A Preliminary Investigation of Wastewater Treatment Efficiency and Economic Cost of Subsurface Flow Oyster-Shell-Bedded Constructed Wetland Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.1.1. Site Description

2.1.2. Configuration of the Four Study SSF CWs
| Item | Oyster shells | Gravels |
|---|---|---|
| True density (kg/m3) | 1273 | 2283 |
| Bulk density (kg/m3) | 289 | 1365 |
| Porosity (%) | 77 | 40 |
| Special surface area (m2/kg) | 0.96 | 0.23 |
| Special surface area (m2/m3) | 1217 | 527 |
| Descriptive statistics | BOD | DO | TP | SS | NH4+ | NO3− | pH | Temp |
|---|---|---|---|---|---|---|---|---|
| Average | 14.7 | 2.34 | 0.99 | 32.8 | 9.09 | 0.75 | 7.05 | 28.5 |
| SD | 4.53 | 0.56 | 0.37 | 6.21 | 3.79 | 0.61 | 0.24 | 0.83 |
| Maximum | 27.7 | 3.90 | 1.93 | 65.0 | 24.6 | 2.83 | 7.68 | 32.4 |
| Minimum | 5.48 | 0.20 | 0.49 | 12.0 | 1.88 | 0.04 | 6.74 | 26.9 |
| Removal quantity (g/m3/day) | BOD | DO | TP | SS | NH4+ | NO3− | Q (CMD) | HRT (day) | ||||
| Average wastewater removal quantity in 35 days | ||||||||||||
| HA(oyster shells) | 13.52 | 6.27 | 0.55 | 60.18 | −0.91 | 1.50 | 114.98 | 0.16 | ||||
| HB(gravels) | 9.81 | 5.59 | 0.89 | 49.65 | 2.19 | 0.61 | 101.21 | 0.09 | ||||
| VA(bagged oyster shells) | 9.24 | 3.50 | 0.46 | 39.76 | −0.97 | 0.60 | 122.99 | 0.26 | ||||
| VB(scattered oyster shells) | 8.03 | 3.35 | 0.20 | 37.85 | 0.61 | 0.41 | 111.67 | 0.28 | ||||
| Average wastewater removal quantity in 55 days | ||||||||||||
| HA | 23.17 | 8.89 | 0.35 | 114.65 | 2.00 | 2.42 | 178.79 | 0.09 | ||||
| HB | 12.61 | 5.69 | 0.34 | 64.60 | −0.10 | 0.60 | 110.09 | 0.07 | ||||
| VA | 21.50 | 6.92 | 0.34 | 100.62 | −4.45 | 1.51 | 224.91 | 0.13 | ||||
| VB | 17.59 | 4.32 | 0.10 | 57.76 | −1.55 | 0.54 | 137.36 | 0.19 | ||||
| Rate (%) | BOD | DO | TP | SS | NH4+ | NO3− | ||||||
| Average treatment efficiency in 35 days | ||||||||||||
| HA | 24.23 | 46.97 | 4.23 | 38.83 | −7.53 | 24.32 | ||||||
| HB | 21.97 | 49.01 | 18.20 | 34.23 | 4.93 | 8.87 | ||||||
| VA | 24.97 | 44.29 | 5.96 | 39.18 | −7.15 | 19.42 | ||||||
| VB | 19.13 | 49.66 | −0.90 | 44.10 | −2.67 | 4.82 | ||||||
| Average treatment efficiency in 55 days | ||||||||||||
| HA | 22.14 | 47.46 | 1.89 | 41.28 | −3.43 | 26.86 | ||||||
| HB | 19.68 | 49.70 | 5.48 | 38.73 | −5.03 | 7.42 | ||||||
| VA | 28.61 | 49.30 | 3.15 | 43.68 | −11.00 | 19.98 | ||||||
| VB | 22.22 | 51.42 | −0.87 | 48.11 | −10.60 | 10.90 | ||||||
2.1.3. Cost-Effectiveness Analysis
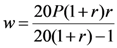
2.1.4. Statistical Analysis
2.2. Simulation Model
| Process rate | Definition | Rate |
|---|---|---|
| C-cycle | ||
| rBOD | Biochemical degradation |  |
| rR_BOD | Microorganism respiration |  |
| rDecay_BOD | Biomass decay |  |
| rB_BOD | Biofilm adsorption |  |
| O-cycle | ||
| rDO | Biochemical degradation |  |
| rN_DO | Nitrification |  |
| rR_DO | Microorganism respiration |  |
| rSOD | Sediment consumption |  |
| rB_DO | Biofilm adsorption |  |
| P-cycle | ||
| rP | Phosphorous utilization by microorganisms |  |
| rSettling_P | Phosphorous settling |  |
| rDecay_P | Biomass decay |  |
| rB_P | Biofilm adsorption |  |
| Suspended solids | ||
| rFitration | Filtration |  |
| rSettling_SS | Settling |  |
