1. Introduction
Chromosomal translocations often result in the formation of fusion proteins that can drive malignant transformation, leading to the emergence of various cancer types [
1,
2]. These aberrant proteins, also known as onco-fusion proteins, may acquire neo-morphic properties which are distinct from their parent counterparts, and frequently hijack the epigenetic machinery to induce cellular transformation from permissive precursor cells [
3]. In keeping with this, the signaling and transcriptional regulatory mechanisms, as well as the oncogenic properties, controlled by the newly formed onco-fusion proteins, heavily rely on their fusion partners and originating cells [
2].
Fusion proteins associated with transcription factors (TFs) are typically created by merging the DNA-binding domain of the TF with the transactivation domain from another protein. While they are likely to retain DNA-binding specificity, their genome-wide occupancy, co-factor preferences, and chromatin activity can differ from their corresponding wild-type (wt) counterparts, providing each fusion protein with distinct neo-morphic properties that vary across tumor entities [
4]. This type of aberrant TF-mediated tumorigenesis is mainly observed in a subset of leukemias and solid tumors, particularly sarcomas [
3,
5] and relies on the expression of the fusion proteins in a permissive cellular context that creates a favorable environment for their oncogenic activity [
6,
7,
8].
Several studies, including from our own group, have examined the biology and mechanisms involved in various translocated sarcoma models and described the distinctive features of specific onco-fusion proteins, including EWS-FLI1, EWS-ATF1, and EWS-WT1, which drive Ewing sarcoma, clear-cell sarcoma, and desmoplastic small round-cell tumors, respectively [
4,
8,
9,
10,
11,
12]. While different aberrant fusion proteins may share some functional properties, a detailed investigation of their activity remains critical to delineate their unique characteristics and their impact on the biology of each sarcoma model, including the less studied CDS tumors.
CIC-DUX4 sarcoma is an aggressive soft tissue tumor that prevalently develops in young adults, and is part of the CIC-rearranged sarcoma category defined by the 5th edition of the WHO classification of soft tissue and bone tumors [
13]. Morphologically, CIC-DUX4 sarcoma is characterized by the proliferation of small blue round cells and defined as small blue round-cell tumors (SBRCT) [
14,
15]. In CDS, the gene
Capicua (
CIC) is fused to the double homeobox 4 gene (
DUX4) to generate the onco-fusion gene
CIC-DUX4.
CIC is a ubiquitously expressed transcriptional repressor involved in inhibiting the MAPK pathway, whereas
DUX4 is a retrotransposed intronless gene located within the D4Z4 microsatellite repeated units that encodes for a pioneer TF expressed during early embryonic development and capable of activating gene transcription through p300 recruitment [
16]. Given that
DUX4 is present as multiple duplications on either chromosome 4 or 10, the resulting fusion gene can be generated by two alternative genetic events between chromosome 19 (
CIC) and either chromosome 4 or 10 (
DUX4). The resulting CIC-DUX4 aberrant TF contains the majority of the N-terminal part of CIC, including its DNA binding domain, and a small portion of the C-terminal part of DUX4 which contain its transactivation domain [
16], and is believed to represent the main genetic driver of this disease, given the lack of additional genetic alterations in the majority of CDS [
17,
18].
Interestingly, the functional conversion of CIC from a canonical repressor into a powerful transcriptional activator occurring in the context of CIC-DUX4 is sufficient to establish a CDS-specific tumorigenic program [
17,
18]. However, despite recent reports assessing CIC-DUX4 direct target genes [
19] and CIC-DUX4 mechanisms of action [
18,
20], the precise molecular underpinnings allowing CIC-DUX4 to activate its direct target gene repertoire remains poorly understood, limiting the identification of new mechanism-based therapeutic options for this aggressive disease. Recent studies have started highlighting the potential of targeted therapies for these tumors [
21], including the inhibition of the G2/M checkpoint regulator WEE1 [
22], the combination of Trabectedin with the AKT/mTOR inhibitor NVPBEZ235 [
23], and the chemical perturbation of the histone acetyltransferase p300 [
20]. Although these works are paving the way for future therapeutic opportunities, the precise functional link between these pathways and CIC-DUX4 activity remains elusive.
