Jun Dimerization Protein 2 (JDP2) Increases p53 Transactivation by Decreasing MDM2
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. DNA Constructs
2.3. Cell Culture
2.4. Transient Transfection
2.5. p53 (14X)RE Luciferase Reporter Assay
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
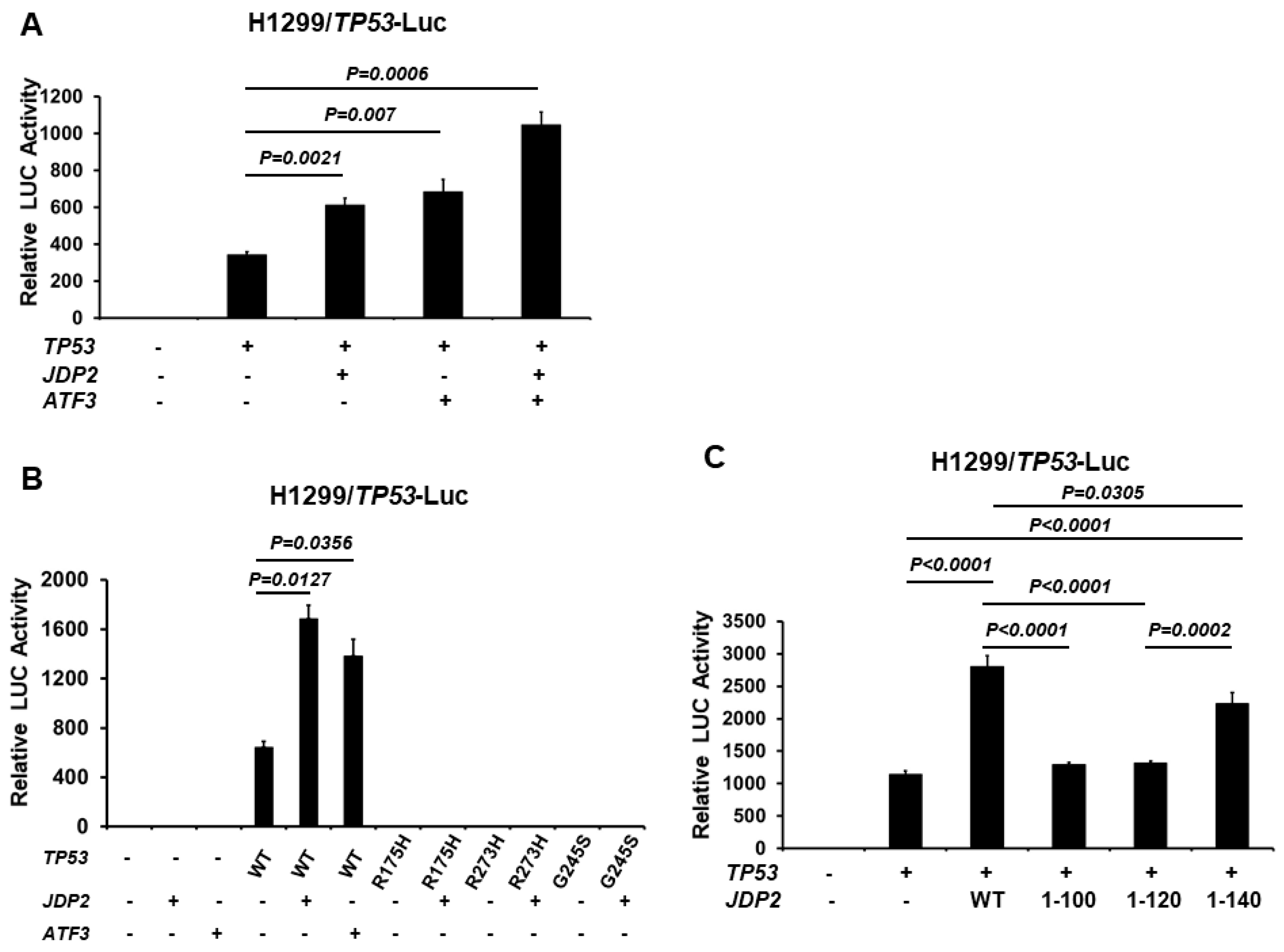
3.1. JDP2 and ATF3 Increase p53 Transactivation
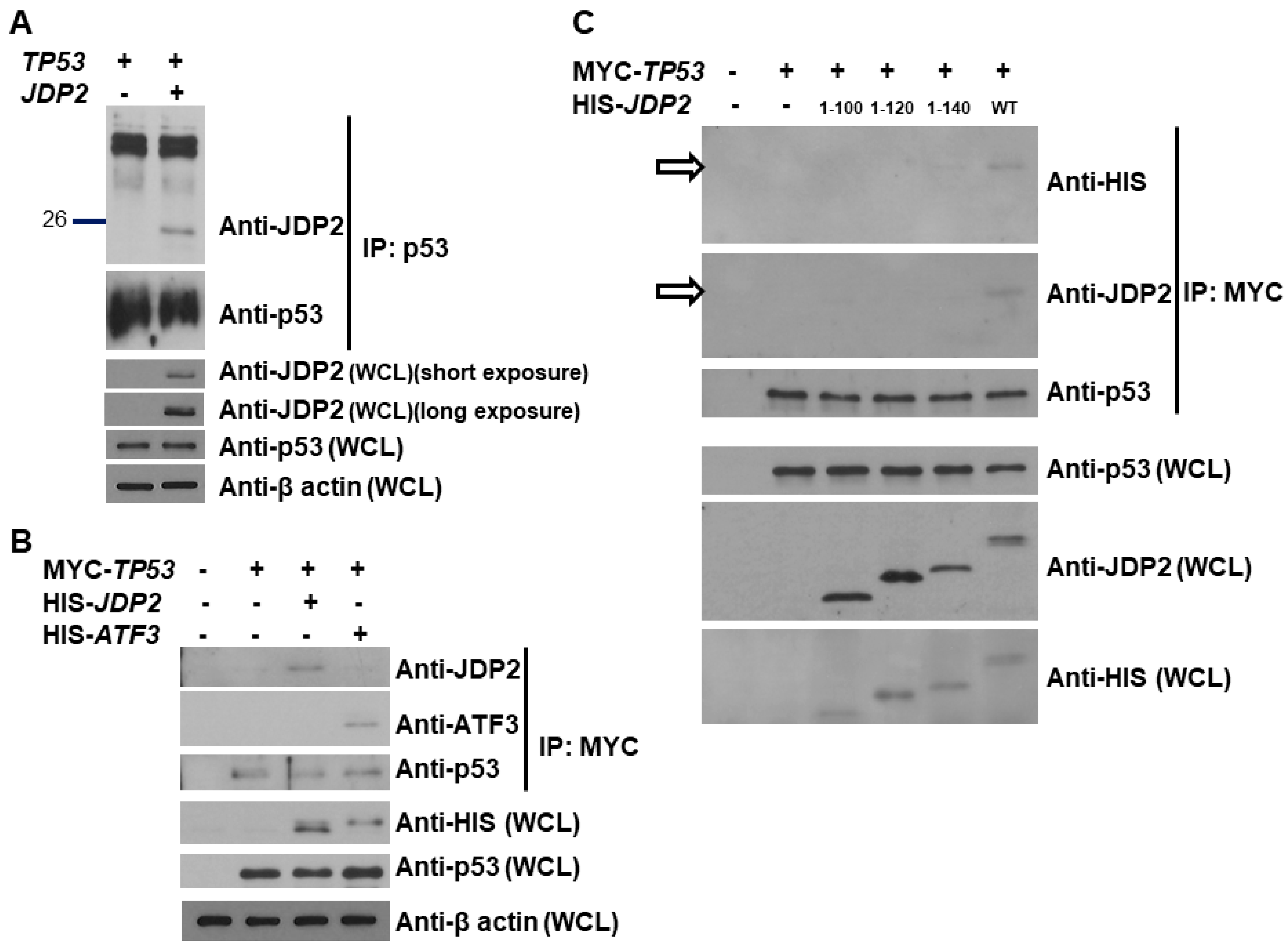
3.2. p53 Binds the C-Terminal Domain of JDP2
3.3. JDP2 and ATF3 Additively Enhance p53 Transactivation and the C-Terminal Domain of JDP2 Is Required for Enhancement of p53 Transactivation
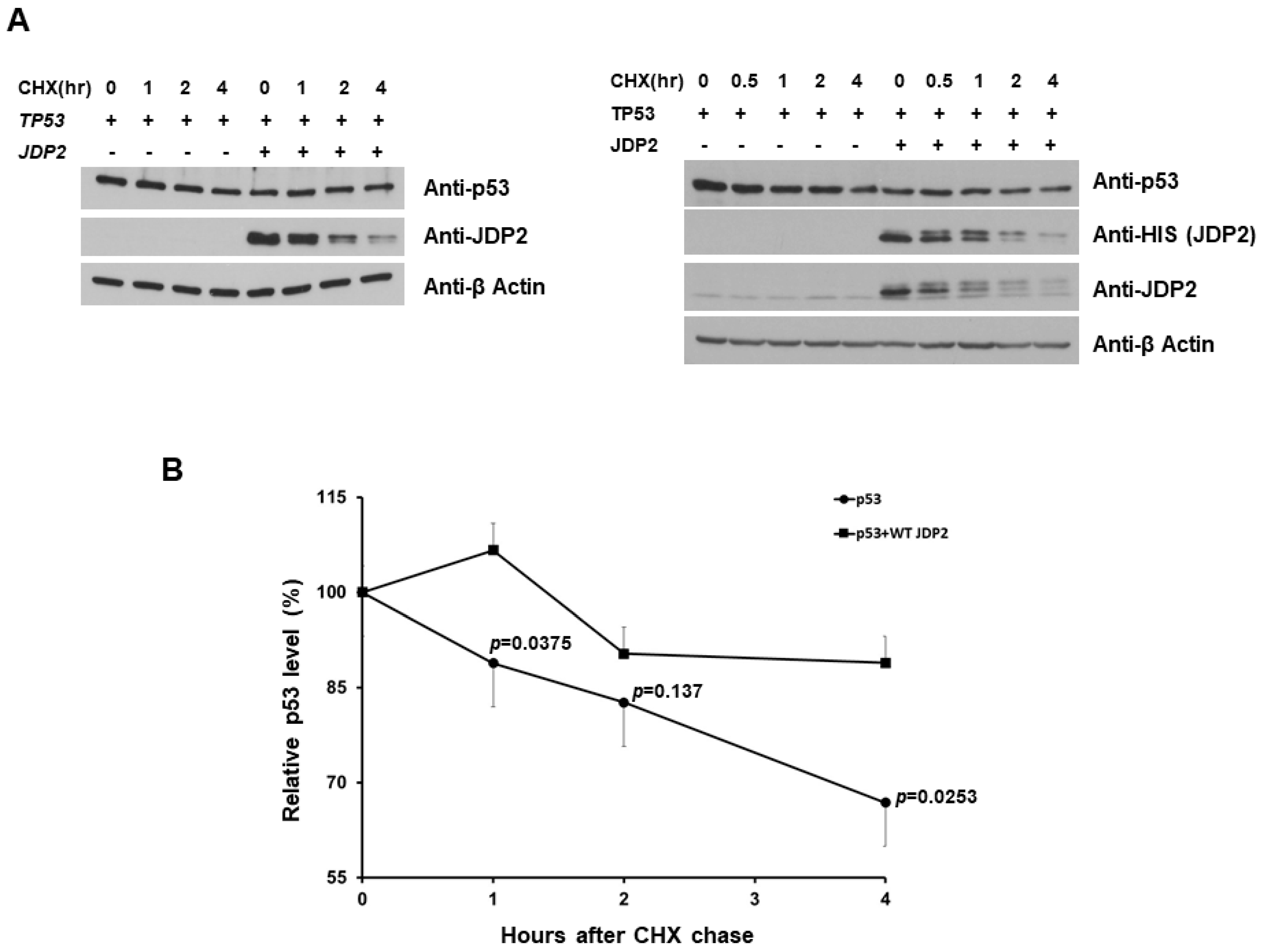
3.4. JDP2 Slightly Increases p53 Stability in H1299 Cells
3.5. JDP2 Decreases MDM2 Levels
3.6. While MDM2 Decreases p53 Transactivation, JDP2 Dose-Dependently Abolishes MDM2-Mediated p53 Repression
3.7. JDP2 Increases p21 Promoter Activity and p21 Protein Level in the Presence of p53
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassin, O.; Oren, M. Drugging p53 in cancer: One protein, many targets. Nat. Rev. Drug Discov. 2023, 22, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Synnott, N.C.; O’Grady, S.; Crown, J. Targeting p53 for the treatment of cancer. Semin. Cancer Biol. 2022, 79, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.M.; Jung, C.H.; Kim, J.; Hwang, S.G.; Park, J.K.; Um, H.D. The p53/p21 Complex Regulates Cancer Cell Invasion and Apoptosis by Targeting Bcl-2 Family Proteins. Cancer Res. 2017, 77, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yu, L.; Chen, W.; Xu, Y.; Wu, M.; Todorova, D.; Tang, Q.; Feng, B.; Jiang, L.; He, J.; et al. Wild-Type p53 Promotes Cancer Metabolic Switch by Inducing PUMA-Dependent Suppression of Oxidative Phosphorylation. Cancer Cell 2019, 35, 191–203.e8. [Google Scholar] [CrossRef] [PubMed]
- Humayun, A.; Fornace, A.J., Jr. GADD45 in Stress Signaling, Cell Cycle Control, and Apoptosis. Adv. Exp. Med. Biol. 2022, 1360, 1–22. [Google Scholar] [PubMed]
