Analysis of (Anti-)Oestrogenic and (Anti-)Androgenic Activities in Wastewater from the Lodz Sewer System
Abstract
:1. Introduction
2. Materials and Methods
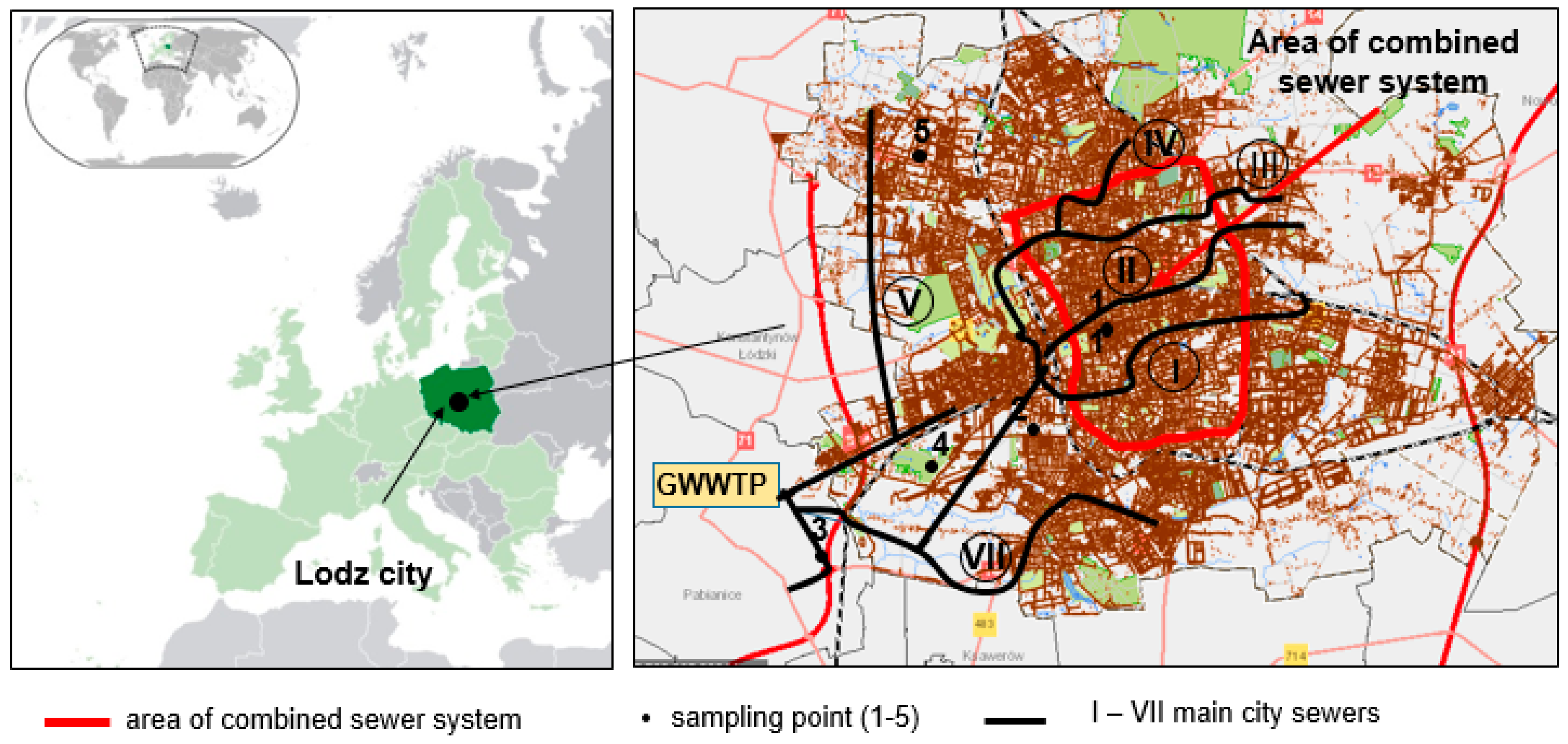
2.1. Characteristics of the Studied Catchment—Lodz City
2.2. Wastewater System of Lodz
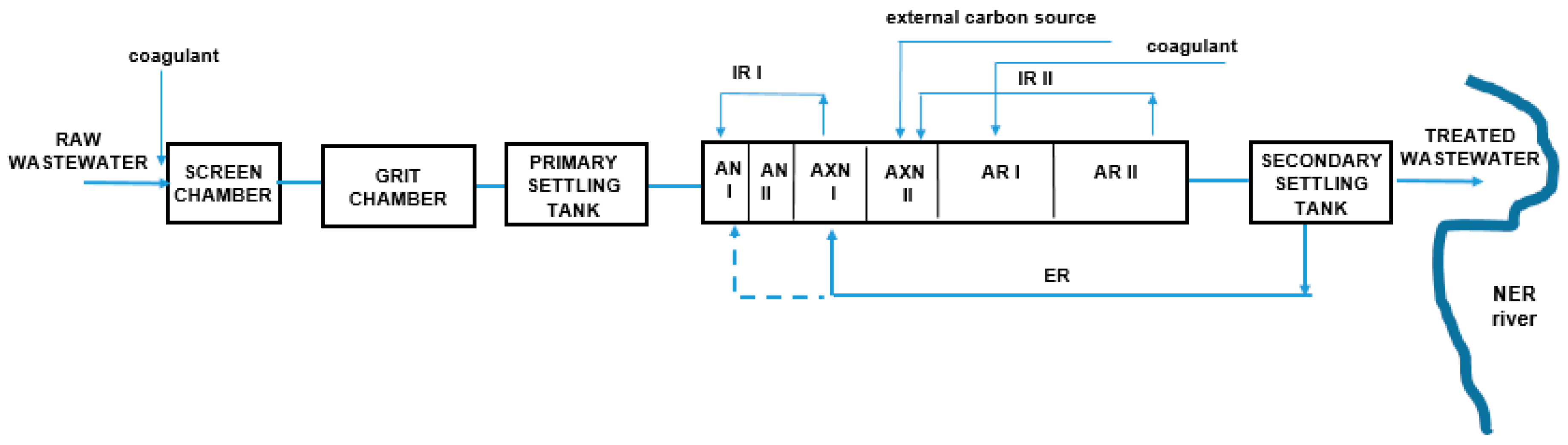
2.3. Group Wastewater Treatment Plant of Lodz
2.4. Sample Collection
2.5. Methodology of Wastewater Tests
3. Results and Discussion
4. Conclusions
- YES/YAS bioassays may be used to assess general levels of endocrine disrupting chemicals and, therefore, could be included, either as an alternative method or complementary to the chemical analysis of wastewater, surface water, as well as for assessing the removal those substances in WWTP.
- The samples of wastewater from the sewer system in Lodz did not exhibit agonistic oestrogenic activity with two exceptions (EEQ = 0.9 and 13.7 ng E2/L). No agonistic androgenic activity was found in any of the tested samples. Oestrogenic antagonistic properties (aEEQ) were found in all wastewater samples collected from the sewer system (max = 8.49 × 105 ng HT/L), as well as in most samples, where androgenic antagonistic properties (aAEQ) were detected (max = 1.70 × 107 ng/L).