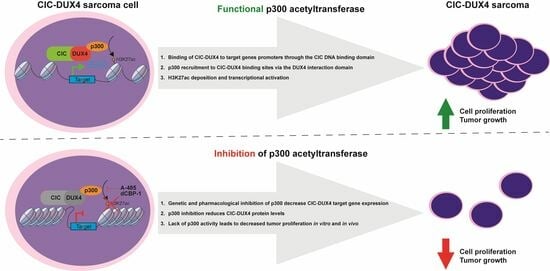
In this study, we investigated the chromatin remodeling properties of CIC-DUX4 in CDS utilizing well-established cell line models and primary human tumors. Our analysis shows that CIC-DUX4 operates as a powerful transcriptional activator at its genomic binding sites, activating a gene expression program that characterizes primary CDS tumors. Furthermore, we combined genetic and pharmacological perturbations with proximity ligation assays to identify the direct interaction of CIC-DUX4 with p300 in vivo, and confirmed the critical role of this chromatin regulator in the establishment of the CDS oncogenic program. Finally, our findings provide compelling mechanistic evidence for the in vivo dependency of CDS tumors on p300 activity, and suggest a potential therapeutic intervention for this deadly malignancy.
2. Materials and Methods
2.1. CIC-DUX4 Sarcoma Primary Tumors
Three primary tumor specimens were collected with approval from the Institutional Review Boards (IRB) of Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, USA), protocol number 02-060. Informed written consent was obtained for all samples collected for this study. Samples were anonymized prior to analysis.
2.2. Cell Lines
The NCC-CDS1-X1(CDS1) and NCC-CDS2-C1(CDS2) cell lines were kindly provided by Tadashi Kondo (Division of rare cancer research head, National Cancer Center Research Institute, Tokyo, Japan). HEK293T cells were obtained from the ATCC. CDS1 and CDS2 cells were grown in RPMI medium (Gibco, Waltham, MA, USA) supplemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin-Streptomycin (PenStrep, Gibco) in adherent cell culture conditions. Cells were passed in RPMI medium (Gibco) supplemented with 20% KO serum (Gibco) one week prior experimentations. LentiX HEK293T, HeLa, and RDES cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS and 1% PenStrep. All cells were cultured at 37 °C with 5% CO2. Cells were maintained and split every 2–4 days (at approximately 80% confluence). Cells were cryopreserved in cryogenic medium [90% FBS with 10% dimethyl sulfoxide (DMSO)] into liquid nitrogen for long-term storage. RD-ES Ewing’s sarcoma cell line was obtained from ATCC, cells were cultured in RPMI medium supplemented with 15% FBS and 1% PenStrep and sub-cultured twice per week.
2.3. Lentiviral Generation
Lentivirus was produced in LentiX HEK293T (Clontech-Takara Bio Europe, Saint-Germain-en-Laye, France)) by FuGene6 (Promega, Madison, WI, USA) transfection with gene delivery vector and packaging vectors GAG/POL and VSV plasmids. Growth medium was changed after 6–8 h from the initial transfection. Viral supernatants were collected 72 h after transfection and concentrated using LentiX concentrator (Clontech-Takara Bio Europe, Saint-Germain-en-Laye, France), following the manufacturer’s instructions. Virus containing pellets were resuspended in PBS and added dropwise on cells in presence of media supplemented with 6 μg/mL polybrene. CDS1 and CDS2 lentivirally infected cells were selected using G418 at a final concentration of 250 μg/mL during 96 h.
2.4. Knock-Down of CIC-DUX4 and p300 by shRNA Transfection
shRNA constructs for P300 knockdown were purchased from Genecopoeia (Genecopoeia, Rockville, MD, USA) (Cat#HSH088760- LVRU6GP, sense sequence: CCAATGGTGGTGATATTAATC and GGATTATGACTTGTGTATCAC, antisense sequence: GATTAATATCACCACCATTGG and GTGATACACAAGTCATAATCC). Negative control shRNA was purchased from Genecopoeia. SiRNAs for depletion of CIC-DUX4 were purchased from Qiagen (Qiagen, Hilden, Germany) (Hs_DUX4_11, sequence: CAGGCGCAACCTCTCCTAGAA) or from Sigma-Aldrich (Sigma-Aldrich, Saint-Louis, MO, USA) (SASI_Hs02_0036094, sense sequence: CCGGCGCCCUGGUCUGCAC [dT][dT], antisense sequence: GUGCAGACCAGGGCGCCGG [dT][dT]). Negative control siRNA (AllStars) was purchased from Qiagen. Oligonucleotides were transfected with INTERFERin siRNA transfection reagent (Polyplus, Illkirch, France) in 150 mm plates using siRNA duplexes at a final concentration of 12 nM and a reverse transfection protocol according to manufacturer recommendations. Cells were harvested 3 days after transfection.