- Fischer, M. Census and evaluation of p53 target genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Li, D.; Ou, Y.; Jiang, L.; Chen, Y.; Zhao, Y.; Gu, W. Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016, 17, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, L.M.; Durell, S.R.; Mazur, S.J.; Appella, E. p53 N-terminal phosphorylation: A defining layer of complex regulation. Carcinogenesis 2012, 33, 1441–1449. [Google Scholar]
- Zheng, H.B.; Fu, Y.T.; Pan, Z.X.; Yang, T.W.; Sun, L.H.; Chen, Y.B.; Tong, T. The effect of mesenchymal stem cells on the p53 methylation in irradiation-induced thymoma in C57BL/6 mice. J. Cancer Res. Ther. 2015, 11, 403–408. [Google Scholar]
- Marcos-Villar, L.; Pérez-Girón, J.V.; Vilas, J.M.; Soto, A.; de la Cruz-Hererra, C.F.; Lang, V.; Collado, M.; Vidal, A.; Rodríguez, M.S.; Muñoz-Fontela, C.; et al. SUMOylation of p53 mediates interferon activities. Cell Cycle 2013, 12, 2809–2816. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Yan, H.; Wu, J.; Yang, Y.; He, J.; Chen, J.; Jiang, Z.; Wu, F.; Jiang, Z. Downregulation of CPT2 promotes proliferation and inhibits apoptosis through p53 pathway in colorectal cancer. Cell Signal. 2022, 92, 110267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Ho, T.L.F.; Hariharan, A.; Goh, H.C.; Wong, Y.L.; Verkaik, N.S.; Lee, M.Y.; Tam, W.L.; van Gent, D.C.; Venkitaraman, A.R.; et al. Rapid recruitment of p53 to DNA damage sites directs DNA repair choice and integrity. Proc. Natl. Acad. Sci. USA 2022, 119, e2113233119. [Google Scholar] [CrossRef]
- Rahman, M.A.; Park, M.N.; Rahman, M.H.; Rashid, M.M.; Islam, R.; Uddin, M.J.; Hannan, M.A.; Kim, B. p53 Modulation of Autophagy Signaling in Cancer Therapies: Perspectives Mechanism and Therapeutic Targets. Front. Cell Dev. Biol. 2022, 10, 761080. [Google Scholar] [CrossRef] [PubMed]
- Loughran, R.M.; Emerling, B.M. Mechanistic roles of mutant p53 governing lipid metabolism. Adv. Biol. Regul. 2022, 83, 100839. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.R.E.; Wang, D.; Zhang, Y.; Ferrari, M.; Okon, A.; Cleary, M.P.; Wagner, C.R.; Yang, D.Q. Induction of the p53 Tumor Suppressor in Cancer Cells through Inhibition of Cap-Dependent Translation. Mol. Cell. Biol. 2018, 38, e00367-17. [Google Scholar] [CrossRef]