| rDecay_SS | Biomass decay |  |
| rB_SS | Biofilm adsorption |  |
| N-cycle | ||
| rN_N | Nitrification |  |
| rG_NH4 | Ammonia utilization by microorganisms |  |
| rG_NO3 | Nitrate utilization by microorganisms |  |
| rReg | Ammonia regeneration |  |
| rMin | Mineralization |  |
| rDN | Denitrification |  |
| rDecay_N | Biomass decay |  |
| rB_N | Biofilm adsorption |  |
| CT* | Temp. dependent factor |  |
| CpH* | pH growth-limiting factor | If pH<7.2 then (1-0.833•(7.2-pH) ) else 1 |
| Parameter | Description | Literature range | Unit | Source |
|---|---|---|---|---|
| Experimental data | ||||
| A | Cross-sectional area | - | m2 | Field monitoring data |
| AS | Special surface area of media | - | m2/m3 | [9] |
| BODd | Dissolve BOD | - | mg/L | Field monitoring data |
| BODs | Suspended BOD | - | mg/L | Field monitoring data |
| dc | Diameter of collector | - | m | Field monitoring data |
| H | Depth | - | m | Field monitoring data |
| p | Porosity | - | % | Field monitoring data |
| Qin | Inflow | - | m3/day | Field monitoring data |
| SNagger | Nitrogen in aggregates | - | mg/L | Field monitoring data |
| SON | Organic nitrogen | - | mg/L | Field monitoring data |
| C-cycle | ||||
| kBOD | Biochemical degradation rate of BOD | 0.3 | day−1 | [23] |
| kDecay_BOD | Biomass decay rate | 0.15 | day−1 | [24] |
| ksob_BOD | Biofilm adsorption coefficient of BOD | - | m−3day−1 | - |
| RBOD | Microorganisms respiration coefficient | - | - | - |
| μBOD | Max growth rate of hetero. at 20 °C | 0.8–6 | day−1 | [25] |
| φBOD | Empirical constant of BOD | 0.098 | °C−1 | [20] |
| O-cycle | ||||
| HSDO | Sediment oxygen demand constant | 2.5 | mg/L | [24] |
| kd_C | Degradation rate for BODd | 0.3 | day−1 | [23] |
| kN | Nitrification rate at 20 °C | 0.05 | day−1 | [23] |
| ks_C | Degradation rate for BODs | 0.3 | day−1 | [23] |
| ksed | Sedimentation coefficient | 0.1 | - | [23] |
| ksob_DO | Biofilm adsorption coefficient of DO | - | m−3day−1 | - |
| RDO | Heterotrophic respiration coefficient | 0.1 | - | - |
| SOD | Sediment oxygen demand | 0.1 | gO2/m2day | [23] |
| μDO | Max growth rate of hetero. at 20 °C | 0.015–0.2 | day−1 | [26,27] |
| φDO | Empirical constant of DO | 0.098 | °C−1 | [20] |
| P-cycle | ||||
| iP,BM | Phosphorus content of biomass | 0.02 | mgP/mgBM | [28] |
| kDecay_P | Biomass decay rate | 0.15 | day−1 | [24] |
| kP | Biochemical degradation rate | - | day−1 | - |
| kSettling_P | Phosphorous settling coefficient | 0.03 | m−1day−1 | - |
| ksob_P | Biofilm adsorption coefficient of TP | - | m−3day−1 | - |
| φP | Empirical constant of TP | 0.098 | °C−1 | [20] |
| Suspended solids | ||||
| dSS | Diameter of settling particle | 0.1–4 | mm | [29] |
| kDecay_SS | Biomass decay rate | 0.15 | day−1 | [24] |
| kF | Filtration coefficient | - | - | - |
| kSettling_SS | Settling coefficient | - | m−3 | - |
| ksob_SS | Biofilm adsorption coefficient of SS | - | m−3day−1 | - |
| α | Sticking coefficient | 0.0008–0.012 | - | [30] |
| ρs | Density of settling particle | 1050–1500 | kg/m3 | [31] |
| ρW | Density of water | 995.69 | kg/m3 | [31] |
| νW | Kinematic viscosity of water | 0.0867 | m2/day | [32] |
| φSS | Empirical constant of SS | 0.098 | °C−1 | [20] |
| N-cycle | ||||