2.5. RNA Extraction, cDNA Synthesis, and Real-Time Quantitative PCR
RNA was extracted using the RNeasy RNA isolation kit (Qiagen, cat#74104, Hilden, Germany), DNAse treatment was performed following the manufacturer’s instructions. For cDNA synthesis, 500 ng of template total RNA were reverse transcribed using the high-capacity cDNA reverse transcription kit (Thermofisher scientific, Waltham, MA, USA, cat#4368814). Real-time qPCR amplification was performed using PowerUp SYBR
® Green Master Mix (Applied Biosystems, Waltham, MA, USA, cat#A25742) and specific PCR primers in a QuantStudio 5 Real-Time PCR System (ThermoFisher Scientific, Waltham, MA, USA). Oligonucleotides used are provided in
Table S1. Relative quantification of each target, normalized to an endogenous control (GAPDH or TBP), was performed using the comparative Ct method (Applied Biosystems, Waltham, MA, USA). Error bars indicate SD of three technical replicates.
2.6. Western Blot Analysis
Cell lysis, SDS-PAGE, and immunoblotting were performed using standard procedures. A measure of 40 μg of proteins were loaded per lane for Western blot and samples underwent electrophoresis through an 8% polyacrylamide gel at 125 mV for 1 h 30. Primary antibodies used for Western blotting are listed in
Table S2. Secondary antibodies were HRP-conjugated goat anti-mouse (GE healthcare, Chicago, Il, USA) and goat anti-rabbit (Dako-Agilent, Santa Clara, CA, USA) antibodies. Protein signals were revealed by SuperSignal West Pico and Femto Chemiluminescent Substrate (Thermo Scientific, Waltham, MA, USA) and captured with the Fusion FX device (Vilber Lourmat, Marne-la-Vallée, France).
2.7. Proximity Ligation Assay (PLA)
The proximity ligation assay was performed using a Duolink II Fluorescence PLA kit (Olink Bioscience, Uppsala, Sweden), as instructed by the manufacturer. Cells were seeded at 70% confluence in 0.2 cm2 dishes, fixed in 4% paraformaldehyde in PIPES buffer for 13 minutes at RT, and permeabilized with 0.3% triton in PBS for 3 minutes. Primary antibodies, anti P300 (cell signaling, Danvers, MA, USA), and anti DUX4 P4H2 (Thermofisher scientific were used with a 1:2000 dilution. PLA amplification was labeled with Alexa Fluor 594 (Olink Bioscience, Uppsala, Sweden). Slides were counterstained with DAPI, mounted, and imaged using the Zeiss (Oberkochen, Germany) Confocal Fluorescent Microscope LSM710, with oil immersion objective 63X, NA 1.4. For each channel the pin hole was set to 0.9 AU. For each sample, the Z-stack was acquired, with a line averaging 2 passages and with a sampling in the XYZ according to the optimal Nyquist criteria. Before analysis the Z-stack was converted with maximum intensity projection. The resulting images were analyzed using ImageJ software version 1.52 R 26 October 2019 (
http://rsbweb.nih.gov/ij/, accessed on 31 October 2019) with a script that defines as region of interest (ROI) the DAPI stained nuclei and counts the included PLA fluorescent foci. For statistical analysis fluorescent foci were counted for each sample in 4 different fields each containing an average of 8–10 cells.