- Zafar, A.; Wang, W.; Liu, G.; Xian, W.; McKeon, F.; Zhou, J.; Zhang, R. Targeting the p53-MDM2 pathway for neuroblastoma therapy: Rays of hope. Cancer Lett. 2021, 496, 16–29. [Google Scholar] [CrossRef]
- Song, D.; Lian, Y.; Zhang, L. The potential of activator protein 1 (AP-1) in cancer targeted therapy. Front. Immunol. 2023, 14, 1224892. [Google Scholar] [CrossRef]
- Jin, C.; Li, H.; Murata, T.; Sun, K.; Horikoshi, M.; Chiu, R.; Yokoyama, K.K. JDP2, a repressor of AP-1, recruits a histone deacetylase 3 complex to inhibit the retinoic acid-induced differentiation of F9 cells. Mol. Cell. Biol. 2002, 22, 4815–4826. [Google Scholar] [CrossRef]
- Darlyuk-Saadon, I.; Weidenfeld-Baranboim, K.; Yokoyama, K.K.; Hai, T.; Aronheim, A. The bZIP repressor proteins, c-Jun dimerization protein 2 and activating transcription factor 3, recruit multiple HDAC members to the ATF3 promoter. Biochim. Biophys. Acta 2012, 1819, 1142–1153. [Google Scholar] [CrossRef]
- Blazek, E.; Wasmer, S.; Kruse, U.; Aronheim, A.; Aoki, M.; Vogt, P.K. Partial oncogenic transformation of chicken embryo fibroblasts by Jun dimerization protein 2, a negative regulator of TRE- and CRE-dependent transcription. Oncogene 2003, 22, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Jin, C.; Murata, T.; Yokoyama, K.K. Sequence specific transcription factor, JDP2 interacts with histone and inhibits p300-mediated histone acetylation. Nucleic Acids Symp. Ser. 2003, 3, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Kato, K.; Chimura, T.; Yamasaki, T.; Nakade, K.; Murata, T.; Li, H.; Pan, J.; Zhao, M.; Sun, K.; et al. Regulation of histone acetylation and nucleosome assembly by transcription factor JDP2. Nat. Struct. Mol. Biol. 2006, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Nakade, K.; Pan, J.; Yoshiki, A.; Ugai, H.; Kimura, M.; Liu, B.; Li, H.; Obata, Y.; Iwama, M.; Itohara, S.; et al. JDP2 suppresses adipocyte differentiation by regulating histone acetylation. Cell Death Differ. 2007, 14, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Nakade, K.; Pan, J.; Yamasaki, T.; Murata, T.; Wasylyk, B.; Yokoyama, K.K. JDP2 (Jun Dimerization Protein 2)-deficient mouse embryonic fibroblasts are resistant to replicative senescence. J. Biol. Chem. 2009, 284, 10808–10817. [Google Scholar] [CrossRef]