| iN,BM | Nitrogen content of biomass | 0.07 | mgN/mgBM | [28] |
| kDecay_N | Biomass decay rate | 0.15 | day−1 | [24] |
| kDN | Denitrification rate at 20 °C | 0–1 | day−1 | [33] |
| kG_NH4 | NH4+ uptake preference factor | - | - | - |
| kG_NO3 | NO3− uptake preference factor | - | - | - |
| kMin | Mineralization rate | 0.0005–0.143 | day−1 | [34] |
| kN_N | Growth rate of nitrosomonas by nitrification | 0.33–2.21 | day−1 | [35] |
| kReg | NH4+ regeneration rate | 0.085 | day−1 | [36] |
| ksob_N | Biofilm adsorption coefficient of NH4+ and NO3− | - | m−3day−1 | - |
| Yn | Nitrosomonas yield coefficient | 0.03–0.13 | mgVSS/mgN | [37] |
| μmax,20 | Max. growth rate of bacteria at 20 °C | 0.18 | day−1 | [38] |
| φN | Empirical constant | 0.098 | °C−1 | [20] |
| Biofilms | ||||
| bX1 | Microorganism heterotroph decay rate | 0.3 | day−1 | - |
| bX2 | Microorganism nitrosomonas decay rate | 0.3 | day−1 | - |
| DNH4 | Diffusion coefficient of NH4+ | 1.71 × 10−4 | m2/day | [39] |
| DNO3 | Diffusion coefficient of NO3− | (4.5–27.9) × 10−6 | m2/day | [40] |
| DTOC | TOC diffusion coefficient | 1.56 × 10−5 | m2/day | [41] |
| DX | Microorganism diffusion coefficient | - | m2/day | - |
| LFmodel | Biofilms thickness | - | m | Biofilm model result |
| XH | Heterotrophic organisms | - | mg/L | Biofilm model result |
| Y1 | Yield constant of heterotroph | 0.6 | - | [27] |
| Y2 | NH4+ yield constant of nitrosomonas | 0.13 | - | [27] |
| Y3 | NO3− yield constant of nitrosomonas | 0.03 | - | [27] |
| μX1 | Max growth rate of heterotroph | 3–6 | day−1 | [28,42] |
| μX2 | Max growth rate of nitrosomonas | 0.33–2.21 | day−1 | [35] |
| Temperature coefficient | ||||
| θBOD | Temp. coefficient of degradation | 1.09 | - | [23] |
| θDecay | Temp. coefficient of biomass decay | - | - | - |
| θR | Temp. coefficient of respiration | - | - | - |
| θDN | Temp. coefficient of denitrification | 1.15 | - | [43] |
| θgrowth | Temp. coefficient of microorganisms growth | 1.08–1.12 | - | [31] |
| θN | Temp. coefficient of nitrification | 1.1 | - | [23] |
| Half- saturation (Half-sat.) constant | ||||
| KBOD | Half-sat. constant of BOD | 2 | mg/L | [23] |
| KDO | Half-sat. constant of DO | 2 | gO2/m3 | [23] |
| KP | Half-sat. constant of TP | 0.02 | mg/L | [44] |
| Kn | Half-sat. constant of NH4+ nitrosomonas | 0.05 | mg/L | [23] |
| KnDO | Half-sat. constant of DO nitrosomonas | 0.13–1.3 | mg/L | [35] |
| KNH4 | Half-sat. constant of NH4+ | 2 | gCOD/m3 | [27] |
| KNO3 | Half-sat. constant of NO3− | 0.15–0.5 | gN/m3 | [26,45] |
2.2.1. Carbon Cycle
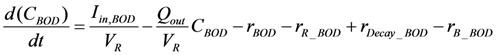
2.2.2. Oxygen Cycle
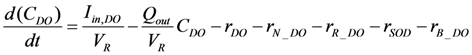
2.2.3. Phosphorus Cycle
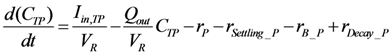
2.2.4. Suspended Solids
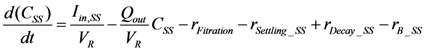
2.2.5. Nitrogen Cycle
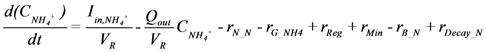
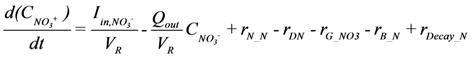
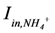 is loading of NH4+ into the reactor (mass per unit per time),
is loading of NH4+ into the reactor (mass per unit per time), 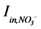 is loading of NO3− into the reactor (mass per unit per time),
is loading of NO3− into the reactor (mass per unit per time), 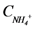 is the concentration of NH4+, and
