Biomonitoring of Epilobium hirsutum L. Health Status to Assess Water Ecotoxicity in Constructed Wetlands Treating Mixtures of Contaminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aims of the Experimental Design
2.2. Planted Microcosms Set-up
2.3. Chemicals and Pollutant Exposure
| Chemicals | Targeted Concentrations (mg/L) | |
|---|---|---|
| Phase 1 | Phases 2–3 | |
| Al | 2.5 | 25 |
| As | 0.05 | 0.5 |
| Cd | 0.2 | 2 |
| Cr | 0.5 | 5 |
| Cu | 0.5 | 5 |
| Fe | 2.5 | 25 |
| Mn | 1 | 10 |
| Ni | 0.5 | 5 |
| Pb | 0.5 | 5 |
| Sn | 2 | 20 |
| Zn | 2 | 20 |
| PHE | 0.05 | 0.5 |
| PYR | 0.05 | 0.5 |
| THC | 10 | 100 |
| LAS | 10 | 10 |
2.4. Chemical Analysis in Water, Substrate, and Plants
2.5. Microcosm and Plant Parameter Monitoring
2.6. Statistical Analysis
3. Results and Discussion
3.1. Metal and As Accumulation in Biomass
| Environ. | Cond. | Al | As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | THC | PHE | PYR | LAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pozzolan (mg/kg) | Control | 23.103 ± 1.103 | n.m. | 7.3 ± 0.3 | 272 ± 7 | 25 ± 1 | 33.103 ± 1.103 | 541±27 | 227±9 | 5.4 ± 0.3 | 42 ± 1 | 45.65 | 0.0231 | 0.028 | n.m. |
| MPM | 23.103 ± 1.103 | n.m. | 5.1 ± 0.1 | 268 ± 10 | 22 ± 1 | 33.103 ± 1.103 | 522±18 | 225±4 | 5.2 ± 0.2 | 49 ± 1 | n.m. | n.m. | n.m. | n.m. | |
| OPM | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | 47.16 | 0.0253 | 0.0298 | n.m. | |
| OMPM | 22.103 ± 1.103 | n.m. | 4.9 ± 0.3 | 241 ± 7 | 21 ± 1 | 32.103 ± 2.103 | 499 ± 17 | 209 ± 4 | 5.1 ± 0.6 | 46 ± 0 | 50.95 | 0.0232 | 0.0298 | n.m. | |
| Water (mg/L) | Control | <0.1 | <0.03 | <0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | < 0.04 | 0.03 | 0.198 | 0.00025 | 0.0001 | n.m. |
| MPM | 1.23 | < 0.03 | 0.09 | 0.04 | 0.08 | 0.69 | 0.07 | 0.43 | 0.05 | 0.83 | n.m. | n.m. | n.m. | n.m. | |
| OPM | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | n.m. | 0.0003 | 0.16 | 0.0004 | 0.31 | |
| OMPM | 0.13 | <0.03 | <0.01 | <0.02 | 0.01 | 0.13 | 0.24 | 0.18 | <0.04 | 0.06 | 0.0007 | 1.06 | 0.0006 | 0.19 |