- Oestrogen and androgen agonist activity was not found in wastewater from the treatment plant. Both oestrogenic and androgenic antagonistic properties were identified in the inflow and outflow from the treatment plant. In the treatment process, they were reduced from 39.4 to 99.2%, depending on the type of activity. Those results confirm that in the case of conventional wastewater treatment plants, a constant high reduction of hormonal pollutants is not guaranteed.
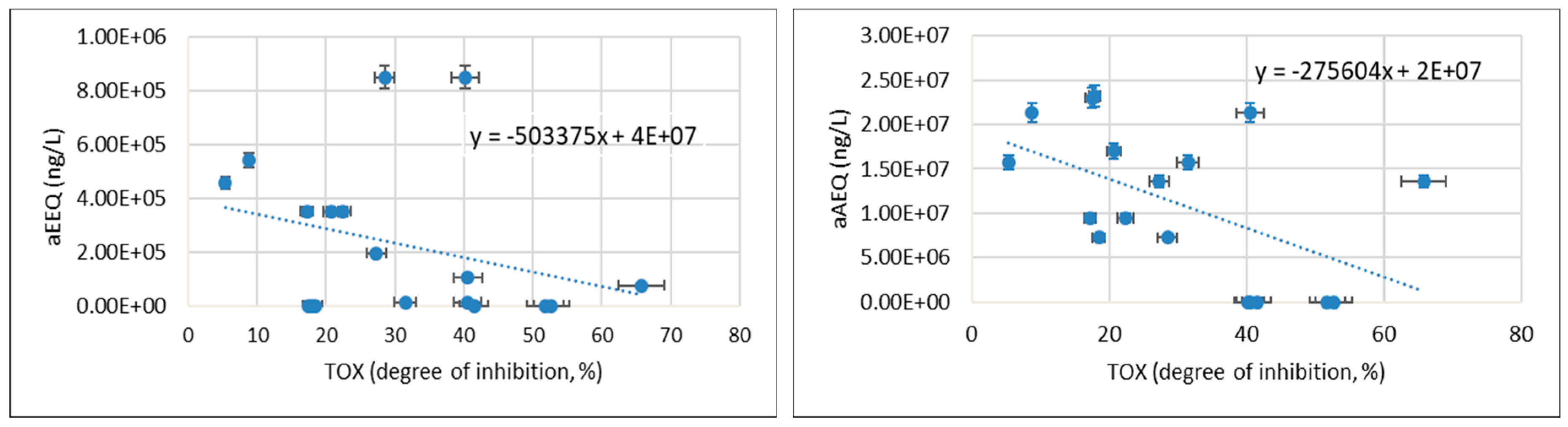
- YES/YAS bioassays used to analyse wastewater containing mixtures of both agonists and antagonists provided general information about the presence of all active chemical compounds with oestrogenic and androgenic effects. No correlation was found between the hormonal activity of wastewater and their basic physicochemical parameters, which could facilitate the identification of oestrogenic and androgenic contaminates that pose a threat to the aquatic environment.
