Surgery of Primary Melanomas
Abstract
:1. Introduction
2. The Diagnosis of the Melanoma (Excisional Biopsy)
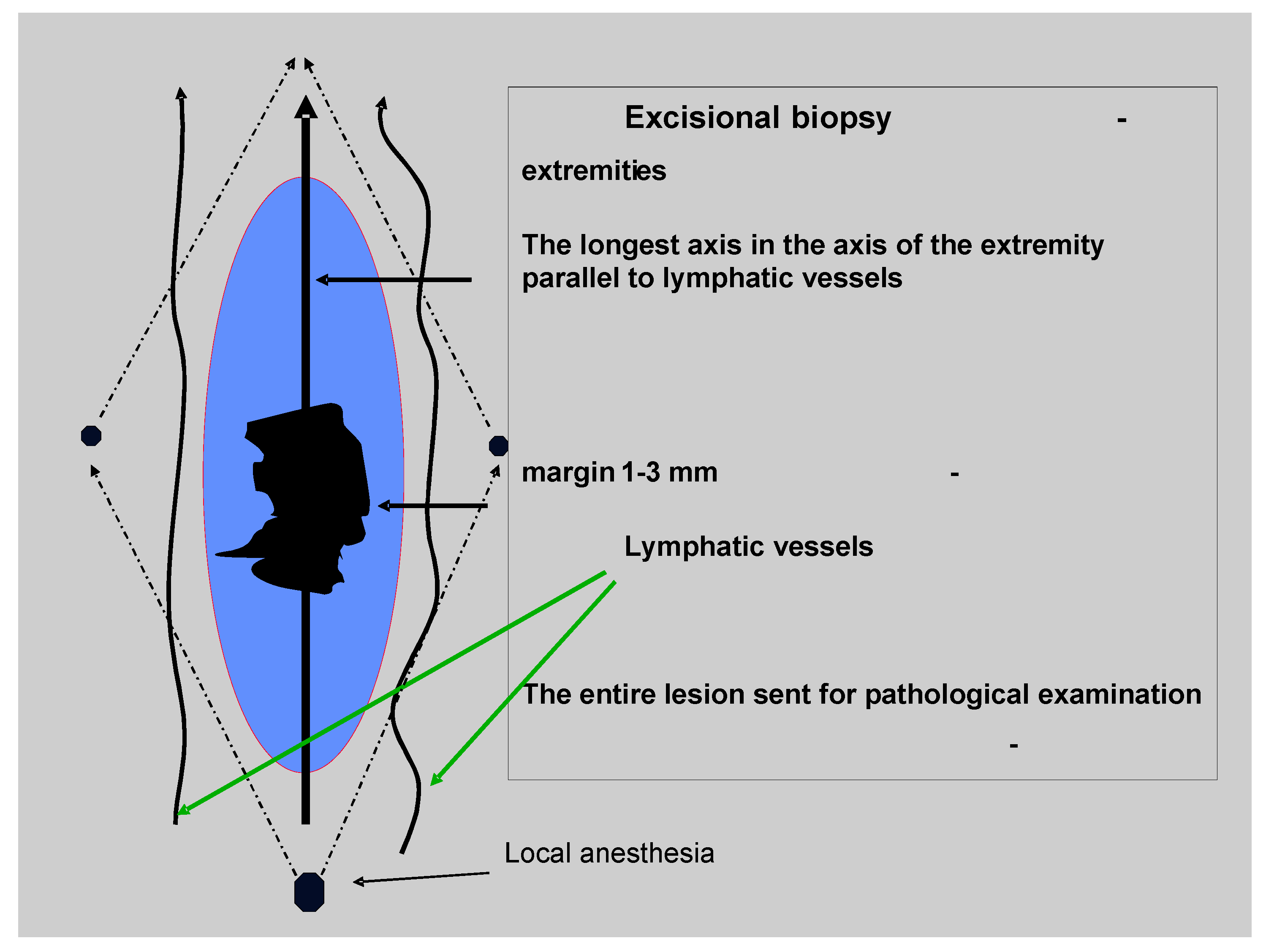
3. The Radical Surgery of the Primary Tumor
| Breslow thickness [mm] | Clinical study | Patients(No.) | Margins [cm] | Overall survival (at years) | Ref. |
|---|---|---|---|---|---|
| French Cooperative Group | 336 | 2 vs. 5 | No difference(10-year) | [25,28] | |
| ≤2 | Swedish Melanoma Group | 989 | 2 vs. 5 | No difference(5-year) | [23,29] |
| WHO Melanoma Group Trial No. 10 | 612 | 1 vs. 3 | No difference(10-year) | [30,31] | |
| 1–4 | Intergroup Melanoma Surgical Trial | 486 | 2 vs. 4 | No difference(6-year) | [26,32,33] |
| ≥2 | UK Melanoma Study Group | 900 | 1 vs. 3 | Not reported; hazard ratio for death was similar in both groups (5-year) | [27] |
| Swedish Melanoma Trial Group | 1,000 | 2 vs. 4 | Final results not reported; preliminary results indicated no difference (5-year) | [34] |
4. Primary Melanoma of Specific Sites
4.1. Melanoma of Mucosal Surfaces
4.2. Subungual Melanoma of the Hand or Foot
4.3. Melanoma of the Face and Scalp
5. Ocular Melanoma
6. Final Considerations
References
- Rigel, D.S.; Carucci, J.A. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA Cancer J. Clin. 2000, 50, 215–236. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer Statistics, 2009. CA Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- MacLennan, R.; Green, A.C.; McLeod, G.R.; Martin, N.G. Increasing incidence of cutaneous melanoma in Queensland, Australia. J. Natl. Cancer Inst. 1992, 84, 1427–1432. [Google Scholar] [CrossRef]
- Diepgen, T.L.; Mahler, V. The epidemiology of skin cancer. Br. J. Dermatol. 2002, 146 (Suppl. 61), 1–6. [Google Scholar]
- Garbe, C.; McLeod, G.R.; Buettner, P.G. Time trends of cutaneous melanoma in Queensland, Australia and Central Europe. Cancer 2000, 89, 1269–1278. [Google Scholar] [CrossRef]
- Markovic, S.N.; Erickson, L.A.; Rao, R.D.; Weenig, R.H.; Pockaj, B.A.; Bardia, A.; Vachon, C.M.; Schild, S.E.; McWilliams, R.R.; Hand, J.L.; Laman, S.D.; Kottschade, L.A.; Maples, W.J.; Pittelkow, M.R.; Pulido, J.S.; Cameron, J.D.; Creagan, E.T. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin. Proc. 2007, 82, 364–380. [Google Scholar]
- Magnus, K. Prognosis in malignant melanoma of the skin. Significance of stage of disease, anatomical site, sex, age and period of diagnosis. Cancer 1977, 40, 389–397. [Google Scholar] [CrossRef]
- Sahin, S.; Rao, B.; Kopf, A.W.; Lee, E.; Rigel, D.S.; Nossa, R.; Rahman, I.J.; Wortzel, H.; Marghoob, A.A.; Bart, R.S. Predicting ten-year survival of patients with primary cutaneous melanoma: corroboration of a prognostic model. Cancer 1997, 80, 1426–1431. [Google Scholar] [CrossRef]
- Kopf, A.W.; Salopek, T.G.; Slade, J.; Marghoob, A.A.; Bart, R.S. Techniques of cutaneous examination for the detection of skin cancer. Cancer 1995, 75, 684–690. [Google Scholar] [CrossRef]