| Type of Biomass | Treatment | Metal Concentration in Dry Biomass (mg/kg DW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | ||
| BG | Control | 2372 ± 53a | 1.6 ± 0.1a | 1.6 ± 0.1a | 23 ± 1a | 19 ± 5a | 5425 ± 102a | 734 ± 13a | 30 ± 1a | 4.5 ± 0.5a | 126 ± 3a |
| MPM | 3801 ± 561b | 21 ± 3ab | 187 ± 14b | 160 ± 14ab | 199 ± 19b | 6984 ± 863ab | 1370 ± 121ab | 653 ± 35b | 74 ± 7ab | 1317 ± 82b | |
| OMPM | 3107 ± 161ab | 77 ± 2b | 85 ± 2ab | 194 ± 8b | 122 ± 4ab | 11867 ± 565b | 2960 ± 68b | 348 ± 13ab | 144 ± 4b | 643 ± 26ab | |
| AG | Control | 714 ± 21b | <2.5 | <0.5 | 38 ± 1b | 15 ± 7 | 791 ± 31b | 115 ± 2a | 36 ± 3b | 1.9 ± 0.2 | 64 ± 22 |
| MPM | 140 ± 7a | <2.5 | 5.2 ± 0.1 | 3.5 ± 0.5a | 12 ± 1 | 239 ± 5a | 253 ± 2ab | 26 ± 1ab | 2.0 ± 0.2 | 138 ± 3 | |
| OMPM | 284 ± 7ab | <2.5 | 2.1 ± 0.1 | 19 ± 1ab | 14 ± 1 | 532 ± 33ab | 452 ± 8b | 21 ± 1a | 3.3 ± 0.1 | 95 ± 15 | |
| Metal Concentration in Plant Dry Biomass (mg/kg DW) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | As | Cd | Cr | Cu | Fe | Mn | Ni | Pb | Zn | ||
| Normal Composition 1 | 80 | n.g. | 0.05 | 1.5 | 10 | 150 | 200 | 1.5 | 1.0 | 50 | |
| Possibly Harmful Concentration 2 | n.g. | 5–20 | 5–30 | 5–30 | 20–100 | n.g. | 300–500 | 10–100 | 30–300 | 100–400 | |
| E. hirsutum | Control | 1171 ± 30 | n.c. | n.c. | 34 ± 1 | 16 ± 7 | 2070 ± 51 | 286 ± 5 | 34 ± 2 | 2.6 ± 0.3 | 81 ± 17 |
| MPM | 845 ± 113 | n.c. | 40 ± 3 | 34 ± 3 | 48 ± 4 | 1538 ± 170 | 468 ± 25 | 147 ± 7 | 16 ± 1 | 365 ± 18 | |
| OMPM | 1240 ± 59 | n.c. | 30 ± 0 | 77 ± 3 | 50 ± 2 | 4369 ± 213 | 1301 ± 28 | 131 ± 5 | 51 ± 1 | 281 ± 19 | |
3.2. Toxicity Symptoms due to Mixtures of Pollutants
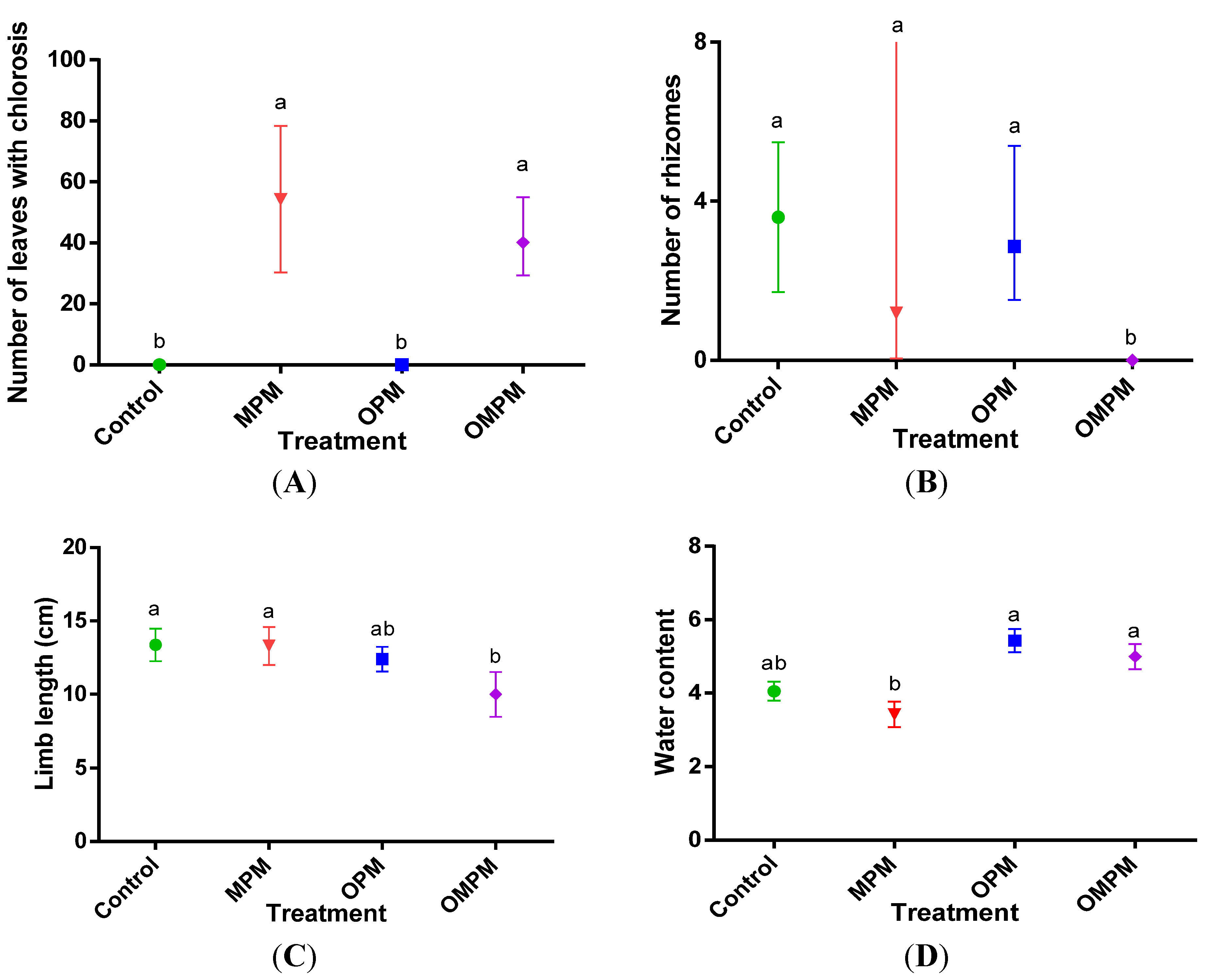



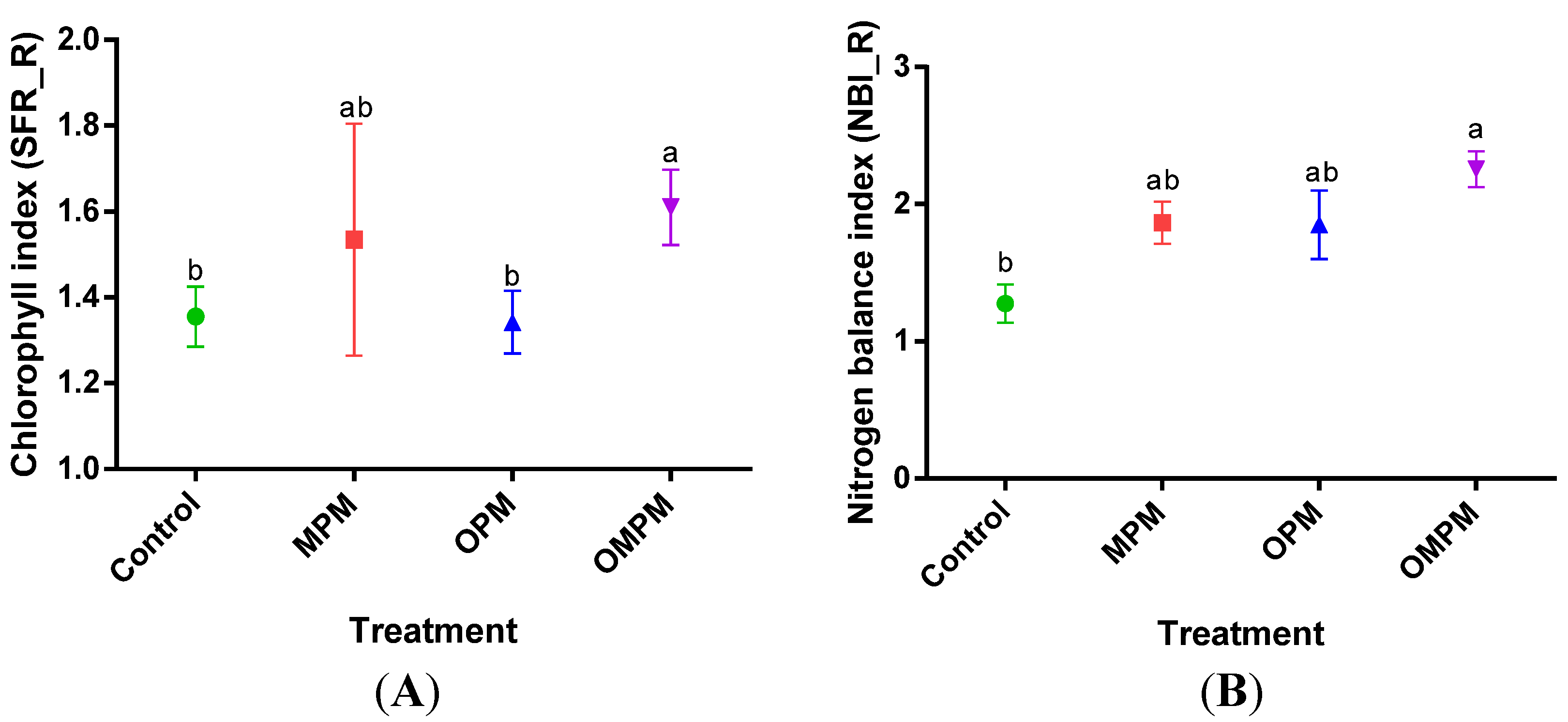
| Treatment | BG FW (g) | BG DW (g) | AG FW (g) | AG DW (g) |
|---|---|---|---|---|
| Control | 26 ± 3 | 3.0 ± 0.6 | 29 ± 4 | 7.7 ± 0.9 |
| MPM | 21 ± 12 | 2.4 ± 1.2 | 35 ± 20 | 10.1 ± 5.9 |
| OPM | 35 ± 13 | 3.2 ± 1.2 | 29 ± 10 | 6.9 ± 2.6 |