- The results of the YES/YAS assay indicate that due to the CSOs activity in Lodz, with discharge of untreated wastewater into small urban rivers, there is the possibility of threats to the aquatic ecosystem, resulting from the presence of endocrine disrupting chemicals. Reducing the risk to the receiving waters from endocrine disruptors contained in wastewater can be achieved both by eliminating/reducing significant point sources of pollution in the catchment, and by reducing CSO activities and the modernisation of existing wastewater treatment plants to increase their micropollutant removal efficiency.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.; Farnazo, D.M. Toxicity characteristics of sewage treatment effluents and potential contribution of micropollutant residuals. J. Ecol. Environ. 2017, 41, 39. [Google Scholar] [CrossRef] [Green Version]
- Gholami-Borujeni, F.; Nejatzadeh-Barandozi, F.; Aghdasi, H. Data on effluent toxicity and physicochemical parameters of municipal wastewater treatment plant using Daphnia Magna. Data Brief 2018, 19, 1837–1843. [Google Scholar] [CrossRef]
- Bhuvaneshwari, M.; Eltzov, E.; Veltman, B.; Shapiro, O.; Sadhasivam, G.; Borisover, M. Toxicity of chlorinated and ozonated wastewater effluents probed by genetically modified bioluminescent bacteria and cyanobacteria Spirulina sp. Water Res. 2019, 164, 114910. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.M.; Nogueira, V.; Lopes, I.; Rocha-Santos, T.; Pereira, R. Evaluation of the potential toxicity of effluents from the textile industry before and after treatment. Appl. Sci. 2019, 9, 3804. [Google Scholar] [CrossRef] [Green Version]
- Cooper, R.; David, A.; Kudoh, T.; Tyler, C.R. Seasonal variation in oestrogenic potency and biological effects of wastewater treatment works effluents assessed using ERE-GFP transgenic zebrafish embryo-larvae. Aquat. Toxicol. 2021, 237, 105864. [Google Scholar] [CrossRef]
- Ruggiero, R.J.; Likis, F.E. Estrogen: Physiology, pharmacology, and formulations for replacement therapy. J. Midwifery Women’s Health 2010, 47, 130–138. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Zhang, H.; Luo, Z.; Yan, C. Occurrence, distribution, and seasonal variation of estrogenic compounds and antibiotic residues in 25 Jiulongjiang River, South China. Environ. Sci. Pollut. Res. 2012, 19, 1392–1404. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate and removal of pharmaceutical residues in the aquatic environment. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Filby, A.L.; Neuparth, T.; Thorpe, K.L.; Owen, R.; Galloway, T.S.; Tyler, C.R. Health impacts of estrogens in the environment, considering complex mixture effects. Environ. Health Perspect. 2007, 115, 1704–1710. [Google Scholar] [CrossRef] [Green Version]
- Leonard, J.A.; Cope, W.G.; Hammer, E.J.; Barnhart, M.C.; Bringolf, R.B. Extending the toxicity-testing paradigm for freshwater mussels: Assessing chronic reproductive effects of the synthetic estrogen 17a-ethinylestradiol on the unionid mussel Elliptio complanata. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 191, 14–25. [Google Scholar] [CrossRef]
- Rossier, N.M.; Chew, G.; Zhang, K.; Riva, F.; Fent, K. Activity of binary mixtures of drospirenone with progesterone and 17a-ethinylestradiol in vitro and in vivo. Aquat. Toxicol. 2016, 174, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Šauer, P.; Tumová, J.; Steinbach, C.; Golovko, O.; Komen, H.; Maillot-Maréchal, E.; Máchová, J.; Grabic, R.; Aït-Aïssa, S.; Kocour Kroupová, H. Chronic simultaneous exposure of common carp (Cyprinus carpio) from embryonic to juvenile stage to drospirenone and gestodene at low ng/L level caused intersex. Ecotox. Environ. Safe. 2020, 188, 109912. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Bohra, A.; Bhateja, S. Carcinogenesis and sex hormones: A review. Endocrinol. Metab. Syndr. 2015, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Lu, X.; Lin, M.; Lin, A.Y. A Review on Sex Steroid Hormone Estrogen Receptors in Mammals and Fish. Int. J. Endocrinol. 2020, 2020, 5386193. [Google Scholar] [CrossRef] [Green Version]
- Bolong, N.; Ismail, A.F.; Salim, M.R.; Matsuura, T. A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 2009, 239, 229–246. [Google Scholar] [CrossRef]
- Sumpter, J.P.; Jobling, S. The occurrence, causes, and consequences of estrogens in the aquatic environment. Environ. Toxicol. Chem. 2013, 32, 249–251. [Google Scholar] [CrossRef]