is the concentration of NH4+, and 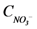 is the concentration of NO3−.
is the concentration of NO3−.2.2.6. Biofilm Reactor Compartment
2.2.7. Sensitivity Analysis
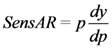
3. Results and Discussion
3.1. Field Experiment
3.1.1. Cost-Effectiveness Analysis
| Cost | HA | HB | VA | VB |
|---|---|---|---|---|
| Capital cost | ||||
| Suppose engineering | 916 | 987 | 1046 | 1046 |
| Civil engineering | 570 | 614 | 651 | 651 |
| Pumping well | 254 | 273 | 290 | 290 |
| Aeration pond | 851 | 916 | 971 | 971 |
| Diversion cut | 1740 | 1880 | 0 | 0 |
| Reverse-flushing system | 1260 | 1167 | 0 | 0 |
| Water distribution pipe | 0 | 0 | 560 | 560 |
| Sludge pipe | 0 | 0 | 1700 | 1700 |
| Antiseep engineering | 1406 | 1514 | 1606 | 1606 |
| Collection drains | 1960 | 2111 | 2239 | 2239 |
| Media paving | 282 | 303 | 322 | 322 |
| Water quality monitoring pipe | 133 | 133 | 100 | 100 |
| Gravels | 0 | 3360 | 0 | 0 |
| Oyster shell transport | 1007 | 0 | 1007 | 1007 |
| Bagged | 0 | 0 | 984 | 0 |
| Original capital cost (US$) | 10379 | 13258 | 11475 | 10491 |
| Capital cost—20-year annuity (US$/yr) | 545 | 696 | 602 | 551 |
| O&M cost | ||||
| 55-day-operation-period (US$) | 332 | 328 | 335 | 329 |
| Per year (US$/yr) | 2205 | 2173 | 2226 | 2186 |
| Total cost | ||||
| 55-day-operation-period (US$) | 10711 | 13586 | 11810 | 10820 |
| 20-year annuity (US$/yr) | 2749 | 2869 | 2828 | 2737 |
| Total waste removal quantity of BOD during the operation time | ||||
| 55-day-operation-period (kg) | 31.77 | 17.29 | 64.97 | 37.92 |
| Per year (kg/yr) | 210.83 | 114.74 | 431.17 | 251.63 |
| Cost-effectiveness value (Cost per mass BOD removed) | ||||
| 55-day-operation-period (US$/kg) | 337.15 | 785.79 | 181.78 | 285.38 |
| 20-year annuity (US$/kg) | 13.04 | 25.01 | 6.56 | 10.88 |
3.1.2. Treatment Efficiency Analysis
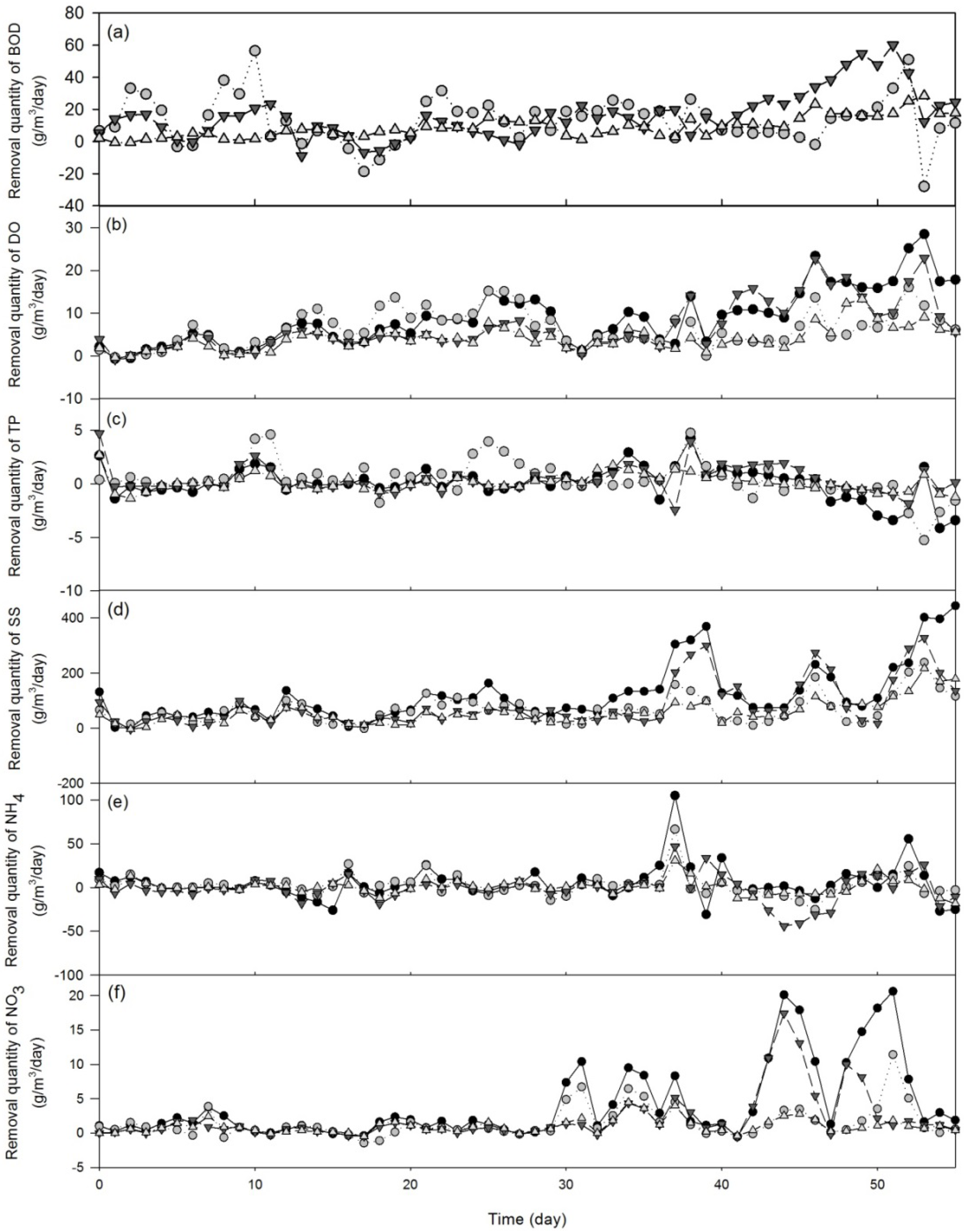
| Wastewater parameter | F-value | P-value |
|---|---|---|
| BOD | 2.655 | 0.049* |
| DO | 8.498 | 0.000* |
| TP | 0.380 | 0.767 |
| SS | 6.727 | 0.000* |
| NH4+ | 1.388 | 0.247 |
| NO3− | 4.233 | 0.006* |
| Parameters | SensAR | SensAR | SensAR | SensAR | |||||
|---|---|---|---|---|---|---|---|---|---|
| RMS | Mean | RMS | Mean | RMS | Mean | RMS | Mean | ||
| HA | HB | VA | VB | ||||||
| ksob_BOD | 3.535 | −3.086 | 0.920 | −0.794 | 3.341 | −3.053 | 3.338 | −3.179 | |
| kDecay_BOD | 1.578 | 1.392 | 0.571 | 0.538 | 1.753 | 1.663 | 0.958 | 0.930 | |
| kBOD | 0.556 | −0.492 | 1.856 | −1.713 | 0.098 | 0.083 | 0.280 | −0.257 | |
| RBOD | 0.274 | −0.231 | 0.009 | 0.004 | 0.146 | −0.241 | 0.204 | −0.187 | |
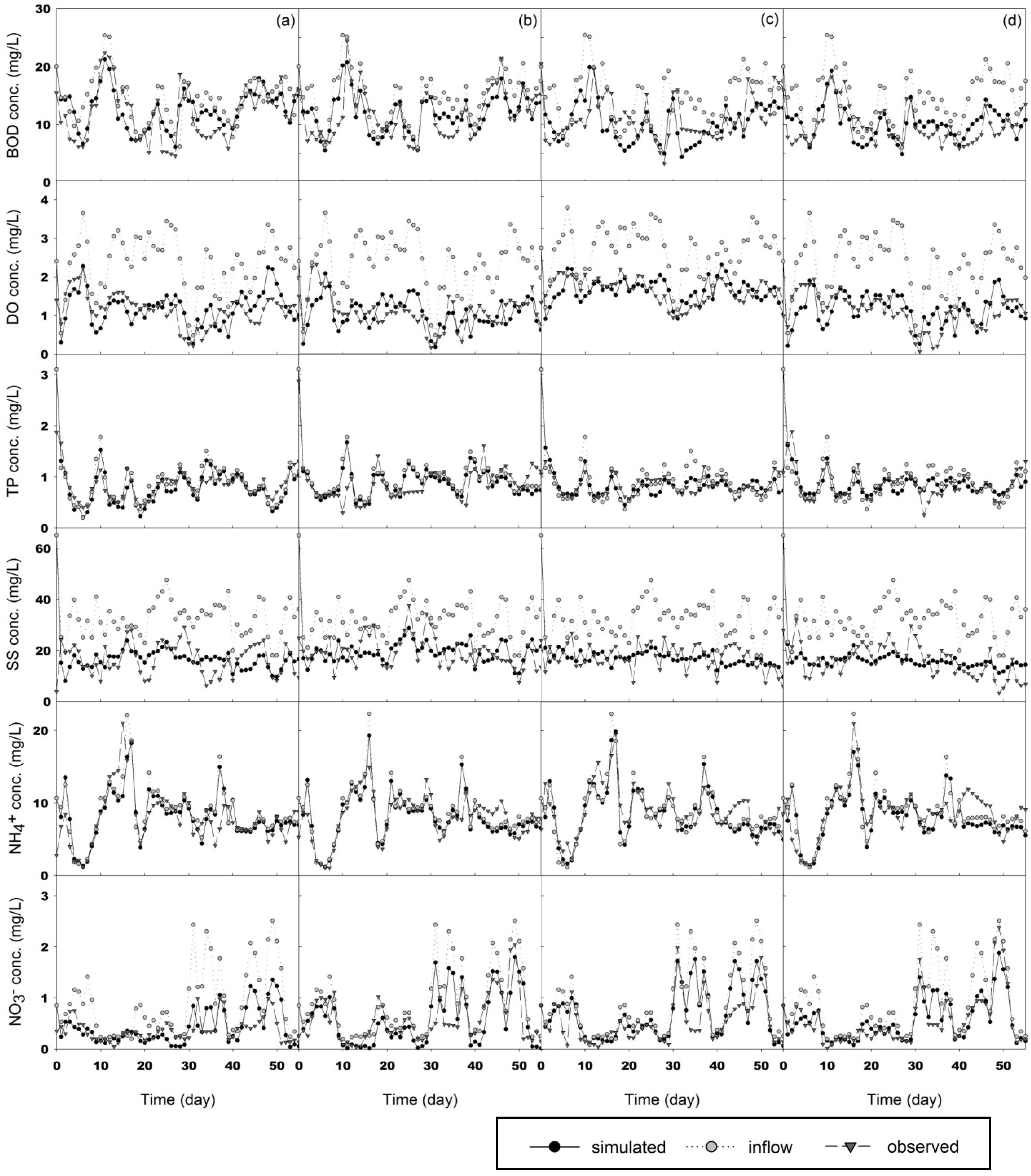
3.2. Simulation
3.2.1. Sensitivity Analysis
3.2.2. Feasible Range of Parameters in Oyster-Shell-Bedded CWs
| Submodel | Parameter | HA | VA | VB | Feasible range |
|---|---|---|---|---|---|
| C-cycle | kBOD | 0.680 | 0.894 | 0.752 | 0.680–0.894 |
| kDecay_BOD | 5.977 | 8.764 | 9.335 | 5.977–9.335 | |
| ksob_BOD | 12.65 | 34.13 | 32.69 | 12.65–34.13 | |
| RBOD | 6.704 | 17.97 | 13.08 | 6.704–17.97 | |
| θBOD | 0.931 | 0.898 | 0.826 | 0.826–0.931 | |
| θDecay | 0.794 | 0.764 | 0.706 | 0.706–0.794 | |