| OMPM | 20 ± 12 | 1.9 ± 1.1 | 14 ± 6 | 3.8 ± 1.7 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, Y.; Zhou, Q.; Xu, Y.; Wang, L.; Liang, X. Phytoremediation for co-contaminated soils of benzo[a]pyrene (B[a]P) and heavy metals using ornamental plant Tagetes patula. J. Hazard. Mater. 2011, 186, 2075–2082. [Google Scholar]
- Zhang, Z.; Rengel, Z.; Chang, H.; Meney, K.; Pantelic, L.; Tomanovic, R. Phytoremediation potential of Juncus subsecundus in soils contaminated with cadmium and polynuclear aromatic hydrocarbons (PAHs). Geoderma 2012, 175–176, 1–8. [Google Scholar]
- Groudeva, V.I.; Groudev, S.N.; Doycheva, A.S. Bioremediation of waters contaminated with crude oil and toxic heavy metals. Int. J. Miner. Process. 2001, 62, 293–299. [Google Scholar]
- Terzakis, S.; Fountoulakis, M.S.; Georgaki, I.; Albantakis, D.; Sabathianakis, I.; Karathanasis, A.D.; Kalogerakis, N.; Manios, T. Constructed wetlands treating highway runoff in the central Mediterranean region. Chemosphere 2008, 72, 141–149. [Google Scholar]
- Tu, Y.T.; Chiang, P.C.; Yang, J.; Chen, S.H.; Kao, C.M. Application of a constructed wetland system for polluted stream remediation. J. Hydrol. 2014, 510, 70–78. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor and Francis Group, CRC Press: Boca Raton, FL, USA, 2011; p. 505. [Google Scholar]
- Zhang, Z.; Rengel, Z.; Meney, K.; Pantelic, L.; Tomanovic, R. Polynuclear aromatic hydrocarbons (PAHs) mediate cadmium toxicity to an emergent wetland species. J. Hazard. Mater. 2011, 189, 119–126. [Google Scholar]
- Chen, Y.X.; Lin, Q.; He, Y.F.; Tian, G.M. Behavior of Cu and Zn under combined pollution of 2,4-dichlorophenol in the planted soil. Plant Soil 2004, 261, 127–134. [Google Scholar]
- Lin, Q.; Shen, K.L.; Zhao, H.M.; Li, W.H. Growth response of Zea mays L. in pyrene-copper co-contaminated soil and the fate of pollutants. J. Hazard. Mater. 2008, 150, 515–521. [Google Scholar]
- Riis, V.; Babel, W.; Pucci, O.H. Influence of heavy metals on the microbial degradation of diesel fuel. Chemosphere 2002, 49, 559–568. [Google Scholar]