- Salopek, T.G.; Slade, J.; Marghoob, A.A.; Rigel, D.S.; Kopf, A.W.; Bart, R.S.; Friedman, R.J. Management of cutaneous malignant melanoma by dermatologists of the American Academy of Dermatology. I. Survey of biopsy practices of pigmented lesions suspected as melanoma. J. Am. Acad. Dermatol. 1995, 33, 441–450. [Google Scholar] [CrossRef]
- Argenziano, G.; Ferrara, G.; Francione, S.; Di Nola, K.; Martino, A.; Zalaudek, I. Dermoscopy--the ultimate tool for melanoma diagnosis. Semin. Cutan. Med. Surg. 2009, 28, 142–148. [Google Scholar]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; Eggermont, A.M.; Flaherty, K.T.; Gimotty, P.A.; Kirkwood, J.M.; McMasters, K.M.; Mihm, M.C., Jr.; Morton, D.L.; Ross, M.I.; Sober, A.J.; Sondak, V.K. Final version of 2009 AJCC melanoma staging and classification. CA J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Martin, R.C., 2nd; Scoggins, C.R.; Ross, M.I.; Reintgen, D.S.; Noyes, R.D.; Edwards, M.J.; McMasters, K.M. Is incisional biopsy of melanoma harmful? Am. J. Surg. 2005, 190, 913–917. [Google Scholar]
- Drzewiecki, K.T.; Ladefoged, C.; Christensen, H.E. Biopsy and prognosis for cutaneous malignant melanomas in clinical stage I. Scand. J. Plast. Reconstr. Surg. 1980, 14, 141–144. [Google Scholar] [CrossRef]
- Heenan, P.J.; Ghaznawie, M. The pathogenesis of local recurrence of melanoma at the primary excision site. Br. J. Plast. Surg. 1999, 52, 209–213. [Google Scholar] [CrossRef]
- Urist, M.M.; Balch, C.M.; Soong, S.; Shaw, H.M.; Milton, G.W.; Maddox, W.A. The influence of surgical margins and prognostic factors predicting the risk of local recurrence in 3445 patients with primary cutaneous melanoma. Cancer 1985, 55, 1398–1402. [Google Scholar] [CrossRef]
- Singletary, S.E.; Tucker, S.L.; Boddie, A.W., Jr. Multivariate analysis of prognostic factors in regional cutaneous metastases of extremity melanoma. Cancer 1988, 61, 1437–1440. [Google Scholar] [CrossRef]
- Leon, P.; Daly, J.M.; Synnestvedt, M.; Schultz, D.J.; Elder, D.E.; Clark, W.H., Jr. The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch. Surg. 1991, 126, 1461–1468. [Google Scholar] [CrossRef]
- Cook, J. Surgical margins for resection of primary cutaneous melanoma. Clin. Dermatol. 2004, 22, 228–233. [Google Scholar] [CrossRef]
- Harrist, T.J.; Rigel, D.S.; Day, C.L., Jr.; Sober, A.J.; Lew, R.A.; Rhodes, A.R.; Harris, M.N.; Kopf, A.W.; Friedman, R.J.; Golomb, F.M.; et al. "Microscopic satellites" are more highly associated with regional lymph node metastases than is primary melanoma thickness. Cancer 1984, 53, 2183–2187. [Google Scholar] [CrossRef]
- Day, C.L., Jr.; Harrist, T.J.; Gorstein, F.; Sober, A.J.; Lew, R.A.; Friedman, R.J.; Pasternack, B.S.; Kopf, A.W.; Fitzpatrick, T.B.; Mihm, M.C., Jr. Malignant melanoma. Prognostic significance of "microscopic satellites" in the reticular dermis and subcutaneous fat. Ann. Surg. 1981, 194, 108–112. [Google Scholar]