| θR | 0.729 | 0.771 | 0.745 | 0.729–0.771 | |
| μBOD | 3.000 | 3.000 | 3.000 | 3.000 | |
| φBOD | 0.081 | 0.104 | 0.097 | 0.081–0.104 | |
| O-cycle | HSDO | 3.000 | 3.000 | 3.000 | 3.000 |
| kd_C | 0.076 | 0.268 | 0.010 | 0.010–0.268 | |
| kN | 0.001 | 0.001 | 0.001 | 0.001 | |
| ks_C | 0.578 | 1.001 | 0.572 | 0.572–1.001 | |
| ksed | 0.921 | 0.742 | 0.897 | 0.742–0.897 | |
| ksob_DO | 23.36 | 26.14 | 45.01 | 23.36–45.01 | |
| RDO | 10.55 | 23.02 | 8.224 | 8.224–10.56 | |
| SOD | 0.100 | 0.100 | 0.100 | 0.100 | |
| θBOD | 0.905 | 0.759 | 0.854 | 0.759–0.905 | |
| θN | 0.688 | 0.600 | 0.600 | 0.600–0.688 | |
| θR | 0.881 | 0.836 | 0.799 | 0.799–0.881 | |
| μDO | 0.163 | 0.163 | 0.163 | 0.163 | |
| φDO | 0.010 | 0.010 | 0.010 | 0.010 | |
| P-cycle | iP, BM | 0.020 | 0.020 | 0.020 | 0.020 |
| kDecay_P | 5.662 | 11.68 | 10.83 | 5.662–11.68 | |
| kP | 0.472 | 2.127 | 0.921 | 0.472–2.127 | |
| kSettling_P | 0.020 | 0.025 | 0.024 | 0.020–0.025 | |
| ksob_P | 0.058 | 0.593 | 0.309 | 0.058–0.593 | |
| θDecay | 0.725 | 0.903 | 0.848 | 0.725–0.903 | |
| θR | 0.757 | 0.718 | 0.742 | 0.718–0.757 | |
| φP | 0.130 | 0.127 | 0.092 | 0.092–0.130 | |
| SS | dSS | 0.001 | 0.001 | 0.001 | 0.001 |
| kDecay_SS | 1.514 | 3.937 | 2.413 | 1.514–3.937 | |
| kF | 0.007 | 0.017 | 0.007 | 0.007–0.017 | |
| kSettling_SS | 0.100 | 0.300 | 0.315 | 0.100–0.315 | |
| ksob_SS | 8.390 | 16.74 | 28.24 | 8.390–28.24 | |
| Sg | 1500 | 1500 | 1500 | 1500 | |
| α | 0.007 | 0.007 | 0.007 | 0.007 | |
| ρS | 1300 | 1300 | 1300 | 1300 | |
| ρW | 995.7 | 995.7 | 995.7 | 995.7 | |
| νW | 0.087 | 0.087 | 0.087 | 0.087 | |
| θDecay | 1.043 | 0.996 | 1.028 | 0.996–1.043 | |
| φSS | 0.018 | 0.134 | 0.010 | 0.010–0.134 | |
| N-cycle | iN, BM | 0.070 | 0.070 | 0.070 | 0.070 |
| kDecay_N | 0.035 | 0.086 | 0.178 | 0.035–0.178 | |
| kDN | 1.582 | 0.051 | 0.771 | 0.051–1.582 | |
| kG_NH4 | 0.354 | 0.032 | 0.032 | 0.032–0.354 | |
| kG_NO3 | 0.727 | 2.934 | 2.477 | 0.727–2.934 | |
| kMin | 0.100 | 0.569 | 0.228 | 0.100–0.569 | |
| kN_N | 0.873 | 0.808 | 0.915 | 0.808–0.915 | |
| kReg | 0.100 | 0.731 | 0.291 | 0.100–0.731 | |
| ksob_N | 0.934 | 1.498 | 1.347 | 0.934–1.498 | |
| Yn | 0.130 | 0.130 | 0.130 | 0.130 | |
| φP | 0.130 | 0.127 | 0.092 | 0.092–0.130 | |
| θDecay | 0.894 | 0.852 | 0.882 | 0.852–0.884 | |
| θDN | 1.181 | 1.198 | 0.958 | 0.958–1.198 | |
| θGrowth | 0.871 | 0.903 | 0.900 | 0.871–0.903 | |
| θN | 0.939 | 0.768 | 0.77 | 0.768–0.939 | |
| μmax,20 | 0.180 | 0.180 | 0.180 | 0.180 | |
| φN | 0.098 | 0.098 | 0.098 | 0.098 |
3.2.3. Applications of Our Model
4. Conclusions
- (1)
- The four study SSF CWs showed a significant difference in the waste removal quantity of BOD, DO, NO3−, and SS. The waste removal quantity of the horizontal SSF oyster-shell-bedded CW (HA) was significantly higher than the horizontal SSF gravel-bedded CW (HB) but similar to the vertical SSF oyster-shell CW (VB). Comparison between bagged (VA) and scattered (VB) arrangement oyster-shell-bedded CWs indicated that the waste removal quantity and treatment efficiency between these two wetlands were generally similar. However, VA wetland demonstrated significantly highest BOD removal capacity among all study sites but also showing the lowest cost per mass BOD removed (6.56 US$/kg) as compared to other three CWs (10.88–25.01 US$/kg). Therefore, VA was determined as the best option for SFF CW in terms of waste treatment efficiency and cost-effectiveness.