- Rostkowski, P.; Horwood, J.; Shears, J.A.; Lange, A.; Oladapo, F.O.; Besselink, H.T.; Tyler, C.R.; Hill, E.M. Bioassay-Directed Identification of Novel Antiandrogenic Compounds in Bile of Fish Exposed to Wastewater Effluents. Environ. Sci. Technol. 2011, 45, 10660–10667. [Google Scholar] [CrossRef]
- Andaluri, G.; Suri, R.P.S.; Kumar, K. Occurrence of estrogen hormones in biosolids, animal manure and mushroom compost. Environ. Monit. Assess. 2012, 184, 1197–1205. [Google Scholar] [CrossRef]
- Belhaj, D.; Athmouni, K.; Jerbi, B.; Kallel, M.; Ayadi, H.; Zhou, J.L. Estrogenic compounds in Tunisian urban sewage treatment plant: Occurrence, removal and ecotoxicological impact of sewage discharge and sludge disposal. Ecotoxicology 2016, 25, 1849–1857. [Google Scholar] [CrossRef]
- Wojnarowski, K.; Podobiński, P.; Cholewińska, P.; Smoliński, J.; Dorobisz, K. Impact of Estrogens Present in Environment on Health and Welfare of Animals. Animals 2021, 11, 2152. [Google Scholar] [CrossRef]
- Yuan, X.; Li, T.; Zhou, L.; Zhao, X. Characteristics and Risk Assessment of Estrogenic Compounds in Rivers of Southern Jiangsu Province, China. International Conference on Environment Systems Science and Engineering. IERI Procedia 2014, 9, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Yang, X.; Zhao, F.; Hu, X.; Fan, F.; Ren, H.; Geng, J. Occurrence and removal of progestogens from wastewater treatment plants in China: Spatiotemporal variation and process comparison. Water Res. 2022, 211, 118038. [Google Scholar] [CrossRef] [PubMed]
- Barel-Cohen, K.; Shore, L.S.; Shemesh, M.; Wenzel, A.; Mueller, J.; Kronfeld-Schor, N. Monitoring of natural and synthetic hormones in a polluted river. J. Environ. Manag. 2006, 78, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Puckowski, A.; Mioduszewska, K.; Łukaszewicz, P.; Borecka, M.; Caban, M.; Maszkowska, J.; Stepnowski, P. Bioaccumulation and analytics of pharmaceutical residues in theenvironment: A review. J. Pharm. Biomed. Anal. 2016, 127, 232–255. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; van Aerle, R.; Santos, E.; Brighty, G. Predicted exposures to steroid estrogens in U.K. Rivers correlate with widespread sexual disruption in wild fish populations. Environ. Health Perspect. 2005, 114, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drewes, J.E.; Hemming, J.; Ladenburger, S.J.; Schauer, J.; Sonzogni, W. An assessment of endocrine disrupting activity changes during wastewater treatment through the use of bioassays and chemical measurements. Water Environ. Res. 2005, 77, 12–23. [Google Scholar] [CrossRef]
- Franco, M.E.; Burket, S.R.; Sims, J.L.; Lovin, L.M.; Scarlett, K.R.; Stroski, K.; Steenbeek, R.; Ashcroft, C.; Luers, M.; Brooks, B.W.; et al. Multi-approach assessment for the evaluation of spatio-temporal estrogenicity in fish from effluent-dominated surface waters under low instream flow. Environ. Pollut. 2020, 265, 115122. [Google Scholar] [CrossRef]
- Arlos, M.J.; Parker, W.J.; Bicudo, J.R.; Law, P.; Marjan, P.; Andrews, S.A.; Servos, M.R. Multi-year prediction of estrogenicity in municipal wastewater effluents. Sci. Total Environ. 2018, 610–611, 1103–1112. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Pessoa, G.P.; de Souza, N.C.; Vidal, C.B.; Alves, J.A.C.; Firmino, P.J.M.; Nascimento, R.F.; dos Santos, A.B. Occurrence and removal of estrogens in Brazilian wastewater treatment plants. Sci. Total Environ. 2014, 490, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, D.; Baccar, R.; Jaabiri, I.; Bouzid, J.; Kallel, M.; Ayadi, H.; Zhou, J.L. Fate of selected estrogenic hormones in an urban sewage treatment plant in Tunisia (North Africa). Sci. Total Environ. 2015, 505, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Shinohara, H.; Murata, A.; Kiri, K.; Managaki, S.; Sato, N.; Takada, H. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. 2007, 41, 4373–4382. [Google Scholar] [CrossRef]
- Chen, J.L.; Ravindran, S.; Swift, S.; Wright, L.J.; Singhal, N. Catalytic oxidative degradation of 17α-ethinylestradiol by FeIII-TAML/H2O2: Estrogenicities of the products of partial, and extensive oxidation. Water Res. 2012, 46, 6309–6318. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taoufik, N.; Boumya, W.; Achak, M.; Sillanpää, M.; Barka, N. Comparative overview of advanced oxidation processes and biological approaches for the removal pharmaceuticals. J. Environ. Manag. 2021, 288, 112404. [Google Scholar] [CrossRef]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Xue, W.; Wu, C.; Xiao, K.; Huang, X.; Zhou, H.; Tsuno, H.; Tanaka, H. Elimination and fate of selected microorganic pollutants in a full-scale anaerobic/anoxic/aerobic process combined with membrane bioreactor for municipal wastewater reclamation. Water Res. 2010, 44, 5999–6010. [Google Scholar] [CrossRef]