- Veronesi, U.; Cascinelli, N.; Adamus, J.; Balch, C.; Bandiera, D.; Barchuk, A.; Bufalino, R.; Craig, P.; De Marsillac, J.; Durand, J.C.; et al. Thin stage I primary cutaneous malignant melanoma. Comparison of excision with margins of 1 or 3 cm. N. Engl. J. Med. 1988, 318, 1159–1162. [Google Scholar] [CrossRef]
- Cohn-Cedermark, G.; Rutqvist, L.E.; Andersson, R.; Breivald, M.; Ingvar, C.; Johansson, H.; Jonsson, P.E.; Krysander, L.; Lindholm, C.; Ringborg, U. Long term results of a randomized study by the Swedish Melanoma Study Group on 2-cm versus 5-cm resection margins for patients with cutaneous melanoma with a tumor thickness of 0.8–2.0 mm. Cancer 2000, 89, 1495–1501. [Google Scholar]
- Banzet, P.; Thomas, A.; Vuillemin, E. Wide versus narrow surgical excision in thin (<2 mm) stage I primary cutaneous malignant melanoma: long term results of a French multicentric prospective randomized trial on 319 patients. Proc. Am. Assoc. Clin. Oncol. 1993, 12, 387. [Google Scholar]
- Khayat, D.; Rixe, O.; Martin, G.; Soubrane, C.; Banzet, M.; Bazex, J.A.; Lauret, P.; Verola, O.; Auclerc, G.; Harper, P.; Banzet, P. Surgical margins in cutaneous melanoma (2 cm versus 5 cm for lesions measuring less than 2.1-mm thick). Cancer 2003, 97, 1941–1946. [Google Scholar]
- Balch, C.M.; Urist, M.M.; Karakousis, C.P.; Smith, T.J.; Temple, W.J.; Drzewiecki, K.; Jewell, W.R.; Bartolucci, A.A.; Mihm, M.C., Jr.; Barnhill, R.; et al. Efficacy of 2-cm surgical margins for intermediate-thickness melanomas (1 to 4 mm). Results of a multi-institutional randomized surgical trial. Ann. Surg. 1993, 218, 262–267; discussion 267–269. [Google Scholar]
- Thomas, J.M.; Newton-Bishop, J.; A'Hern, R.; Coombes, G.; Timmons, M.; Evans, J.; Cook, M.; Theaker, J.; Fallowfield, M.; O'Neill, T.; Ruka, W.; Bliss, J.M. Excision margins in high-risk malignant melanoma. N. Engl. J. Med. 2004, 350, 757–766. [Google Scholar] [CrossRef]
- Banzet, P.; Thomas, A.; Vuillemin, E. Wide versus narrow surgical excision in thin (<2 mm) stage I primary cutaneous melanoma: long term results of a French multicentric prospective randomized trial on 319 patients. Proc. Am. Assoc. Clin. Oncol. 1993, 12, 387. [Google Scholar]
- Ringborg, U.; Andersson, R.; Eldh, J.; Glaumann, B.; Hafstrom, L.; Jacobsson, S.; Jonsson, P.E.; Johansson, H.; Krysander, L.; Lagerlof, B. Resection margins of 2 versus 5 cm for cutaneous malignant melanoma with a tumor thickness of 0.8 to 2.0 mm: randomized study by the Swedish Melanoma Study Group. Cancer 1996, 77, 1809–1814. [Google Scholar]
- Veronesi, U.; Cascinelli, N. Narrow excision (1-cm margin). A safe procedure for thin cutaneous melanoma. Arch. Surg. 1991, 126, 438–441. [Google Scholar] [CrossRef]
