [Google Scholar] [CrossRef] [PubMed]
- Grunze, M.; Eisert, F.; Himmelhaus, M.; Buck, M.; Wöll, C.; Gauss, I. Adsorption of docosanethiol from solution on polycrystalline silver surfaces: An XPS and NEXAFS study. J. Electron. Spectros. Relat. Phenomena 1998, 92, 139–149. [Google Scholar]
- Takagi, N.; Minami, N.; Furukawa, T.; Nishijima, M. The growth of ice clusters on the Si (100)(2Xl)-H(D) surface: Electron energy loss spectroscopy and thermal desorption studies. Surf. Sci. Lett. 1993, 297, L43–L47. [Google Scholar] [CrossRef]
- Klyachko, D.; Rowntree, P.; Sanche, L. Oxidation of hydrogen-passivated silicon surfaces induced by dissociative electron attachment to physisorbed H 2 O. Surf. Sci. 1996, 346, L49–L54. [Google Scholar] [CrossRef]
- Guilbert, S.; Guittet, M.J.; Barré, N.; Gautier-Soyer, M.; Trocellier, P.; Gosset, D.; Andriambololona, Z. Dissolution of UO2 in Boom clay water in oxidizing conditions: An XPS study. J. Nucl. Mater. 2000, 282, 75–82. [Google Scholar] [CrossRef]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.U.; Mahanty, B.; Kim, C.G. Adsorptive Removal of Carbamazepine and Diatrizoate in Iron Oxide Nanoparticles Amended Sand Column Mimicing Managed Aquifer Recharge. Water 2017, 9, 250. https://doi.org/10.3390/w9040250
Yoon SU, Mahanty B, Kim CG. Adsorptive Removal of Carbamazepine and Diatrizoate in Iron Oxide Nanoparticles Amended Sand Column Mimicing Managed Aquifer Recharge. Water. 2017; 9(4):250. https://doi.org/10.3390/w9040250
Chicago/Turabian StyleYoon, Soon Uk, Biswanath Mahanty, and Chang Gyun Kim. 2017. "Adsorptive Removal of Carbamazepine and Diatrizoate in Iron Oxide Nanoparticles Amended Sand Column Mimicing Managed Aquifer Recharge" Water 9, no. 4: 250. https://doi.org/10.3390/w9040250