- Ho, L.; Grasset, C.; Hoefel, D.; Dixon, M.B.; Leusch, F.D.L.; Newcombe, G.; Saint, C.P.; Brookes, J.D. Assessing granular media filtration for the removal of chemical contaminants from wastewater. Water Res. 2011, 45, 3461–3472. [Google Scholar] [CrossRef]
- Chen, Z.; Li, M.; Wen, Q. Comprehensive evaluation of three sets of advanced wastewater treatment trains for treating secondary effluent: Organic micro-pollutants and bio-toxicity. Chemosphere 2017, 189, 426–434. [Google Scholar] [CrossRef]
- Avberšek, M.; Šömen, J.; Heath, E. Dynamics of steroid estrogen daily concentrations in hospital effluent and connected wastewater treatment plant. J. Environ. Monit. 2011, 13, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- Rechsteiner, D.; Schrade, S.; Zähner, M.; Müller, M.; Hollender, J.; Bucheli, T.D. Occurrence and Fate of Natural Estrogens in Swiss Cattle and Pig Slurry. J. Agric. Food Chem. 2020, 68, 5545–5554. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, W.; Yang, L.; Du, B.; Chen, S.; Sun, W.; Jiang, H.; Xie, M.; Tang, J. Occurrence and Fate of Steroid Estrogens in a Chinese Typical Concentrated Dairy Farm and Slurry Irrigated Soil. J. Agric. Food Chem. 2021, 69, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, F.X.; Casey, H.; Hakk, D.J.; Smith, G.; Padmanabhan, G. Fate and transformation of an estrogen conjugate and its metabolites in agricultural soils. Environ. Sci. Technol. 2012, 46, 11047–11053. [Google Scholar] [CrossRef]
- Ihara, M.; Ihara, M.O.; Kumar, V.; Narumiya, M.; Hanamoto, S.; Nakada, N.; Yamashita, N.; Miyagawa, S.; Iguchi, T.; Tanaka, H. Co-occurrence of estrogenic and antiestrogenic activities in wastewater: Quantitative evaluation of balance by in vitro ER alpha reporter gene assay and chemical analysis. Environ. Sci. Technol. 2014, 48, 6366–6373. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Yiang, G.-G.; Yang, B.; Liu, S.; Zhou, L.-J.; Chen, Z.-F.; Lai, H.-J. Screening of multiple hormonal activities in surface water and sediment from the Pearl River system, South China, using effect-directed in vitro bioassays. Environ. Toxicol. Chem. 2011, 30, 2208–2215. [Google Scholar] [CrossRef]
- Bain, P.A.; Williams, M.; Kumar, A. Assessment of multiple hormonal activities in wastewater at different stages of treatment. Environ. Toxicol. Chem. 2014, 33, 2297–2307. [Google Scholar] [CrossRef]
- Escher, B.I.; Allison, M.; Altenburger, R.; Bain, P.A.; Balaguer, B.; Busch, W.; Crago, J.; Denslow, N.D.; Dopp, E.; Hilscherova, K.; et al. Benchmarking Organic Micropollutants in Wastewater, Recycled Water and Drinking Water with In Vitro Bioassays. Environ. Sci. Technol. 2014, 48, 1940–1956. [Google Scholar] [CrossRef]
- König, M.; Beate, I.; Escher, B.I.; Neale, P.A.; Krauss, M.; Hilscherová, K.; Novák, J.; Teodorović, I.; Schulze, T.; Seidensticker, S.; et al. Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environ. Pollut. 2017, 220, 1220–1230. [Google Scholar] [CrossRef]
- Urbatzka, R.; van Cauwenberge, A.; Maggioni, S.; Viganò, L.; Mandich, A.; Benfenati, E.; Lutz, I.; Kloas, W. Androgenic and antiandrogenic activities in water and sediment samples from the river Lambro, Italy, detected by yeast androgen screen and chemical analyses. Chemosphere 2007, 67, 1080–1087. [Google Scholar] [CrossRef]
- Leusch, F.D.L.; Khan, S.J.; Laingam, S.; Prochazka, E.; Froscio, S.; Trinh, T.; Chapman, H.F.; Humpage, A. Assessment of the application of bioanalytical tools as surrogate measure of chemical contaminants in recycled water. Water Res. 2014, 49, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Chou, P.-H. Detection of endocrine active substances in the aquatic environment in southern Taiwan using bioassays and LC–MS/MS, 214-220. Chemosphere 2016, 152, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 as Regards Priority Substances in the Field of Water Policy. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:226:0001:0017:EN:PDF (accessed on 12 May 2023).
- Directive 2008/105/EC OF the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0105 (accessed on 12 May 2023).
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060 (accessed on 12 May 2023).
- Commission Implementing Decision (EU) 2022/1307 of 22 July 2022 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council (Notified Under Document C (2022) 5098). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32022D1307 (accessed on 12 May 2023).
- Statistical Office in Lodz. 2022. Available online: https://lodz.stat.gov.pl/ (accessed on 4 May 2023).