- Cascinelli, N.; Belli, F.; Santinami, M.; Fait, V.; Testori, A.; Ruka, W.; Cavaliere, R.; Mozzillo, N.; Rossi, C.R.; MacKie, R.M.; Nieweg, O.; Pace, M.; Kirov, K. Sentinel lymph node biopsy in cutaneous melanoma: the WHO Melanoma Program experience. Ann. Surg. Oncol. 2000, 7, 469–474. [Google Scholar] [CrossRef]
- Karakousis, C.P.; Balch, C.M.; Urist, M.M.; Ross, M.M.; Smith, T.J.; Bartolucci, A.A. Local recurrence in malignant melanoma: long-term results of the multiinstitutional randomized surgical trial. Ann. Surg. Oncol. 1996, 3, 446–452. [Google Scholar] [CrossRef]
- Balch, C.M.; Soong, S.J.; Smith, T.; Ross, M.I.; Urist, M.M.; Karakousis, C.P.; Temple, W.J.; Mihm, M.C.; Barnhill, R.L.; Jewell, W.R.; Wanebo, H.J.; Desmond, R. Long-term results of a prospective surgical trial comparing 2 cm vs. 4 cm excision margins for 740 patients with 1–4 mm melanomas. Ann. Surg. Oncol. 2001, 8, 101–108. [Google Scholar]
- Ringborg, U.; Brahme, E.M.; Drewiecki, K. Randomized trial of a resection margin of 2 cm versus 4 cm for cutaneous malignant melanoma with a tumor thickness of more than 2 mm. In World Congress on Melanoma, Vancouver, BC, Canada, 6–10 September 2005.
- Heaton, K.M.; Sussman, J.J.; Gershenwald, J.E.; Lee, J.E.; Reintgen, D.S.; Mansfield, P.F.; Ross, M.I. Surgical margins and prognostic factors in patients with thick (>4mm) primary melanoma. Ann. Surg. Oncol. 1998, 5, 322–328. [Google Scholar] [CrossRef]
- Cohen, L.M.; McCall, M.W.; Hodge, S.J.; Freedman, J.D.; Callen, J.P.; Zax, R.H. Successful treatment of lentigo maligna and lentigo maligna melanoma with Mohs' micrographic surgery aided by rush permanent sections. Cancer 1994, 73, 2964–2970. [Google Scholar] [CrossRef]
- Zitelli, J.A.; Brown, C.D.; Hanusa, B.H. Surgical margins for excision of primary cutaneous melanoma. J. Am. Acad. Dermatol. 1997, 37, 422–429. [Google Scholar] [CrossRef]
- Zitelli, J.A.; Mohs, F.E.; Larson, P.; Snow, S. Mohs micrographic surgery for melanoma. Dermatol. Clin. 1989, 7, 833–843. [Google Scholar]
- Quinn, M.J.; Crotty, K.A.; Thompson, J.F.; Coates, A.S.; O'Brien, C.J.; McCarthy, W.H. Desmoplastic and desmoplastic neurotropic melanoma: experience with 280 patients. Cancer 1998, 83, 1128–1135. [Google Scholar] [CrossRef]
- Olsen, G. Some views on the treatment of melanomas of the skin. Arch. Chir. Neerl. 1970, 22, 79–90. [Google Scholar]
- Kenady, D.E.; Brown, B.W.; McBride, C.M. Excision of underlying fascia with a primary malignant melanoma: effect on recurrence and survival rates. Surgery 1982, 92, 615–618. [Google Scholar]
- Holmstrom, H. Surgical management of primary melanoma. Semin. Surg. Oncol. 1992, 8, 366–369. [Google Scholar] [CrossRef]
- The Collaborative Ocular Melanoma Study Group, Mortality in Patients with Small Choroidal Melanoma. COMS report no. 4. American Medical Association: Chicago, IL, USA, 1997; Arch. Ophthalmol. 1997; 115, 886–893.