- Ostrovsky, O.; Bengal, E.; Aronheim, A. Induction of terminal differentiation by the c-Jun dimerization protein JDP2 in C2 myoblasts and rhabdomyosarcoma cells. J. Biol. Chem. 2002, 277, 40043–40054. [Google Scholar] [CrossRef] [PubMed]
- Kawaida, R.; Ohtsuka, T.; Okutsu, J.; Takahashi, T.; Kadono, Y.; Oda, H.; Hikita, A.; Nakamura, K.; Tanaka, S.; Furukawa, H. Jun dimerization protein 2 (JDP2), a member of the AP-1 family of transcription factor, mediates osteoclast differentiation induced by RANKL. J. Exp. Med. 2003, 197, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Heger, J.; Bornbaum, J.; Würfel, A.; Hill, C.; Brockmann, N.; Gáspár, R.; Pálóczi, J.; Varga, Z.V.; Sárközy, M.; Bencsik, P.; et al. JDP2 overexpression provokes cardiac dysfunction in mice. Sci. Rep. 2018, 8, 7647. [Google Scholar] [CrossRef]
- Wardell, S.E.; Boonyaratanakornkit, V.; Adelman, J.S.; Aronheim, A.; Edwards, D.P. Jun dimerization protein 2 functions as a progesterone receptor N-terminal domain coactivator. Mol. Cell. Biol. 2002, 22, 5451–5466. [Google Scholar] [CrossRef]
- Hill, K.K.; Roemer, S.C.; Jones, D.N.; Churchill, M.E.; Edwards, D.P. A progesterone receptor co-activator (JDP2) mediates activity through interaction with residues in the carboxyl-terminal extension of the DNA binding domain. J. Biol. Chem. 2009, 284, 24415–24424. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Noda, C.; Saito, S.; Kawashima, D.; Sugimoto, A.; Isomura, H.; Kanda, T.; Yokoyama, K.K.; Tsurumi, T. Involvement of Jun dimerization protein 2 (JDP2) in the maintenance of Epstein-Barr virus latency. J. Biol. Chem. 2011, 286, 22007–22016. [Google Scholar] [CrossRef]
- Maruyama, K.; Fukasaka, M.; Vandenbon, A.; Saitoh, T.; Kawasaki, T.; Kondo, T.; Yokoyama, K.K.; Kidoya, H.; Takakura, N.; Standley, D.; et al. The transcription factor Jdp2 controls bone homeostasis and antibacterial immunity by regulating osteoclast and neutrophil differentiation. Immunity 2012, 37, 1024–1036. [Google Scholar] [CrossRef]