- Sakson, G.; Brzezinska, A.; Bandzierz, D.; Olejnik, D.; Jedrzejczak, J.; Gryglik, D.; Ewa Badowska, E. Monitoring of wastewater quality in Lodz sewage system (Poland)—Do the current solutions enable the protection of WWTP and receiving water? Int. J. Energy Environ. Eng. 2021, 13, 713–727. [Google Scholar] [CrossRef]
- Xenometrix, A.G. Swiss Commitment for Bioassays, 2019, XenoScreen YES/YAS, Instruction for Use; Version 3.11; Xenometrix, A.G.: Allschwil, Switzerland, 2019. [Google Scholar]
- Gasperi, J.; Kafi–Benyahiab, M.; Lorgeouxc, C.; Moillerona, R.; Gromairec, M.-C.; Chebbo, G. Wastewater quality and pollutant loads in combined sewers during dry weather periods. Urban. Water J. 2008, 5, 305–314. [Google Scholar] [CrossRef]
- Alavi, J.; Ewees, A.A.; Ansari, S.; Shahid, S.; Yaseen, Z.M. A new insight for real-time wastewater quality prediction using hybridized kernel-based extreme learning machines with advanced optimization algorithms. Environ. Sci. Pollut. Res. 2022, 29, 20496–20516. [Google Scholar] [CrossRef]
- Gasperi, J.; Gromaire, M.C.; Kafi, M.; Moilleron, R.; Chebbo, G. Contributions of wastewater, runoff and sewer deposit erosion to wet weather pollutant loads in combined sewer systems. Water Res. 2010, 44, 5875–5886. [Google Scholar] [CrossRef]
- Hannouche, A.; Chebbo, G.; Joannis, C. Assessment of the contribution of sewer deposits to suspended solids loads in combined sewer systems during rain events. Environ. Sci. Pollut. Res. 2014, 21, 5311–5317. [Google Scholar] [CrossRef]
- Ratajczyk, W. Environmental Hazards Resulting from Discharge of Treated Sewage into Marine Waters. Ph.D. Thesis, Medical University of Gdańsk, Division of Environmental Toxicology, Gdańsk, Poland, 2017. (In Polish). [Google Scholar]
- Leusch, F.D.L.; Neale, P.A.; Hebert, A.; Scheurer, M.; Schriks, C.M.M. Analysis of the sensitivity of in vitro bioassays for androgenic, progestagenic, glucocorticoid, thyroid and estrogenic activity: Suitability for drinking and environmental waters. Environ. Int. 2017, 99, 120–130. [Google Scholar] [CrossRef]
- Duong, C.N.; Ra, J.S.; Cho, J.; Kim, S.D.; Choi, H.K.; Park, J.-H.; Kim, K.W.; Inam, E.; Kim, S.D. Estrogenic chemicals and estrogenicity in river waters of South Korea and seven Asian countries. Chemosphere 2010, 78, 286–293. [Google Scholar] [CrossRef]
- Leusch, F.; van den Heuvel, M.R.; Heather, F.; Chapman, H.F.; Gooneratne, S.R.; Eriksson, A.M.E.; Tremblay, A.L. Development of methods for extraction and in vitro quantification of estrogenic and androgenic activity of wastewater samples. Comp. Biochem. Part C Toxicol. Pharmacol. 2006, 143, 117–126. [Google Scholar] [CrossRef]
- Jarosova, B.L.; Blaha, J.P.; Giesy, J.; Hilscherova, K. What level of estrogenic activity determined by in vitro assays in municipal wastewaters can be considered as safe? Environ. Int. 2014, 64, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 42, 123–155. [Google Scholar] [CrossRef] [PubMed]