2.8. Chromatin Immunoprecipitation Followed by Sequencing (ChIP-seq)
ChIP assays were carried out on approximately 2–5 million cells or 5–10 mg frozen tumor tissue per sample and per epitope, following the protocol described previously [
8]. Briefly, chromatin from formaldehyde-fixed cells was fragmented to a size range of 200–600 bases using a Branson 250 sonicator. Solubilized chromatin was immunoprecipitated with the indicated antibodies (listed in
Table S3) overnight at 4 °C. Antibody–chromatin complexes were pulled down with protein G-Dynabeads (Invitrogen, Waltham, MA, USA), washed, and then eluted. After crosslink reversal, RNase A, and proteinase K treatment, immunoprecipitated DNA was extracted with AMP Pure beads (Beckman Coulter, Brea, CA, USA). ChIP DNA was quantified with Qubit (Life technologies, Carlsbad, CA, USA). A measure of 1 to 5 ng of ChIP DNA was used to prepare sequencing libraries, and ChIP DNA and input controls were sequenced with the Hi-seq Illumina (San Diego, CA, USA) genome analyzer.
2.9. TF and Histone Mark ChIP-seq Data Analysis
Single-end reads were trimmed using TrimGalore version (v) 0.6.4 (
https://github.com/FelixKrueger/TrimGalore, accessed on 31 October 2019) and aligned to the human genome assembly GRCh37 (hg19) using STAR v 2.5.0a58. Multiple matches, as well as regions present in the ENCODE project DAC Exclusion List Regions (dataset ENCSR636HFF), were removed and a maximum of three redundant reads were kept for subsequent analyses.
Significantly enriched genomic regions for TF and histone mark ChIP-seq were detected using MACS2 v 2.2.659 with an FDR set to 0.05. To reduce the amount of false-positive enrichment, we applied another method to identify significantly enriched genomic regions60. Briefly, the whole genome was split into 400-bp bins and the scores (log2 of the bins reads counts) of these regions were calculated for both the IP and the input samples. A pseudo count was added to avoid NAs. Bins were then split into quantiles based on the mean scores of the IP and input ((IP + Input)/2); for each quantile, the Z-scores were calculated using the mean and SD of the bins fold change (log2(IP)-log2(Input)) within the quantile. p-values were calculated using the pnorm function in R and adjusted with the Benjamini–Hochberg method. In each quantile, bins with an FDR below 0.001 were considered as enriched. Only the MACS2 peaks that intersected with the enriched genomic regions identified by the above-mentioned method were kept for further analysis. The BEDtools v 2.27.1 intersect command, with the -u option of unicity, was used to extract the enriched regions common between the two methods61. This process was applied to each ChIP-seq sample separately. For the generation of the DUX4 reference peak set, all DUX4 peaks identified in the four ChIP-seq experiments (two replicates each of the CDS1 and CDS2 cell lines) were merged, as well as regions overlapping within +/− 150 bp. A total of 810 DUX4 peaks were identified. The peaks within +/− 1 kb of a known gene’s TSS (ucsc_RefFlat_07_08_ 2016) were labeled as TSS. The remnant peaks were evaluated for H3K4me3 enrichment as above and labeled as TSS if H3K4me3-enriched regions were identified in five ChIP-seq experiments, giving in total 428 TSS-associated DUX4 peaks. All the other peaks were labeled as Distal (n = 382). For the genomic distributions, the Distal peaks were annotated as intragenic or intergenic according to the ucsc_RefFlat_07_08_2016 list. IGV was used to visualize ChIP-seq tracks62.
CPM-normalized BigWig files were generated using bamCoverage63, with an extension of ChIP-seq reads to 300 bp fragment length, and used for the ChIP signal heat maps and composite plots (generated with the deepTools scripts computeMatrix, plotHeatmap, and plotProfile). Heat maps were sorted based on the DUX4 mean signal in the four cell line replicate ChIP-seq experiments.
For ChIP-seq peak score calculation, the read counts per peak was calculated using bedTools with the –c option, normalizing to 20 M reads per sample (the approximate mean reads across all samples) and scaling to 500 bp peak width (the approximate DUX4 reference peak mean width). Each peak score is given by log2(IP + 1)-log2(Input + 1), where +1 is an additional pseudo-count, mean values of the replicates. For each dataset, the mean peak read count in the input sample was used as the lower threshold. The peaks in the IP sample with a read count below the threshold were set to zero. Correlations between ChIP-seq scores were calculated by Pearson’s correlation coefficient (r).