- Barbarov, Y.; Timaner, M.; Alishekevitz, D.; Hai, T.; Yokoyama, K.K.; Shaked, Y.; Aronheim, A. Host JDP2 expression in the bone marrow contributes to metastatic spread. Oncotarget 2015, 6, 37737–37749. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Nakade, K.; Huang, Y.C.; Zhu, Z.W.; Masuzaki, S.; Hasegawa, H.; Murata, T.; Yoshiki, A.; Yamaguchi, N.; Lee, C.H.; et al. Suppression of cell-cycle progression by Jun dimerization protein-2 (JDP2) involves downregulation of cyclin-A2. Oncogene 2011, 29, 6245–6256. [Google Scholar] [CrossRef]
- Nakade, K.; Lin, C.S.; Chen, X.Y.; Tsai, M.H.; Wuputra, K.; Zhu, Z.W.; Pan, J.Z.; Yokoyama, K.K. Jun dimerization protein 2 controls hypoxia-induced replicative senescence via both the p16Ink4a-pRb and Arf-p53 pathways. FEBS Open Bio 2017, 7, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jin, C.; Liu, Z.; Pan, J.; Li, H.; Zhang, Z.; Bi, S.; Yokoyama, K.K. Cloning and characterization of the mouse JDP2 gene promoter reveal negative regulation by p53. Biochem. Biophys. Res. Commun. 2014, 450, 1531–1536. [Google Scholar] [CrossRef]
- Heinrich, R.; Livne, E.; Ben-Izhak, O.; Aronheim, A. The c-Jun dimerization protein 2 inhibits cell transformation and acts as a tumor suppressor gene. J. Biol. Chem. 2004, 279, 5708–5715. [Google Scholar] [CrossRef]
- Yuanhong, X.; Feng, X.; Qingchang, L.; Jianpeng, F.; Zhe, L.; Kejian, G. Downregulation of AP-1 repressor JDP2 is associated with tumor metastasis and poor prognosis in patients with pancreatic carcinoma. Int. J. Biol. Markers 2010, 25, 136–140. [Google Scholar] [CrossRef]
- Järvinen, A.K.; Autio, R.; Kilpinen, S.; Saarela, M.; Leivo, I.; Grénman, R.; Mäkitie, A.A.; Monni, O. High-resolution copy number and gene expression microarray analyses of head and neck squamous cell carcinoma cell lines of tongue and larynx. Genes Chromosomes Cancer 2008, 47, 500–509. [Google Scholar] [CrossRef]
- Bitton-Worms, K.; Pikarsky, E.; Aronheim, A. The AP-1 repressor protein, JDP2, potentiates hepatocellular carcinoma in mice. Mol. Cancer 2010, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Price, K.; Cardoso, L.; Yang, W.H.; Wang, C.M.; Yang, R.H. Abstract 5723: JDP2 interacts with p53 and enhances p53 transactivation and stability. In Proceedings of the American Association for Cancer Research Annual Meeting 2022, New Orleans, LA, USA, 8–13 April 2022; AACR: Philadelphia, PA, USA, 2022. [Google Scholar]