- Välitalo, P.; Perkola, N.; Seiler, T.-B.; Sillanpää, M.; Kuckelkorn, J.; Mikola, A.; Hollert, H.; Schultz, E. Estrogenic activity in Finnish municipal wastewater effluents. Water Res. 2016, 88, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, S.; Ternes, T.A.; Bonerz, M.; Rastall, A.C.; Erdinger, L.; Braunbeck, T. Estrogenicity of solid phase-extracted water samples from two municipal sewage treatment plant effluents and river Rhine water using the yeast estrogen screen. Toxicol. In Vitro 2004, 18, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Brownawell, B.J. Analysis of estrogens in sediment from a sewage-impacted urban estuary using high-performance liquid chromatography/time-of-flight mass spectrometry. Environ. Toxicol. Chem. 2005, 24, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Argolo, A.; Gomes, G.; Bila, D.M. (Anti)estrogenic activity impacted by complex environmental matrices: A DOM and multiphase distribution approach. Chemosphere 2023, 310, 136917. [Google Scholar] [CrossRef]
- Dingemans, M.M.L.; Baken, K.A.; van der Oost, R.; Schriks, M.; van Wezel, A.P. Risk-based approach in the revised European Union drinking water legislation: Opportunities for bioanalytical tools. Integr. Environ. Assess. Manag. 2019, 15, 126–134. [Google Scholar] [CrossRef]
- Schuijt, L.M.; Peng, F.J.; van den Berg, S.J.P.; Dingemans, M.M.L.; van den Brink, P.J. (Eco)toxicological tests for assessing impacts of chemical stress to aquatic ecosystems: Facts, challenges, and future. Sci. Total 2021, 795, 148776. [Google Scholar] [CrossRef]
- Gomez, L.; Niegowska, M.; Navarro, A.; Amendola, L.; Arukwe, A.; Ait-Aissa, S.; Balzamo, S.; Barreca, S.; Belkin, S.; Bittner, M.; et al. Estrogenicity of chemical mixtures revealed by a panel of bioassays. Sci. Total Environ. 2021, 785, 147284. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Martínez, R.; Navarro-Martín, L.; Kamstra, J.H.; Schwendt, A.; Reynaud, S.; Chalifour, L. Emerging Concepts and Opportunities for Endocrine Disruptor Screening of the Non-EATS Modalities. Environ. Res. 2022, 204, 111904. [Google Scholar] [CrossRef]
- Könemann, S.; Kase, R.; Simon, E.; Swart, K.; Buchinger, S.; Schlüsener, M.; Hollert, H.; Escher, B.I.; Werner, I.; Aït-Aïssa, S.; et al. Effect-based and chemical analytical methods to monitor estrogens under the European Water Framework Directive. TrAC Trends Anal. Chem. 2018, 102, 225–235. [Google Scholar] [CrossRef]
- Leusch, F.D.L.; De Jager, C.; Levi, Y.; Lim, R.; Puijker, L.; Sacher, F.; Tremblay, L.A.; Wilson, V.S.; Chapman, H.F. Comparison of Five In Vitro Bioassays to Measure Estrogenic Activity in Environmental Waters. Environ. Sci. Technol. 2010, 44, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, G.; Yin, H.; Dang, Z. Estimated human excretion rates of natural estrogens calculated from their concentrations in raw municipal wastewater and its application. Environ. Sci. Pollut. Res. 2015, 22, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Forrez, I.; Carballa, M.; Noppe, H.; De Brabander, H.; Boon, N.; Verstraete, W. Influence of manganese and ammonium oxidation on the removal of 17α-ethinylestradiol (EE2). Water Res. 2009, 43, 77–86. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Proposal for a Directive of The European Parliament and of the Council Concerning Urban Wastewater Treatment (Recast); Brussels, 26.10.2022 COM(2022) 541 final 2022/0345 (COD); European Commission: Brussels, Belgium, 2022. [Google Scholar]

| Parameter | Value (%) |
|---|---|
| feminisation coefficient | 119 |
| masculinisation coefficient | 84 |
| SP | pH | BOD5 mgO2/L | COD mgO2/L | TSS mg/L | PC mg/L | TOX Degree of Inhibition—DI (%) |
|---|---|---|---|---|---|---|
| GWWTP | 7.80 | 575 | 1093 | 586 | 0.03 | 40.5 |
| 1. | 8.53 | 70 | 316 | 158 | - | 65.8 |
| 2. | 7.50 | 472 | 790 | 440 | 0.08 | 31.5 |
| 3. | 7.62 | 575 | 1014 | 438 | 0.038 | - |