- Sandrin, T.R.; Maier, R.M. Impact of metals on the biodegradation of organic pollutants. Environ. Health Perspect. 2003, 111, 1093–1101. [Google Scholar]
- Guittonny-Philippe, A.; Masotti, V.; Höhener, P.; Boudenne, J.L.; Viglione, J.; Laffont-Schwob, I. Constructed wetlands to reduce metal pollution from industrial catchments in aquatic Mediterranean ecosystems: A review to overcome obstacles and suggest potential solutions. Environ. Int. 2014, 64, 1–16. [Google Scholar]
- Imfeld, G.; Braeckevelt, M.; Kuschk, P.; Richnow, H.H. Monitoring and assessing processes of organic chemicals removal in constructed wetlands. Chemosphere 2009, 74, 349–362. [Google Scholar]
- Shelef, O.; Gross, A.; Rachmilevitch, S. Role of plants in a constructed wetland: Current and new perspectives. Water 2013, 5, 405–419. [Google Scholar]
- Beharrell, M. Planting, Selection and Plant Establishment in Constructed Wetlands in a Tropical Environment. In Wetlands Ecosystems in Asia: Function and Management; Wong, M.H., Ed.; Elsevier B.V.: Amsterdam, Netherlands, 2004; Volume 1, pp. 311–329. [Google Scholar]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar]
- Bonanno, G.; lo Giudice, R. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar]
- Polechońska, L.; Klink, A. Trace metal bioindication and phytoremediation potentialities of Phalaris arundinacea L. (reed canary grass). J. Geochem. Explor. 2014, 146, 27–33. [Google Scholar]
- Klink, A.; Macioł, A.; Wisłocka, M.; Krawczyk, J. Metal accumulation and distribution in the organs of Typha latifolia L. (cattail) and their potential use in bioindication. Limnol. Ecol. Manag. Inland Waters 2013, 43, 164–168. [Google Scholar]
- Bonanno, G. Arundo donax as a potential biomonitor of trace element contamination in water and sediment. Ecotoxicol. Environ. Saf. 2012, 80, 20–27. [Google Scholar]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar]
- Shelef, O.; Golan-Goldhirsh, A.; Gendler, T.; Rachmilevitch, S. Physiological parameters of plants as indicators of water quality in a constructed wetland. Environ. Sci. Pollut. Res. 2011, 18, 1234–1242. [Google Scholar]