- Yang, W.H.; Wang, C.M.; Yang, R.H.; Yang, W.H. ODP281 JDP2 Interacts with MDM2 and Decreases MDM2-Mediated p53 Repression. J. Endocr. Soc. 2022, 6 (Suppl. S1), A450. [Google Scholar] [CrossRef]
- Yang, W.H.; George, A.P.; Wang, C.M.; Yang, R.H.; Duncan, A.M.; Patel, D.; Neil, Z.D.; Yang, W.H. Tumor suppressor p53 down-regulates programmed cell death protein 4 (PDCD4) expression. Curr. Oncol. 2023, 30, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Brennan, V.C.; Gutierrez, N.M.; Wang, X.; Wang, L.; Yang, W.H. SUMOylation of ATF3 alters its transcriptional activity on regulation of TP53 gene. J. Cell. Biochem. 2013, 114, 589–598. [Google Scholar] [CrossRef]
- Wang, C.M.; Yang, W.H.; Cardoso, L.; Gutierrez, N.; Yang, R.H.; Yang, W.H. Forkhead Box Protein P3 (FOXP3) Represses ATF3 Transcriptional Activity. Int. J. Mol. Sci. 2021, 22, 11400. [Google Scholar] [CrossRef]
- Yan, C.; Lu, D.; Hai, T.; Boyd, D.D. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J. 2005, 24, 2425–2435. [Google Scholar] [CrossRef]
- Yoo, Y.G.; Yeo, M.G.; Kim, D.K.; Park, H.; Lee, M.O. Novel function of orphan nuclear receptor Nur77 in stabilizing hypoxia-inducible factor-1alpha. J. Biol. Chem. 2004, 279, 53365–53373. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Dassaye, R.; Naidoo, S.; Cerf, M.E. Transcription factor regulation of pancreatic organogenesis, differentiation and maturation. Islets 2016, 8, 13–34. [Google Scholar] [CrossRef]
- Wilanowski, T.; Dworkin, S. Transcription Factors in Cancer. Int. J. Mol. Sci. 2022, 23, 4434. [Google Scholar] [CrossRef]
- Kant, R.; Manne, R.K.; Anas, M.; Penugurti, V.; Chen, T.; Pan, B.S.; Hsu, C.C.; Lin, H.K. Deregulated transcription factors in cancer cell metabolisms and reprogramming. Semin. Cancer Biol. 2022, 86, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Al-Nusaif, M.; Chen, X.; Li, S.; Le, W. Roles of Transcription Factors in the Development and Reprogramming of the Dopaminergic Neurons. Int. J. Mol. Sci. 2022, 23, 845. [Google Scholar] [CrossRef] [PubMed]