- Conley, J.; Pack, G.T. Melanoma of the mucous membranes of the head and neck. Arch. Otolaryngol. 1974, 99, 315–319. [Google Scholar] [CrossRef]
- Cooper, P.H.; Mills, S.E.; Allen, M.S., Jr. Malignant melanoma of the anus: report of 12 patients and analysis of 255 additional cases. Dis. Colon. Rectum. 1982, 25, 693–703. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Elashoff, R.; Essner, R.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Reintgen, D.S.; Coventry, B.J.; Glass, E.C.; Wang, H.J. Sentinel-node biopsy or nodal observation in melanoma. N. Engl. J. Med. 2006, 355, 1307–1317. [Google Scholar] [CrossRef]
- Moroi, Y. Significance of sentinel lymph node biopsy in malignant melanoma: overview of international data. Int. J. Clin. Oncol. 2009, 14, 485–489. [Google Scholar] [CrossRef]
- Voit, C.; Kron, M.; Schafer, G.; Schoengen, A.; Audring, H.; Lukowsky, A.; Schwurzer-Voit, M.; Sterry, W.; Winter, H.; Rademaker, J. Ultrasound-guided fine needle aspiration cytology prior to sentinel lymph node biopsy in melanoma patients. Ann. Surg. Oncol. 2006, 13, 1682–1689. [Google Scholar] [CrossRef]
- Voit, C.A.; van Akkooi, A.C.; Schafer-Hesterberg, G.; Schoengen, A.; Schmitz, P.I.; Sterry, W.; Eggermont, A.M. Rotterdam Criteria for sentinel node (SN) tumor burden and the accuracy of ultrasound (US)-guided fine-needle aspiration cytology (FNAC): can US-guided FNAC replace SN staging in patients with melanoma? J. Clin. Oncol. 2009, 27, 4994–5000. [Google Scholar] [CrossRef]
- Bastiaannet, E.; Wobbes, T.; Hoekstra, O.S.; van der Jagt, E.J.; Brouwers, A.H.; Koelemij, R.; de Klerk, J.M.; Oyen, W.J.; Meijer, S.; Hoekstra, H.J. Prospective comparison of [18F].fluorodeoxyglucose positron emission tomography and computed tomography in patients with melanoma with palpable lymph node metastases: diagnostic accuracy and impact on treatment. J. Clin. Oncol. 2009, 27, 4774–4780. [Google Scholar]
- Jimenez-Requena, F.; Delgado-Bolton, R.C.; Fernandez-Perez, C.; Gambhir, S.S.; Schwimmer, J.; Perez-Vazquez, J.M.; Carreras-Delgado, J.L. Meta-analysis of the performance of (18)F-FDG PET in cutaneous melanoma. Eur. J. Nucl. Med. Mol. Imag. 2010, 37, 284–300. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Testori, A.; Marsden, J.; Hersey, P.; Quirt, I.; Petrella, T.; Gogas, H.; MacKie, R.M.; Hauschild, A. Utility of adjuvant systemic therapy in melanoma. Ann. Oncol. 2009, 20, 30–34. [Google Scholar] [CrossRef]
- Glaspy, J.; Ribas, A.; Chmielowski, B. Interferon alfa in the postsurgical management of high-risk melanoma: is it worth it? J. Clin. Oncol. 2009, 27, 2896–2897. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Manola, J.; Ibrahim, J.; Sondak, V.; Ernstoff, M.S.; Rao, U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin. Cancer. Res. 2004, 10, 1670–1677. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Suciu, S.; Santinami, M.; Testori, A.; Kruit, W.H.; Marsden, J.; Punt, C.J.; Sales, F.; Gore, M.; Mackie, R.; Kusic, Z.; Dummer, R.; Hauschild, A.; Musat, E.; Spatz, A.; Keilholz, U. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet. 2008, 372, 117–126. [Google Scholar] [CrossRef] [Green Version]
- McBride, C.M.; Sugarbaker, E.V.; Hickey, R.C. Prophylactic isolation-perfusion as the primary therapy for invasive malignant melanoma of the limbs. Ann Surg. 1975, 182, 316–324. [Google Scholar] [CrossRef]
- Grunhagen, D.J.; de Wilt, J.H.; van Geel, A.N.; Eggermont, A.M. Isolated limb perfusion for melanoma patients--a review of its indications and the role of tumour necrosis factor-alpha. Eur. J. Surg. Oncol. 2006, 32, 371–380. [Google Scholar] [CrossRef]