- (2)
- The total costs of the four study CWs ranged from 2,737 (VB) to 2,869 (HB) US$/yr in 20-year annuity whereas they were between 10,711 (HA) and 13,586 (HB) US$ for only 55-day operation period. Also, the relative importance of capital costs to the total costs of all CWs for long-term operation (20-year annuity) was only one fifth of that for 55 days’ operation. Therefore, results of the cost-effectiveness analysis highlighted that the economic returns of CWs would be higher for long-term operation.
- (3)
- The average waste removal quantity of most wastewater parameters increased slightly from 35-day to 55-day-periods but the average treatment efficiency of all wastewater parameters remained fairly constant between 35-day and 55-day-periods. Our findings suggested that establishment time could be critical for the success of CWs with respect to wastewater treatment efficiency.
- (4)
- The results of our numerical water quality model demonstrated that, biofilm adsorption played the most essential role in the wastewater treatment processes in oyster-shell-bedded CWs but biochemical degradation was the most significant mechanism in gravel-bedded CW.
- (5)
- The feasible range of each water quality parameter in oyster-shell bedded wetlands was identified in the present study, and it was obtained by a regression model using the field monitoring data. These feasible ranges could be used for water quality simulations in the CWs and this could help characterizing different CWs by determining the quantitative importance of different biochemical treatment processes in SSF CWs.
Acknowledgements
References
- Economopoulou, M.A.; Tsihrintzis, V.A. Design methodology and area sensitivity analysis of horizontal subsurface flow constructed wetlands. Water Resour. Manag. 2003, 17, 147–174. [Google Scholar] [CrossRef]
- Vymazal, J.; Brix, H.; Cooper, P.F.; Haberl, R.; Perfler, R.; Laber, J. Removal Mechanisms and Types of Constructed Wetlands. In Constructed Wetlands for Wastewater Treatment in Europe; Backhuys: Leiden, The Netherland, 1998; pp. 17–66. [Google Scholar]
- Vymazal, J. Constructed wetlands for wastewater treatment. Water 2010, 2, 530–549. [Google Scholar] [CrossRef]
- Teng, C.J.; Leu, S.Y.; Ko, C.H.; Fan, C.; Sheu, Y.S.; Hu, H.Y. Economic and environmental analysis of using constructed riparian wetlands to support urbanized municipal wastewater treatment. Ecol. Eng. 2012, 44, 249–258. [Google Scholar] [CrossRef]
- Chen, G.Q.; Shao, L.; Chen, Z.M.; Li, Z.; Zhang, B.; Chen, H.; Wu, Z. Low-carbon assessment for ecological wastewater treatment by a constructed wetland in Beijing. Ecol. Eng. 2011, 37, 622–628. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA), Wastewater Technology Fact Sheet Wetlands: Subsurface Flow; EPA 832-F-00-023; Office of Water, USEPA: Washington, DC, USA, 2000.
- U.S. Environmental Protection Agency (USEPA), Manual Constructed Wetlands Treatment of Municipal Wastewaters; EPA 625-R-99-010; USEPA: Cincinnati, OH, USA, 2000.