- Vale, M.; Nguyen, C.; Dambrine, E.; Dupouey, J. Microbial activity in the rhizosphere soil of six herbaceous species cultivated in a greenhouse is correlated with shoot biomass and root C concentrations. Soil Biol. Biochem. 2005, 37, 2329–2333. [Google Scholar]
- Vymazal, J. Plants in constructed, restored and created wetlands. Ecol. Eng. 2013, 61, 501–504. [Google Scholar]
- Vymazal, J. Vegetation development in subsurface flow constructed wetlands in the Czech Republic. Ecol. Eng. 2013, 61P, 575–581. [Google Scholar]
- Nazrul-Islam, A.K.M. Effects of interaction of calcium and manganese on the growth and nutrition of Epilobium hirsutum L. Soil Sci. Plant Nutr. 1986, 32, 161–168. [Google Scholar]
- Wheeler, B.D.; Al-Farraj, M.; Cook, R.E.D. Iron toxicity to plants in base-rich wetlands: Comparative effects on the distribution and growth of Epilobium hirsutum L. and Juncus subnodulosus Schrank. New Phytol. 1985, 100, 653–669. [Google Scholar]
- Roman, I.; Rusu, M.A.; Puică, C.; Borşa, M. Citotoxic effects of three species of Epilobium (Onagraceae) herbal extracts in rats. Stud. Univ. Vasile Goldis Ser. Stiint. Vietii (Life Sci. Ser.) 2010, 20, 19–23. [Google Scholar]
- Wojdyło, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar]
- Battinelli, L.; Tita, B.; Evandri, M.G.; Mazzanti, G. Antimicrobial activity of Epilobium spp. extracts. II Farmaco 2001, 56, 345–348. [Google Scholar]
- Grisey, E.; Laffray, X.; Contoz, O.; Cavalli, E.; Mudry, J.; Aleya, L. The bioaccumulation performance of reeds and cattails in a constructed treatment wetland for removal of heavy metals in landfill leachate treatment (Etueffont, France). Water Air Soil Pollut. 2012, 223, 1723–1741. [Google Scholar]
- Soda, S.; Hamada, T.; Yamaoka, Y.; Ike, M.; Nakazato, H.; Saeki, Y.; Kasamatsu, T.; Sakurai, Y. Constructed wetlands for advanced treatment of wastewater with a complex matrix from a metal-processing plant: Bioconcentration and translocation factors of various metals in Acorus gramineus and Cyperus alternifolius. Ecol. Eng. 2012, 39, 63–70. [Google Scholar]