- Koops, H.S.; Vaglini, M.; Suciu, S.; Kroon, B.B.; Thompson, J.F.; Gohl, J.; Eggermont, A.M.; Di Filippo, F.; Krementz, E.T.; Ruiter, D.; Lejeune, F.J. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J. Clin. Oncol. 1998, 16, 2906–2912. [Google Scholar]
- Siegler, H.F. Mucosal melanoma. J. Surg. Oncol. 2004, 86, 187–188. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Hicks, M.J.; Flaitz, C.M. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000, 36, 152–169. [Google Scholar] [CrossRef]
- Gorsky, M.; Epstein, J.B. Melanoma arising from the mucosal surfaces of the head and neck. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 86, 715–719. [Google Scholar] [CrossRef]
- Narasimhan, K.; Kucuk, O.; Lin, H.S.; Heilbrun, L.K.; Carron, M.; Venkatramanamoorthy, R.; Mathog, R. Sinonasal mucosal melanoma: a 13-year experience at a single institution. Skull Base 2009, 19, 255–262. [Google Scholar] [CrossRef]
- Teppo, H.; Kervinen, J.; Koivunen, P.; Alho, O.P. Incidence and outcome of head and neck mucosal melanoma--a population-based survey from Northern Finland. Int. J. Circumpolar Health 2006, 65, 443–447. [Google Scholar]
- Temam, S.; Mamelle, G.; Marandas, P.; Wibault, P.; Avril, M.F.; Janot, F.; Julieron, M.; Schwaab, G.; Luboinski, B. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer 2005, 103, 313–319. [Google Scholar]
- Wu, A.J.; Gomez, J.; Zhung, J.E.; Chan, K.; Gomez, D.R.; Wolden, S.L.; Zelefsky, M.J.; Wolchok, J.D.; Carvajal, R.D.; Chapman, P.B.; Wong, R.J.; Shaha, A.R.; Kraus, D.H.; Shah, J.P.; Lee, N.Y. Radiotherapy After Surgical Resection for Head and Neck Mucosal Melanoma. Am. J. Clin. Oncol. 2009.
- Irvin, W.P., Jr.; Legallo, R.L.; Stoler, M.H.; Rice, L.W.; Taylor, P.T., Jr.; Andersen, W.A. Vulvar melanoma: a retrospective analysis and literature review. Gynecol. Oncol. 2001, 83, 457–465. [Google Scholar] [CrossRef]
- Bradgate, M.G.; Rollason, T.P.; McConkey, C.C.; Powell, J. Malignant melanoma of the vulva: a clinicopathological study of 50 women. Br. J. Obstet. Gynaecol. 1990, 97, 124–133. [Google Scholar] [CrossRef]
- Look, K.Y.; Roth, L.M.; Sutton, G.P. Vulvar melanoma reconsidered. Cancer 1993, 72, 143–146. [Google Scholar] [CrossRef]
- Ariel, I.M. Malignant melanoma of the female genital system: a report of 48 patients and review of the literature. J. Surg. Oncol. 1981, 16, 371–383. [Google Scholar] [CrossRef]
- Rose, P.G.; Piver, M.S.; Tsukada, Y.; Lau, T. Conservative therapy for melanoma of the vulva. Am. J. Obstet. Gynecol. 1988, 159, 52–55. [Google Scholar]
- Trimble, E.L.; Lewis, J.L., Jr.; Williams, L.L.; Curtin, J.P.; Chapman, D.; Woodruff, J.M.; Rubin, S.C.; Hoskins, W.J. Management of vulvar melanoma. Gynecol. Oncol. 1992, 45, 254–258. [Google Scholar] [CrossRef]
- Phillips, G.L.; Bundy, B.N.; Okagaki, T.; Kucera, P.R.; Stehman, F.B. Malignant melanoma of the vulva treated by radical hemivulvectomy. A prospective study of the Gynecologic Oncology Group. Cancer 1994, 73, 2626–2632. [Google Scholar] [CrossRef]
- Ragnarsson-Olding, B.K.; Nilsson, B.R.; Kanter-Lewensohn, L.R.; Lagerlof, B.; Ringborg, U.K. Malignant melanoma of the vulva in a nationwide, 25-year study of 219 Swedish females: predictors of survival. Cancer 1999, 86, 1285–1293. [Google Scholar] [CrossRef]
- de Bree, E.; Sanidas, E.; Tzardi, M.; Gaki, B.; Tsiftsis, D. Malignant melanoma of the penis. Eur. J. Surg. Oncol. 1997, 23, 277–279. [Google Scholar] [CrossRef]