- Hsu, Y.F.; Pan, W.C. Study on the Production and Labor of Oyster-Cultivating Industry in Tong-Shih: A Periphery in the Commodity Chain. In Nanhua University Policy Research; Nanhua University: Chiayi, Taiwan, 2003; pp. 105–138. [Google Scholar]
- Lin, Y.F.; Jing, S.R. Recycling of Seafood Solid Waste as Substrate Material Used in Constructed Wetland for Wastewater Treatment; National Sciences Council: Taipei, Taiwan, 2006. [Google Scholar]
- Chen, B.; Chen, Z.M.; Zhou, Y.; Zhou, J.B.; Chen, G.Q. Emergy as embodied energy based assessment for local sustainability of a constructed wetland in Beijing. Commun. Nonlinear Sci. Numer. Simul. 2009, 14, 622–635. [Google Scholar]
- Seo, D.C.; Cho, J.S.; Lee, H.J.; Heo, J.S. Phosphorus retention capacity of filter media for estimating the longevity of constructed wetland. Water Res. 2005, 39, 2445–2457. [Google Scholar]
- Park, W.H.; Polprasert, C. Roles of oyster shells in an integrated constructed wetland system designed for P removal. Ecol. Eng. 2008, 34, 50–56. [Google Scholar]
- Huett, D.O.; Morris, S.G.; Smith, G.; Hunt, N. Nitrogen and phosphorus removal from plant nursery runoff in vegetated and unvegetated subsurface flow wetlands. Water Res. 2005, 39, 3259–3272. [Google Scholar]
- Reichert, P. AQUASIM 2.0: Computer Program for the Identification and Simulation of Aquatic Systems; Swiss Federal Institute for Environmental Science and Technology (EAWAG): Dübendorf, Switzerland, 1998. [Google Scholar]
- Environmental Protection Administration, Republic of China (ROCEPA). Environmental Water Quality Information; ROCEPA: Taipei, Taiwan, 2010. Available online: http://wq.epa.gov.tw/WQEPA/Code/?Languages=en (accessed on 1 February 2013).
- Chang, C.F. Economical Analysis of Oyster Shells Contact Bed in Wastewater Treatment. M.S. Thesis, National Taiwan University, Taipei, Taiwan, 2009. [Google Scholar]
- APHA, Standard Methods for Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1998.
- Henrichs, M.; Langergraber, G.; Uhl, M. Modelling of organic matter degradation in constructed wetlands for treatment of combined sewer overflow. Sci. Total Environ. 2007, 380, 196–209. [Google Scholar]
- Yang, C.P.; Kuo, J.T.; Lung, W.S.; Lai, J.S.; Wu, J.T. Water quality and ecosystem modeling of tidal wetlands. J. Environ. Eng. 2007, 133, 711–721. [Google Scholar]
- Downing, A.L. Population Dynamics in Biological System. In Proceedings of 3rd International Conference of Water Pollution Research, Munich, Germany, 1966; Volume 2, pp. 117–137.
- Ottova, V.; Balcarova, J.; Vymazal, J. Microbial characteristics of constructed wetlands. Water Sci. Tech. 1997, 35, 117–123. [Google Scholar]
- Fountoulakis, M.S.; Terzakis, S.; Chatzinotas, A.; Brix, H.; Kalogerakis, N.; Manios, T. Pilot-scale comparison of constructed wetlands operated under high hydraulic, loading rates and attached biofilm reactors for domestic wastewater treatment. Sci. Total Environ. 2009, 407, 2996–3003. [Google Scholar]
- Lopes, J.F.; Silva, C. Temporal and spatial distribution of dissolved oxygen in the Ria de Aveiro lagoon. Ecol. Model. 2006, 197, 67–88. [Google Scholar] [CrossRef]
- Hull, V.; Parrella, L.; Falcucci, M. Modelling dissolved oxygen dynamics in coastal lagoons. Ecol. Model. 2008, 211, 468–480. [Google Scholar]
- Lin, T.S. The Effect of Variation of Tank Volume on Heterotrophic/Nitrifying Species in TNCU3 Activated Sludge Process. M.S. Thesis, Chaoyang University of Technology, Taichung, Taiwan, 2005. [Google Scholar]
- Wynn, T.M.; Liehr, S.K. Development of a constructed subsurface-flow wetland simulation model. Ecol. Eng. 2001, 16, 519–536. [Google Scholar]
- Reichert, P.; Borchardt, D.; Henze, M.; Rauch, W.; Shanahan, P.; Somlyody, L.; Vanrolleghem, P. River water quality model No. 1 (RWQM1): II. Biochemical process equations. Water Sci. Tech. 2001, 43, 11–30. [Google Scholar]
- Henze, M. Wastewater Treatment: Biological and Chemical Processes; Springer: Berlin, Germany, 2002. [Google Scholar]
- Poirier, M.R. Minimum Velocity Required to Transport Solid Particles from the 2H-Evaporator to the Tank Farm. Westinghouse Savannah River Company: Aiken, SC, USA, 2000; WSRC-TR-2000-00263. [Google Scholar]
- Polprasert, C.; Khatiwada, N.R. An integrated kinetic model for water hyacinth ponds used for wastewater treatment. Water Res. 1998, 32, 179–185. [Google Scholar]
- Tchobanoglous, G.; Burton, F.L. Wastewater Engineering:Treatment, Disposal, and Reuse; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Young, D.F.; Munson, B.R.; Okiishi, T.H.; Huebsch, W.W. A Brief Introduction to Fluid Mechanics; John Wiley & Sons: New York, NY, USA, 2010. [Google Scholar]
- Baca, R.G.; Arnett, R.C. A Limnological Model for Eutrophic Lakes and Impoundments; Battelle, Pacific Northwest Laboratories, for USEPA, Office of Research and Development: Richland, WA, USA, 1976. [Google Scholar]