- Weidemüller, P.; Kholmatov, M.; Petsalaki, E.; Zaugg, J.B. Transcription factors: Bridge between cell signaling and gene regulation. Proteomics 2021, 21, e2000034. [Google Scholar] [CrossRef] [PubMed]
- Eischen, C.M. Genome Stability Requires p53. Cold Spring Harb. Perspect. Med. 2016, 6, a026096. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.S.; Uddin, M.A.; Barabutis, N. P53 Regulates the Redox Status of Lung Endothelial Cells. Inflammation 2020, 43, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Pisconti, S.; Della Vittoria Scarpati, G. P53 mutations and cancer: A tight linkage. Ann. Transl. Med. 2016, 4, 522. [Google Scholar] [CrossRef] [PubMed]
- Weidenfeld-Baranboim, K.; Hasin, T.; Darlyuk, I.; Heinrich, R.; Elhanani, O.; Pan, J.; Yokoyama, K.K.; Aronheim, A. The ubiquitously expressed bZIP inhibitor, JDP2, suppresses the transcription of its homologue immediate early gene counterpart, ATF3. Nucleic Acids Res. 2009, 37, 2194–2203. [Google Scholar] [CrossRef]
- Yan, C.; Boyd, D.D. ATF3 regulates the stability of p53: A link to cancer. Cell Cycle 2006, 5, 926–929. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, C.; Kawauchi, J.; Hashimoto, Y.; Tsuchida, N.; Kitajima, S. Transcriptional activation of the human stress-inducible transcriptional repressor ATF3 gene promoter by p53. Biochem. Biophys. Res. Commun. 2002, 297, 1302–1310. [Google Scholar] [CrossRef]
- Wei, S.; Wang, H.; Lu, C.; Malmut, S.; Zhang, J.; Ren, S.; Yu, G.; Wang, W.; Tang, D.D.; Yan, C. The activating transcription factor 3 protein suppresses the oncogenic function of mutant p53 proteins. J. Biol. Chem. 2014, 289, 8947–8959. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Saito, S.; Yokoyama, K.K. Histone chaperone Jun dimerization protein 2 (JDP2): Role in cellular senescence and aging. Kaohsiung J. Med. Sci. 2010, 26, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Lerdrup, M.; Holmberg, C.; Dietrich, N.; Shaulian, E.; Herdegen, T.; Jäättelä, M.; Kallunki, T. Depletion of the AP-1 repressor JDP2 induces cell death similar to apoptosis. Biochim. Biophys. Acta 2005, 1745, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Piu, F.; Aronheim, A.; Katz, S.; Karin, M. AP-1 repressor protein JDP-2: Inhibition of UV-mediated apoptosis through p53 down-regulation. Mol. Cell. Biol. 2001, 21, 3012–3024. [Google Scholar] [CrossRef] [PubMed]