Centered, 500 bp extended peak regions were searched for De Novo motif enrichment using HOMER v 4.11.164, applying the 500 bp target sequence length and chopify options. The De Novo motifs were then clustered using the «compareMotifs.pl» script from HOMER. To find motif half-site enrichment, a second round of motif analysis was performed using shorter motif length (4, 5, 6 nt).
All figures, except heat maps and composite plots, were generated using R (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria,
http://www.R-project.org/, v 4.1.0, accessed on 30 June 2020)).
2.10. RNA-seq Differential Expression Analysis
For RNA-seq analysis, total RNA was extracted from frozen tumor tissue using the TRIzol reagent (Invitrogen) or from cells using the RNeasy Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Minimum 100 ng used for preparing sequencing libraries using the TruSeq mRNA stranded kit (Illumina, San Diego, CA, USA) and sequencing was performed on a Hi-Seq Illumina Genome Analyzer (100 bp single-read). Samples used for RNA-seq profiling included siRNAQ-, siRNAS-, or siCTRL-treated CDS1 cell lines and DMSO- or dCBP-1 100 nM-treated CDS2 cell lines.
Single-end reads were trimmed using TrimGalore v 0.6.4 and aligned to the human genome and transcriptome assembly GRCh37 (hg19) using STAR v 2.5.0a. Transcript quantification was performed using rsem v 1.3.068. The resulting counts matrix was used for subsequent analysis. Analysis was performed in R; gene filtering was performed based on the rule of 1 count per M (cpm) in at least 1 sample; library size scaling was performed using TMM normalization (EdgeR package v 3.32.1)69, Robinson Bioinformatics (2010)); log-transformation was performed using the Limma voom function70. Normalized data were batch-corrected using the Limma removeBatchEffect function and unwanted variation was removed with RUVr (RUVseq package v 1.24.0)71 whenever needed. Statistically significant differentially expressed genes were identified from log-transformed, TMM-scaled values using lmFit of the Limma package by fitting a linear model on the selected samples. P-values were adjusted for multiple testing using the Benjamini–Hochberg correction. Batch and/or RUVr-computed factors were added in the design matrix.
Analysis was performed by considering both cell lines (CDS1 and CDS2) and both siRNAs (siRNAQ and siRNAS) as replicates. Those four samples were tested against CDS1 and CDS2 DMSO-treated samples. All RNAseq-related figures were generated using R v 4.1.0 (
http://www.R-project.org/, R Foundation for Statistical Computing, Vienna, Austria, accessed on 30 June 2020)).
2.11. Cell Viability Assays
CDS1 and CDS2 cells viability tests using CellTiter-Glo® luminescent cell viability assay (Cat# G7571, Promega Corporation, Madison, WI, USA) were performed by plating 20,000 cells/well in triplicates in 96-well cell culture plates. Cell viability was measured after 3, 5, and 7 days. Endpoint luminescence was measured on a SpectraMax M5 plate reader (Molecular Devices, San José, CA, USA).
2.12. In Vitro p300 Pharmacological Inhibition
For pharmacological targeting of p300, the A-485 chemical inhibitor [
24] was dissolved in DMSO. A total of 5000 CDS1 cells and 15,000 CDS2 cells were plated in each well as quadruplicates in a 96-wells’plate and allowed to adhere for 24 h in KO serum complemented medium. The A-485 drug was added at a final concentration of 0.01, 0.05, 0.1, 0.25, 0.5, 1, and 2 μM for CDS1, and 5 and 10 μM were added for CDS2 treatment. The according volumes of DMSO were added to control wells and cells were treated with 2 μM Staurosporine in 4 supplementary wells. The fraction of viable cells was determined using the CellTiter-Glo
® luminescent cell viability assay (Cat# G7571, Promega Corporation, Madison, WI, USA), as described by the manufacturer. To generate dose–response curves, data were normalized by setting the average value of Staurosporine and DMSO control wells to 0% and 100% viability, respectively. The replicate values for each dilution point were averaged and the EC
50 values for each compound were generated in Prism GraphPad by fitting normalized data to a sigmoidal curve model of linear regression. D5 timepoint EC
50 values of each cell line were reported in the published cell screening study, from which the same protocol was followed [
24].