- Martin, J.F.; Reddy, K.R. Interaction and spatial distribution of wetland nitrogen processes. Ecol. Model. 1997, 105, 1–21. [Google Scholar]
- Jørgensen, S.E.; Nielsen, S.N.; Jørgensen, L.A. Handbook of Ecological Parameters and Ecotoxicology; Elsevier: Amsterdam, The Netherland, 1991. [Google Scholar]
- Mayo, A.W.; Bigambo, T. Nitrogen transformation in horizontal subsurface flow constructed wetlands I: Model development. Phys. Chem. Earth. 2005, 30, 658–667. [Google Scholar] [CrossRef]
- Charley, R.C.; Hooper, D.G.; McLee, A.G. Nitrification kinetics in activated-sludge at various temperatures and dissolved-oxygen concentrations. Water Res. 1980, 14, 1387–1396. [Google Scholar]
- Ferrara, R.A.; Harleman, D.R.F. Dynamic nutrient cycle model for waste stabilization ponds. J. Environ. Eng. ASCE. 1980, 106, 37–54. [Google Scholar]
- Thibodeaux, L.J. Environmental Chemodynamics:Movement of Chemicals in Air, Water, and Soil; John. Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- Hill, D. Diffusion-coefficients of nitrate, chloride, sulfate and water in cracked and uncracked chalk. J. Soil Sci. 1984, 35, 27–33. [Google Scholar]
- Domingos, R.F.; Benedetti, M.F.; Croué, J.P.; Pinheiro, J.P. Electrochemical methodology to study labile trace metal/natural organic matter complexation at low concentration levels in natural waters. Anal. Chim. Acta 2004, 521, 77–86. [Google Scholar] [CrossRef]
- Henze, M. Activated Sludge Models ASM1, ASM2, ASM2d and ASM3; IWA Publishing: London, UK, 2000. [Google Scholar]
- Reed, S.C.; Crites, R.W.; Middlebrooks, E.J. Natural Systems for Waste Management and Treatment; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Ghermandi, A.; Vandenberghe, V.; Benedetti, L.; Bauwens, W.; Vanrolleghem, P.A. Model-based assessment of shading effect by riparian vegetation on river water quality. Ecol. Eng. 2009, 35, 92–104. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; Matsuo, T.; Wentzel, M.C.; Marais, G.V.R.; van Loosdrecht, M.C.M. Activated sludge model No.2d, ASM2d. Water Sci. Tech. 1999, 39, 165–182. [Google Scholar]
- Wanner, O.; Reichert, P. Mathematical modeling of mixed-culture biofilms. Biotechnol. Bioeng. 1996, 49, 172–184. [Google Scholar] [CrossRef]
- Wang, Y.C.; Lin, Y.P.; Huang, C.W.; Chiang, L.C.; Chu, H.J.; Ou, W.S. A system dynamic model and sensitivity analysis for simulating domestic pollution removal in a free-water surface constructed wetland. Water Air Soil Pollut. 2012, 223, 2719–2742. [Google Scholar]
- Her, J.J.; Huang, J.S. Influences of carbon surface and C/N ratio on nitrate nitrite denitrification and carbon breakthrough. Bioresour. Technol. 1995, 54, 45–51. [Google Scholar] [CrossRef]
- Luederitz, V.; Eckert, E.; Lange-Weber, M.; Lange, A.; Gersberg, R.M. Nutrient removal efficiency and resource economics of vertical flow and horizontal flow constructed wetlands. Ecol. Eng. 2001, 18, 157–171. [Google Scholar] [CrossRef]
- Bouwer, E.J. Theoretical investigation of particle deposition in biofilm systems. Water Res. 1987, 21, 1489–1498. [Google Scholar] [CrossRef]
- Tufenkji, N.; Elimelech, M. Correlation equation for predicting single-collector efficiency in physicochemical filtration in saturated porous media. Environ. Sci. Technol. 2004, 38, 529–536. [Google Scholar] [CrossRef]
- Dixon, A.; Simon, M.; Burkitt, T. Assessing the environmental impact of two options for small-scale wastewater treatment: Comparing a reedbed and an aerated biological filter using a life cycle approach. Ecol. Eng. 2003, 20, 297–308. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yam, R.S.W.; Hsu, C.-C.; Chang, T.-J.; Chang, W.-L. A Preliminary Investigation of Wastewater Treatment Efficiency and Economic Cost of Subsurface Flow Oyster-Shell-Bedded Constructed Wetland Systems. Water 2013, 5, 893-916. https://doi.org/10.3390/w5030893
Yam RSW, Hsu C-C, Chang T-J, Chang W-L. A Preliminary Investigation of Wastewater Treatment Efficiency and Economic Cost of Subsurface Flow Oyster-Shell-Bedded Constructed Wetland Systems. Water. 2013; 5(3):893-916. https://doi.org/10.3390/w5030893
Chicago/Turabian StyleYam, Rita S.W., Chia-Chuan Hsu, Tsang-Jung Chang, and Wen-Lian Chang. 2013. "A Preliminary Investigation of Wastewater Treatment Efficiency and Economic Cost of Subsurface Flow Oyster-Shell-Bedded Constructed Wetland Systems" Water 5, no. 3: 893-916. https://doi.org/10.3390/w5030893
APA StyleYam, R. S. W., Hsu, C.-C., Chang, T.-J., & Chang, W.-L. (2013). A Preliminary Investigation of Wastewater Treatment Efficiency and Economic Cost of Subsurface Flow Oyster-Shell-Bedded Constructed Wetland Systems. Water, 5(3), 893-916. https://doi.org/10.3390/w5030893