- Faulwetter, J.L.; Gagnon, V.; Sundberg, C.; Chazarenc, F.; Burr, M.D.; Brisson, J.; Camper, A.K.; Stein, O.R. Microbial processes influencing performance of treatment wetlands: A review. Ecol. Eng. 2009, 35, 987–1004. [Google Scholar]
- Guittonny-Philippe, A.; Petit, M.E.; Masotti, V.; Monnier, Y.; Malleret, L.; Coulomb, B.; Combroux, I.; Baumberger, T.; Viglione, J.; Laffont-Schwob, I. Selection of wild macrophytes for their use in constructed wetlands for phytoremediation of contaminant mixtures. J. Environ. Manag. 2015, 147, 108–123. [Google Scholar]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar]
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar]
- Wasi, S.; Tabrez, S.; Ahmad, M. Toxicological effects of major environmental pollutants: An overview. Environ. Monitor. Assess. 2013, 185, 2585–2593. [Google Scholar]
- Banat, K.; Forstner, U.; Muller, G. Experimental mobilization of metals from aquatic sediments by nitrilotriacetic acid. Chem. Geol. 1974, 14, 199–207. [Google Scholar]
- Hernández-Soriano, M.D.C.; Degryse, F.; Smolders, E. Mechanisms of enhanced mobilisation of trace metals by anionic surfactants in soil. Environ. Poll. 2011, 159, 809–816. [Google Scholar]
- Thavamani, P.; Malik, S.; Beer, M.; Megharaj, M.; Naidu, R. Microbial activity and diversity in long-term mixed contaminated soils with respect to polyaromatic hydrocarbons and heavy metals. J. Environ. Manag. 2012, 99, 10–17. [Google Scholar]
- Radić, S.; Stipaničev, D.; Vujčić, V.; Rajčić, M.M.; Širac, S.; Pevalek-Kozlina, B. The evaluation of surface and wastewater genotoxicity using the Allium cepa test. Sci. Total Environ. 2010, 408, 1228–1233. [Google Scholar]
- Council Directive of 4 May 1976 on Pollution Caused by Certain Dangerous Substances Discharged into the Aquatic Environment of the Community (76/464/EEC); European Union: Brussels, Belgium, 18 May 1976.