| 4. | 7.46–7.79 | 435–1250 | 937–2416 | 236–3264 | 0.055–0.125 | 20.7–51.8 |
| 5. | 7.27–7.65 | 380–1000 | 728–1636 | 236–584 | 0.039–0.087 | 40.6–52.7 |
| Limit values for sewage discharged into sewer system * | 6.5–9.5 | 600 | 1200 | 600 | 15 | - |
| Sampling Point | Data | Oestrogenic Activity | Androgenic Activity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Agonistic Oestrogenic Activity | Antagonistic Oestrogenic Activity | Agonistic Androgenic Activity | Antagonistic Androgenic Activity | ||||||
| EEQ, ng E2/L | IR | aEEQ, ng HT/L | IR | AEQ, ng DHT/L | IR | aAEQ, ng FLU/L | IR | ||
| 1. | 09.01.2019 W | ND | − | 7.59 × 104 | + | ND | − | 1.36 × 107 | + |
| 2. | 29.01.2019 D | ND | − | 1.29 × 104 | + | ND | − | 1.57 × 107 | + |
| 3. | 28.11.2019 D | ND | − | 3.52 × 105 | + | ND | − | 9.45 × 106 | + |
| 4. | 05.06.2019 D | 0.90 | + | 3.03 × 102 | + | ND | − | ND | − |
| 4. | 05.12.2019 D | ND | − | 3.50 × 105 | + | ND | − | 1.70 × 107 | + |
| 4. | 09.01.2020 W | 13.70 | + | 8.49 × 105 | + | ND | − | ND | − |
| 5. | 10.12.2020 D | ND | − | 3.01 × 102 | + | ND | − | ND | − |
| 5. | 17.12.2020 D | ND | − | 2.20 × 106 | + | ND | − | ND | − |
| 5. | 13.01.2021 D | ND | − | 1.04 × 105 | + | ND | − | 1.88 × 104 | + |
| Parameter | pH | BOD5 mgO2/L | COD mgO2/L | TSS mg/L | PC mg/L | TOX Degree of Inhibition—DI (%) |
|---|---|---|---|---|---|---|
| Inflow | 7.41–7.66 | 186–700 | 442–1742 | 212–828 | 0.01–0.051 | 5.4–40.5 |
| Limit values for treated sewage ** | - | 15 | 125 | 35 | - | - |
| Oestrogenic Activity | Androgenic Activity | |||||||
|---|---|---|---|---|---|---|---|---|
| Agonistic Oestrogenic Activity | Antagonistic Oestrogenic Activity | Agonistic Androgenic Activity | Antagonistic Androgenic Activity | |||||
| EEQ, ng E2/L | IR | aEEQ, ng HT/L | IR | AEQ, ng DHT/L | IR | aAEQ, ng FLU/L | IR | |
| Min. | ND | − | 2.20 × 102 | + | ND | − | 7.53 × 105 | + |
| Max. | 1.31 | + | 8.49 × 105 | ND | − | 2.32 × 107 | + | |
| Sample/Date | Oestrogenic Activity | Androgenic Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| Agonistic Oestrogenic Activity | Antagonistic Oestrogenic Activity | Agonistic Androgenic Activity | Antagonistic Androgenic Activity | |||||
| EEQ ng E2/L | IR | aEEQ, ng HT/L | IR | AEQ ng DHT/L | IR | aAEQ, ng FLU/L | IR | |
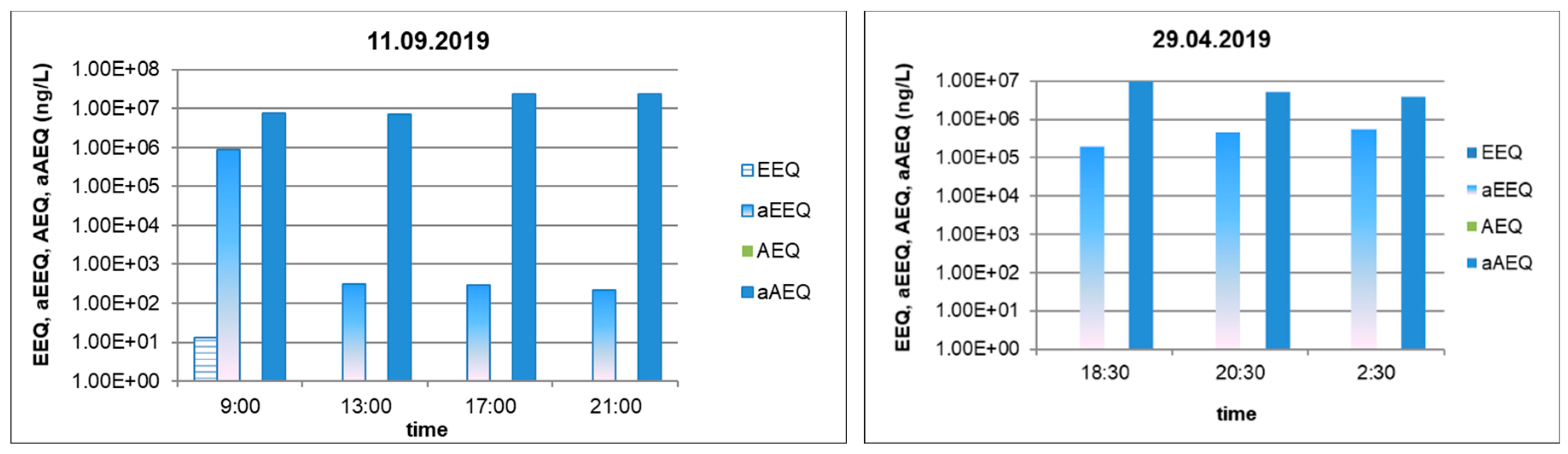
| Inlet 11.09.2019 | 1.31 | + | 8.49 × 105 | + | ND | − | 7.33 × 106 | + |
| Outlet 11.09.2019 | ND | − | 6.73 × 104 | + | ND | − | 3.88 × 106 | + |
| Reduction (%) | 100 | 99.2 | 47.1 | |||||
| Inlet 10.03.2021 | ND | − | 4.66 × 105 | + | ND | − | 2.03 × 107 | + |
| Outlet 10.03.2021 | ND | − | 2.26 × 105 | + | ND | − | 1.23 × 107 | + |
| Reduction (%) | 51.5 | 39.4 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezinska, A.; Sakson, G.; Olejnik, D. Analysis of (Anti-)Oestrogenic and (Anti-)Androgenic Activities in Wastewater from the Lodz Sewer System. Water 2023, 15, 2454. https://doi.org/10.3390/w15132454
Brzezinska A, Sakson G, Olejnik D. Analysis of (Anti-)Oestrogenic and (Anti-)Androgenic Activities in Wastewater from the Lodz Sewer System. Water. 2023; 15(13):2454. https://doi.org/10.3390/w15132454
Chicago/Turabian StyleBrzezinska, Agnieszka, Grazyna Sakson, and Dorota Olejnik. 2023. "Analysis of (Anti-)Oestrogenic and (Anti-)Androgenic Activities in Wastewater from the Lodz Sewer System" Water 15, no. 13: 2454. https://doi.org/10.3390/w15132454