2.13. In Vitro p300 Degrader Assay
dCBP-1 P300 degrader was obtained from (MedChemExpress, Monmouth Junction, NJ, USA
https://doi.org/10.1016/j.chembiol.2020.12.004) [
25]. Cells were cultured for 1–3 days in RPMI containing 20% KnockOut™ Serum Replacement, 1% penistrep, then stimulated with 50 or 100 nM dCBP-1 or vehicle (DMSO) and harvested after 24, 48, 72, and 96 h for Western blot analysis and total RNA extraction. For viability test cells were seeded in 60 mm plates at a concentration of 200,000/plate and cultured for 3 days in RPMI containing 20% KnockOut™ Serum Replacement, 1% penistrep. After controlling the number of cells/well, stimulation with 50 or 100 nM dCBP-1 or vehicle (DMSO) was started and cell count was performed in triplicates at regular intervals over a period of time of 4 days. Trypan blue exclusion was used to evaluate cell viability. Cell counts were performed using an automated cell counter Countess II (Thermo-Fisher, Waltham, MA, USA).
2.14. In Vivo Studies
All mice experiments were approved by the Cantonal Veterinary Office, authorization number VD3021 and VD3437. NOD/SCID c Gamma (NSG) mice aged 5–7 weeks were purchased from Jackson Laboratory, Bar Harbor, ME, USA (stock number 005557). All mice were monitored three times a week for tumor development, and sacrificed using CO2 inhalation and cervical dislocation, when the tumor reached 1000–1500 mm3 volume or at the end of the experiment. Every time, an accurate necropsy of the mice was performed to assess local tumor growth and metastatic spread.
2.15. In Vivo A-485 Treatment
Here, 2 × 106 CDS1 cells were resuspended in 100 uL of 1X PBS prior to bilateral subcutaneous injection in the subcutaneous suprascapular region of NSG mice. The experiment included a cohort of eleven mice, which were allocated between the control-treated (n = 6) or the drug-treated (n = 5) group to normalize their size before starting the treatment. A-485 (MedChemExpress, Cat# HY-107455, Monmouth Junction, NJ, USA) was prepared daily and administered intraperitoneally at a dose of 50 mg/kg twice daily. When the first tumors started to be visible, mice were treated with either 50 mg/kg of A-485 or solvent for 15 days. All mice were monitored daily for signs of distress and were weighed three times a week. Tumor size was measured three times a week with a caliper, and tumor volume was calculated according to the following equation: (length × width2)/2 = tumor volume [mm3]. Control-treated mice received the solvent used for oral administration. Mice were sacrificed, as described above, the day after the last dose of A-485.
2.16. Quantification and Statistical Analysis
Statistical analysis of wet lab experiments (Student’s t test, 2-way ANOVA, Mann–Whitney) were performed through Prism GraphPad Software 8.00. All statistical tests and sample numbers are disclosed in respective Figure Legends.
4. Discussion
Sarcoma-associated fusion proteins drive the development of distinct tumor types arising in a variety of anatomical locations, and involving different epigenetic factors. Yet these chimeric proteins also share a number of functional features that underlie their ability to transform permissive precursors cells. Among these, the most notable is the acquisition of neo-morphic properties altering their chromatin activity, and enabling the establishment of tumor specific programs. In keeping with this, TFs such as FLI1 and ATF1 acquire pioneering properties and bind otherwise-inaccessible genomic regions when fused to EWS in Ewing and clear cell sarcoma, respectively [
4,
8]. Similarly, SS18-SSX expression in synovial sarcoma alters the composition and chromatin binding profile of the BAF complex, inducing an oncogenic transcriptional signature that supports tumor growth [
32]. Our study shows an analogous mechanism in CDS, where the fusion of the DNA binding domain of CIC to the transactivation domain of DUX4 converts a transcriptional repressor into a powerful activator, concomitantly turning a well-established tumor suppressor into a potent oncogene.