- Caldelas, C.; Araus, J.L.; Febrero, A.; Bort, J. Accumulation and toxic effects of chromium and zinc in Iris pseudacorus L. Acta Physiol. Plant. 2012, 34, 1217–1228. [Google Scholar]
- Lotmani, B.; Fatarna, L.; Berkani, A.; Rabier, J.; Prudent, P.; Laffont-Schwob, I. Selection of Algerian populations of the Mediterranean saltbush, Atriplex halimus, tolerant to high concentrations of lead, zinc and copper for phytostabilization of heavy metal-contaminated soils. Eur. J. Plant Sci. Biotechnol. 2011, 5, 20–26. [Google Scholar]
- Dietz, A.; Schnoor, J.L. Advances in phytoremediation. Environ. Health Perspect. 2001, 109, 163–168. [Google Scholar]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar]
- Schowanek, D.; David, H.; Francaviglia, R.; Hall, J.; Kirchmann, H.; Krogh, P.H.; Schraepen, N.; Smith, S.; Wildemann, T. Probabilistic risk assessment for linear alkylbenzene sulfonate (LAS) in sewage sludge used on agricultural soil. Regul. Toxicol. Pharmacol. 2007, 49, 245–259. [Google Scholar]
- Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); ISO 11885:2009; International Organization for Standardization (ISO): Geneva, Switzerland, 2009.
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Methylene Blue Active Substances Method # 5540 C. In American Public Health Association, Standards Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association (APHA): Washington, DC, USA, 1998; pp. 5–47. [Google Scholar]
- Holopainen, J.K.; Heijari, J.; Oksanen, E.; Alessio, G.A. Leaf volatile emissions of Betula pendula during autumn coloration and leaf fall. J. Chem. Ecol. 2010, 36, 1068–1075. [Google Scholar]
- Rabier, J.; Laffont-Schwob, I.; Pricop, A.; Ellili, A.; D’Enjoy-Weinkammerer, G.; Salducci, M.D.; Prudent, P.; Lotmani, B.; Tonetto, A.; Masotti, V. Heavy metal and arsenic resistance of the halophyte Atriplex halimus L. along a gradient of contamination in a French Mediterranean spray-zone. Water Air Soil Pollut. 2014, 225, 1993. [Google Scholar]
- Creus, C.M.; Sueldo, R.J.; Barassi, C.A. Shoot growth and water status in Azospirillum-inoculated wheat plantlets grown under osmotic and salt stresses. Plant Physiol. Biochem. 1997, 35, 939–944. [Google Scholar]
- Prasad, M.N.V. Heavy Metal Stress in Plants: From Biomolecules to Ecosystems, 2nd ed.; Springer-Verlag: Berlin, Germany, 2004; p. 462. [Google Scholar]
- Teuchies, J.; Jacobs, S.; Oosterlee, L.; Bervoets, L.; Meire, P. Role of plants in metal cycling in a tidal wetland: Implications for phytoremediation. Sci. Total Environ. 2013, 445–446, 146–154. [Google Scholar]
- Cheng, S.; Grosse, W.; Karrenbrock, F.; Thoennessen, M. Efficiency of constructed wetlands in decontamination of water polluted by heavy metals. Ecol. Eng. 2002, 18, 317–325. [Google Scholar]
- Liu, J.G.; Li, G.H.; Shao, W.C.; Xu, J.K.; Wang, D.K. Variations in uptake and translocation of copper, chromium and nickel among nineteen wetland plant species. Pedosphere 2010, 20, 96–103. [Google Scholar]