- Slingluff, C.L., Jr.; Vollmer, R.T.; Seigler, H.F. Anorectal melanoma: clinical characteristics and results of surgical management in twenty-four patients. Surgery 1990, 107, 1–9. [Google Scholar]
- Thibault, C.; Sagar, P.; Nivatvongs, S.; Ilstrup, D.M.; Wolff, B.G. Anorectal melanoma--an incurable disease? Dis. Colon. Rectum. 1997, 40, 661–668. [Google Scholar] [CrossRef]
- Quinn, M.J.; Thompson, J.E.; Crotty, K.; McCarthy, W.H.; Coates, A.S. Subungual melanoma of the hand. J. Hand. Surg. Am. 1996, 21, 506–511. [Google Scholar] [CrossRef]
- Finley, R.K., 3rd; Driscoll, D.L.; Blumenson, L.E.; Karakousis, C.P. Subungual melanoma: an eighteen-year review. Surgery 1994, 116, 96–100. [Google Scholar]
- Rayatt, S.S.; Dancey, A.L.; Davison, P.M. Thumb subungual melanoma: is amputation necessary? J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 635–638. [Google Scholar] [CrossRef]
- Orr, D.J.; Hughes, L.E.; Horgan, K. Management of malignant melanoma of the head and neck. Br. J. Surg. 1993, 80, 998–1000. [Google Scholar] [CrossRef]
- Baron, P.L. The surgical management of melanoma: from diagnosis to local treatment. Semin. Oncol. 1996, 23, 714–718. [Google Scholar]
- Farshad, A.; Burg, G.; Panizzon, R.; Dummer, R. A retrospective study of 150 patients with lentigo maligna and lentigo maligna melanoma and the efficacy of radiotherapy using Grenz or soft X-rays. Br. J. Dermatol. 2002, 146, 1042–1046. [Google Scholar] [CrossRef]
- Arlette, J.P.; Trotter, M.J.; Trotter, T.; Temple, C.L. Management of lentigo maligna and lentigo maligna melanoma: seminars in surgical oncology. J. Surg. Oncol. 2004, 86, 179–186. [Google Scholar] [CrossRef]
- Tsang, R.W.; Liu, F.F.; Wells, W.; Payne, D.G. Lentigo maligna of the head and neck. Results of treatment by radiotherapy. Arch. Dermatol. 1994, 130, 1008–1012. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Brunner, B.; Konz, B.; Kaudewitz, P.; Wendtner, C.M.; Peter, R.U.; Plewig, G.; Volkenandt, M. Fractionated radiotherapy of lentigo maligna and lentigo maligna melanoma in 64 patients. J. Am. Acad. Dermatol. 2000, 43, 477–482. [Google Scholar]
- Hazan, C.; Dusza, S.W.; Delgado, R.; Busam, K.J.; Halpern, A.C.; Nehal, K.S. Staged excision for lentigo maligna and lentigo maligna melanoma: A retrospective analysis of 117 cases. J. Am. Acad. Dermatol. 2008, 58, 142–148. [Google Scholar] [CrossRef]
- Then, S.Y.; Malhotra, R.; Barlow, R.; Kurwa, H.; Huilgol, S.; Joshi, N.; Olver, J.; Collin, R.; Selva, D. Early cure rates with narrow-margin slow-Mohs surgery for periocular malignant melanoma. Dermatol. Surg. 2009, 35, 17–23. [Google Scholar] [CrossRef]
- Whalen, J.; Leone, D. Mohs micrographic surgery for the treatment of malignant melanoma. Clin. Dermatol. 2009, 27, 597–602. [Google Scholar] [CrossRef]
- Shumaker, P.R.; Kelley, B.; Swann, M.H.; Greenway, H.T., Jr. Modified Mohs micrographic surgery for periocular melanoma and melanoma in situ: long-term experience at Scripps Clinic. Dermatol. Surg. 2009, 35, 1263–1270. [Google Scholar] [CrossRef]
- Jampol, L.M.; Moy, C.S.; Murray, T.G.; Reynolds, S.M.; Albert, D.M.; Schachat, A.P.; Diddie, K.R.; Engstrom, R.E., Jr.; Finger, P.T.; Hovland, K.R.; Joffe, L.; Olsen, K.R.; Wells, C.G. The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma: IV. Local Treatment Failure and Enucleation in the First 5 Years after Brachytherapy. COMS report no. 19. American Medical Association: Chicago, IL, USA, 2002; Ophthalmology, 2002; 109, 2197–2206.