- Kalfon, R.; Friedman, T.; Eliachar, S.; Shofti, R.; Haas, T.; Koren, L.; Moskovitz, J.D.; Hai, T.; Aronheim, A. JDP2 and ATF3 deficiencies dampen maladaptive cardiac remodeling and preserve cardiac function. PLoS ONE 2019, 14, e0213081. [Google Scholar] [CrossRef] [PubMed]
- Haupt, Y.; Maya, R.; Kazaz, A.; Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 1997, 387, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Yang, F.; Xu, L.; Zou, Q. A comprehensive review of the imbalance classification of protein post-translational modifications. Brief Bioinform. 2021, 8, bbab089. [Google Scholar] [CrossRef] [PubMed]
- Keenan, E.K.; Zachman, D.K.; Hirschey, M.D. Discovering the landscape of protein modifications. Mol. Cell 2021, 81, 1868–1878. [Google Scholar] [CrossRef]
- Weidenfeld-Baranboim, K.; Koren, L.; Aronheim, A. Phosphorylation of JDP2 on threonine-148 by the c-Jun N-terminal kinase targets it for proteosomal degradation. Biochem. J. 2011, 436, 661–669. [Google Scholar] [CrossRef]
- Wang, C.M.; Wang, R.X.; Liu, R.; Yang, W.H. Jun Dimerization Protein 2 Activates Mc2r Transcriptional Activity: Role of Phosphorylation and SUMOylation. Int. J. Mol. Sci. 2017, 18, 304. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, K.; Yang, W.H.; Cardoso, L.; Wang, C.-M.; Yang, R.H.; Yang, W.-H. Jun Dimerization Protein 2 (JDP2) Increases p53 Transactivation by Decreasing MDM2. Cancers 2024, 16, 1000. https://doi.org/10.3390/cancers16051000
Price K, Yang WH, Cardoso L, Wang C-M, Yang RH, Yang W-H. Jun Dimerization Protein 2 (JDP2) Increases p53 Transactivation by Decreasing MDM2. Cancers. 2024; 16(5):1000. https://doi.org/10.3390/cancers16051000
Chicago/Turabian StylePrice, Kasey, William H. Yang, Leticia Cardoso, Chiung-Min Wang, Richard H. Yang, and Wei-Hsiung Yang. 2024. "Jun Dimerization Protein 2 (JDP2) Increases p53 Transactivation by Decreasing MDM2" Cancers 16, no. 5: 1000. https://doi.org/10.3390/cancers16051000