To better understand the functional consequences of this event, in this study we leveraged two human patient-derived cell lines and three primary tumors (
Table S6) to investigate the chromatin remodeling patterns associated with CIC-DUX4 expression. Using a combination of chromatin profiling and functional perturbations studies, we defined a robust set of direct binding sites (shared between cell lines and showing similar chromatin patterns in primary tumors), confirmed the activator role of CIC-DUX4 at these sites, and established the corresponding target gene repertoire. Interestingly, motif analyses of the DNA sequences underlying a fraction of these genomic regions revealed enrichment for ETS TFs motifs. Given that CIC-DUX4 has been shown to directly induce the expression of ETV1, ETV4, and ETV5 [
31], which all belong to the ETS TF family, a plausible scenario could be that the fusion protein operates along a spatiotemporal trajectory, starting with the activation of direct targets controlled by canonical HLH motifs, and followed by the induction of additional genes regulated by the co-binding of CIC-DUX4 with its own targets, including ETV1, 4, and 5.
Despite playing critical roles across all stages of tumor evolution, aberrant TFs are hardly targetable using standard pharmacological strategies. Current approaches trying to bypass this limitation include modulation of their downstream target genes, or targeting catalytically active binding partners of the fusion proteins. Since DUX4 has been shown to recruit p300 through its C-terminal portion, which is retained in CIC-DUX4, we explored the possibility that the fusion protein may directly hijack p300 at its binding sites to induce chromatin activation, and evaluated p300 inhibition as a viable therapeutic strategy for the clinical management of these tumors. In keeping with this, we report the direct interaction between CIC-DUX4 and p300 in CDS tumor cells, and confirm the role of this chromatin regulator in sustaining the oncogenic transcriptional program induced by the fusion protein. Our chromatin profiles show that p300 and CIC-DUX4 co-localize at a large fraction of the fusion protein binding sites in the CDS1 and CDS2 lines, a result further substantiated using PLA as an orthogonal approach. Consistent with this notion, inhibition of p300 activity in CDS cells by genetic and pharmacological approaches, recapitulated the transcriptional changes observed upon CIC-DUX4 depletion, and resulted in tumor growth decreases in vitro and in vivo. Altogether these results add mechanistic insight to the observation reported by Bosnakovski, D. et al. concerning the reduction in CDS tumor growth following p300 inhibition [
20], and provide a new tangible therapeutic option that consists of a selective and potent p300 degrader [
25].
An intriguing result of our study is the decrease in CIC-DUX4 protein levels observed upon the genetic or pharmacological inhibition of p300 activity. Although a general reduction in transcriptional expression is to be expected under similar conditions, the lack of corresponding decreases in
CIC-DUX4 transcript levels suggests that alternative mechanisms may underlie these observations. One potential hypothesis warranting future investigation is that direct acetylation of CIC-DUX4 by p300 increases the stability of the fusion protein, as observed for other oncogenic TFs [
33,
34]. In this scenario, inhibiting p300 activity could represent a synergistic strategy that would reduce the transactivation ability of CIC-DUX4, while decreasing the global levels of the fusion protein itself.
Our current work also presents some limitations inherent to biology and to these tumors. First, given the rarity of available CIC-DUX4 sarcoma samples and in vitro models in our study, we used two well-established cell lines (out of the four distinct cell lines reported in the literature), and three primary CIC-DUX4 sarcoma frozen samples. Thus, further validation of our findings in additional lines is warranted before their translation into clinical studies. A second limitation of our current work is the lack of assessment of the long-term in vivo effects of p300 inhibitors/degraders on mice survival and pharmacological toxicity, which will be highly informative before the clinical translation of these findings. Finally, future work should also focus on detailing the interaction between the endogenous CIC-DUX4 and wt CIC proteins in our cell lines, since the current anti-CIC antibodies do not discriminate between these two proteins, which share the majority of the CIC amino-acid sequence. Indeed, the precise role of wt CIC in the context of CDS remains to be investigated, particularly in regard to the potential mechanisms allowing CIC-DUX4 to override the repressor function of CIC. Given that CIC and CIC-DUX4 share the same DNA-binding motif, it stands to reason that the two TFs may compete with each other for the same binding regions and target genes within CDS tumor cells. Future experimental models in which the endogenous wt CIC gene is tagged using a CRSPR editing approach will be ideal in untangling the interaction between these two proteins in the context of CDS cells. Understanding if and how wt CIC and CIC-DUX4 functionally interact in the context of CDS will represent a major step forward in deciphering the pathogenesis of these tumors and beyond, given that CIC inactivating mutations are known to fuel the progression and dissemination of a large panel of cancer types [
35].