- The Collaborative Ocular Melanoma Study Group, Randomized Trial of Pre-enucleation Radiation of Large Choroidal Melanoma II: Initial Mortality Findings. COMS report no. 10. American Medical Association: Chicago, IL, USA, 1998; Am. J. Ophthalmol. 1998; 125, 779–796.
- Hawkins, B.S. The Collaborative Ocular Melanoma Study (COMS) Randomized Trial of Pre-Enucleation Radiation of Large Choroidal Melanoma: IV. Ten-year Mortality Findings and Prognostic Factors. COMS report number 24. American Medical Association: Chicago, IL, USA, 2004; Am. J. Ophthalmol. 2004, 138, 936–951. [Google Scholar]
- The Collaborative Ocular Melanoma Study Group, The COMS Randomized Trial of Iodine 125 Brachytherapy for Choroidal Melanoma: V. Twelve-year Mortality Rates and Prognostic Factors. COMS report No. 28. American Medical Association: Chicago, IL, USA, 2006; Arch. Ophthalmol. 2006, 124, 1684–1693.
- Skalicky, S.E.; Holt, P.E.; Giblin, M.; Taylor, S.; Conway, R.M. Australian Cancer Network clinical practice guidelines for the management of ocular and periocular melanoma: an evidence-based literature analysis. Clin. Exp. Ophthalmol. 2008, 36, 646–658. [Google Scholar]
- Gupta, S.; Bedikian, A.Y.; Ahrar, J.; Ensor, J.; Ahrar, K.; Madoff, D.C.; Wallace, M.J.; Murthy, R.; Tam, A.; Hwu, P. Hepatic artery chemoembolization in patients with ocular melanoma metastatic to the liver: response, survival, and prognostic factors. Am. J. Clin. Oncol. 2009. [Google Scholar] [CrossRef]
- Yang, H.; Jager, M.J.; Grossniklaus, H.E. Bevacizumab Suppresses Establishment of Micrometastases in Experimental Ocular Melanoma. Invest. Ophthalmol. Vis. Sci. 2010. [Google Scholar] [CrossRef]
- Testori, A.; Rutkowski, P.; Marsden, J.; Bastholt, L.; Chiarion-Sileni, V.; Hauschild, A.; Eggermont, A.M. Surgery and radiotherapy in the treatment of cutaneous melanoma. Ann. Oncol. 2009, 20, 22–29. [Google Scholar] [CrossRef]
- Wargo, J.A.; Tanabe, K. Surgical management of melanoma. Hematol, Oncol. Clin. North. Am. 2009, 23, 565–581. [Google Scholar]
- Essner, R. Surgical treatment of malignant melanoma. Surg. Clin. North. Am. 2003, 83, 109–156. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rutkowski, P.; Zdzienicki, M.; Nowecki, Z.I.; Van Akkooi, A.C.J. Surgery of Primary Melanomas. Cancers 2010, 2, 824-841. https://doi.org/10.3390/cancers2020824
Rutkowski P, Zdzienicki M, Nowecki ZI, Van Akkooi ACJ. Surgery of Primary Melanomas. Cancers. 2010; 2(2):824-841. https://doi.org/10.3390/cancers2020824
Chicago/Turabian StyleRutkowski, Piotr, Marcin Zdzienicki, Zbigniew I. Nowecki, and Alexander C. J. Van Akkooi. 2010. "Surgery of Primary Melanomas" Cancers 2, no. 2: 824-841. https://doi.org/10.3390/cancers2020824