- Yadav, A.K.; Abbassi, R.; Kumar, N.; Satya, S.; Sreekrishnan, T.R.; Mishra, B.K. The removal of heavy metals in wetland microcosms: Effects of bed depth, plant species, and metal mobility. Chem. Eng. J. 2012, 211–212, 501–507. [Google Scholar]
- Ghaderian, S.M.; Ghotbi Ravandi, A.A. Accumulation of copper and other heavy metals by plants growing on Sarcheshmeh copper mining area, Iran. J. Geochem. Explor. 2012, 123, 25–32. [Google Scholar]
- López-Millán, A.F.; Sagardoy, R.; Solanas, M.; Abadía, A.; Abadía, J. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ. Exp. Bot. 2009, 65, 376–385. [Google Scholar]
- Mishra, S.; Srivastava, S.; Tripathi, R.D.; Kumar, R.; Seth, C.S.; Gupta, D.K. Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 2006, 65, 1027–1039. [Google Scholar]
- Shaibur, M.R.; Kawai, S. Effect of arsenic on visible symptom and arsenic concentration in hydroponic Japanese mustard spinach. Environ. Exp. Bot. 2009, 67, 65–70. [Google Scholar]
- Singh, A.K. Effect of trivalent and hexavalent chromium on spinach (Spinacea oleracea L). Environ. Ecol. 2001, 19, 807–810. [Google Scholar]
- Rabier, J.; Laffont-Schwob, I.; Bouraïma-Madjèbi, S.; Léon, V.; Prudent, P.; Viano, J.; Nabors, W.M.; Pilon-Smits, E.A.H. Characterization of metal tolerance and accumulation in Grevillea Exul VAR Exul. Int. J. Phytoremediat. 2007, 9, 419–435. [Google Scholar]
- Küpper, H.; Küpper, F.; Spiler, M. Environmental relevance of heavy metal substituted chlorophylls using the example of submersed water plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar]
- Küpper, H.; Šetlík, I.; Spiller, M.; Küpper, F.C.; Prášil, O. Heavy metal-induced inhibition of photosynthesis: Targets of in vivo heavy metal chlorophyll formation. J. Phycol. 2002, 38, 429–441. [Google Scholar]
- Thomas, G.; Stärk, H.J.; Wellenreuther, G.; Dickinson, B.C.; Küpper, H. Effects of nanomolar copper on water plants-Comparison of biochemical and biophysical mechanisms of deficiency and sublethal toxicity under environmentally relevant conditions. Aquat. Toxicol. 2013, 140–141, 27–36. [Google Scholar]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guittonny-Philippe, A.; Masotti, V.; Rabier, J.; Petit, M.-E.; Malleret, L.; Coulomb, B.; Laffont-Schwob, I. Biomonitoring of Epilobium hirsutum L. Health Status to Assess Water Ecotoxicity in Constructed Wetlands Treating Mixtures of Contaminants. Water 2015, 7, 697-715. https://doi.org/10.3390/w7020697
Guittonny-Philippe A, Masotti V, Rabier J, Petit M-E, Malleret L, Coulomb B, Laffont-Schwob I. Biomonitoring of Epilobium hirsutum L. Health Status to Assess Water Ecotoxicity in Constructed Wetlands Treating Mixtures of Contaminants. Water. 2015; 7(2):697-715. https://doi.org/10.3390/w7020697
Chicago/Turabian StyleGuittonny-Philippe, Anna, Véronique Masotti, Jacques Rabier, Marie-Eléonore Petit, Laure Malleret, Bruno Coulomb, and Isabelle Laffont-Schwob. 2015. "Biomonitoring of Epilobium hirsutum L. Health Status to Assess Water Ecotoxicity in Constructed Wetlands Treating Mixtures of Contaminants" Water 7, no. 2: 697-715. https://doi.org/10.3390/w7020697






