Design, Fabrication and Computational Characterization of a 3D Micro-Valve Built by Multi-Photon Polymerization
Abstract
:1. Introduction
2. Design and Fabrication
2.1. Micro-Valve Design
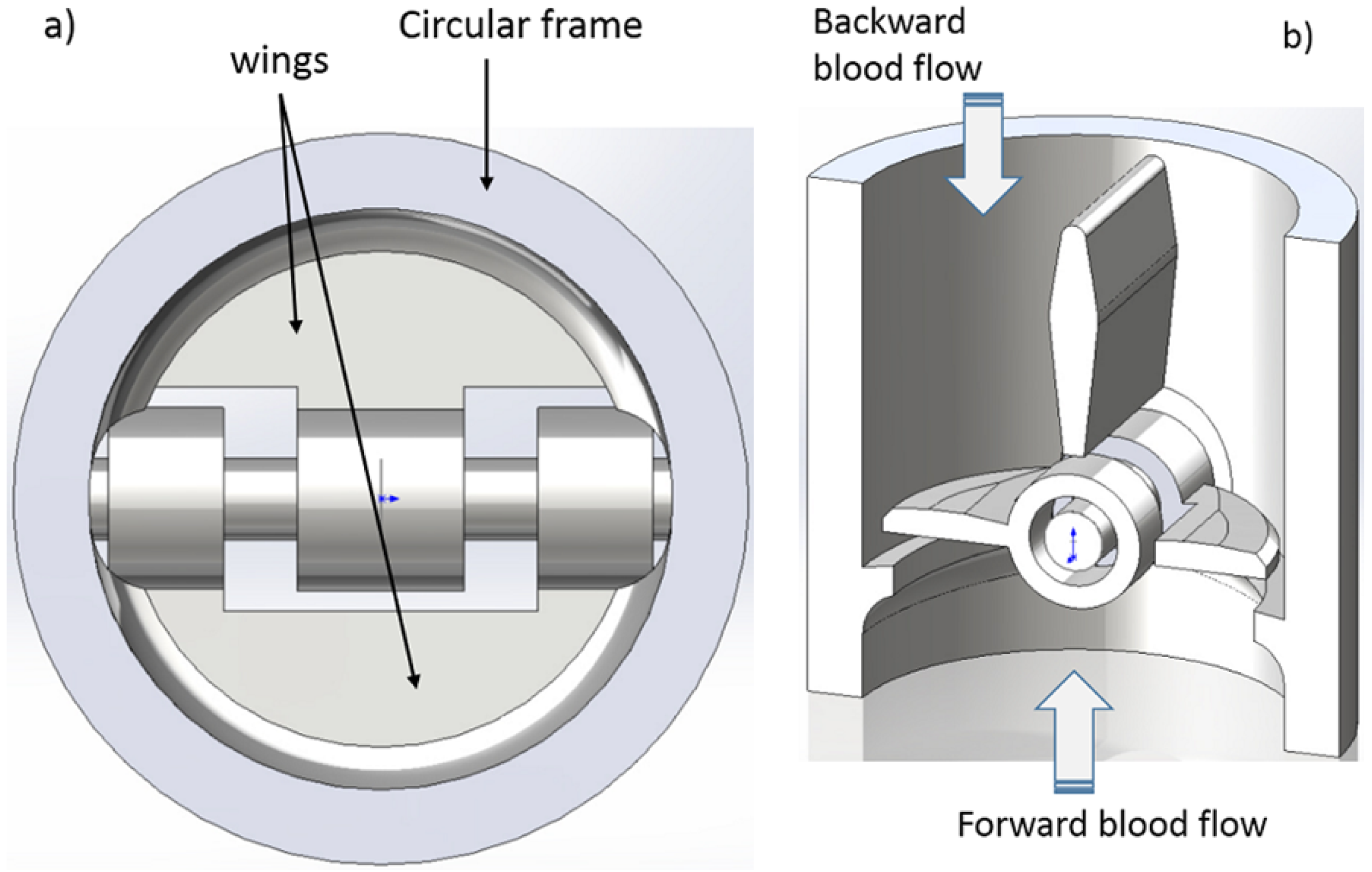
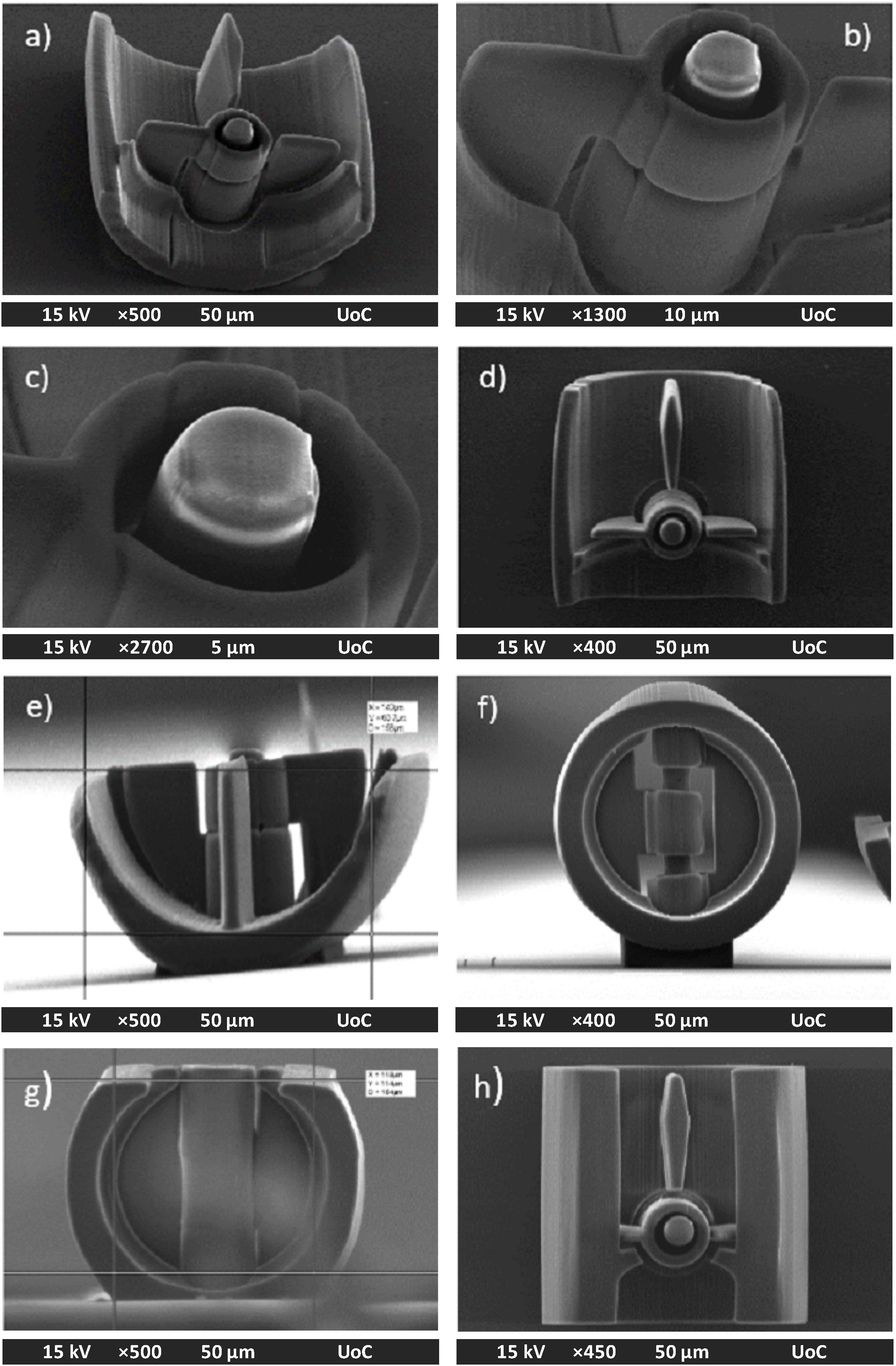
2.2. Micro-Valve Fabrication
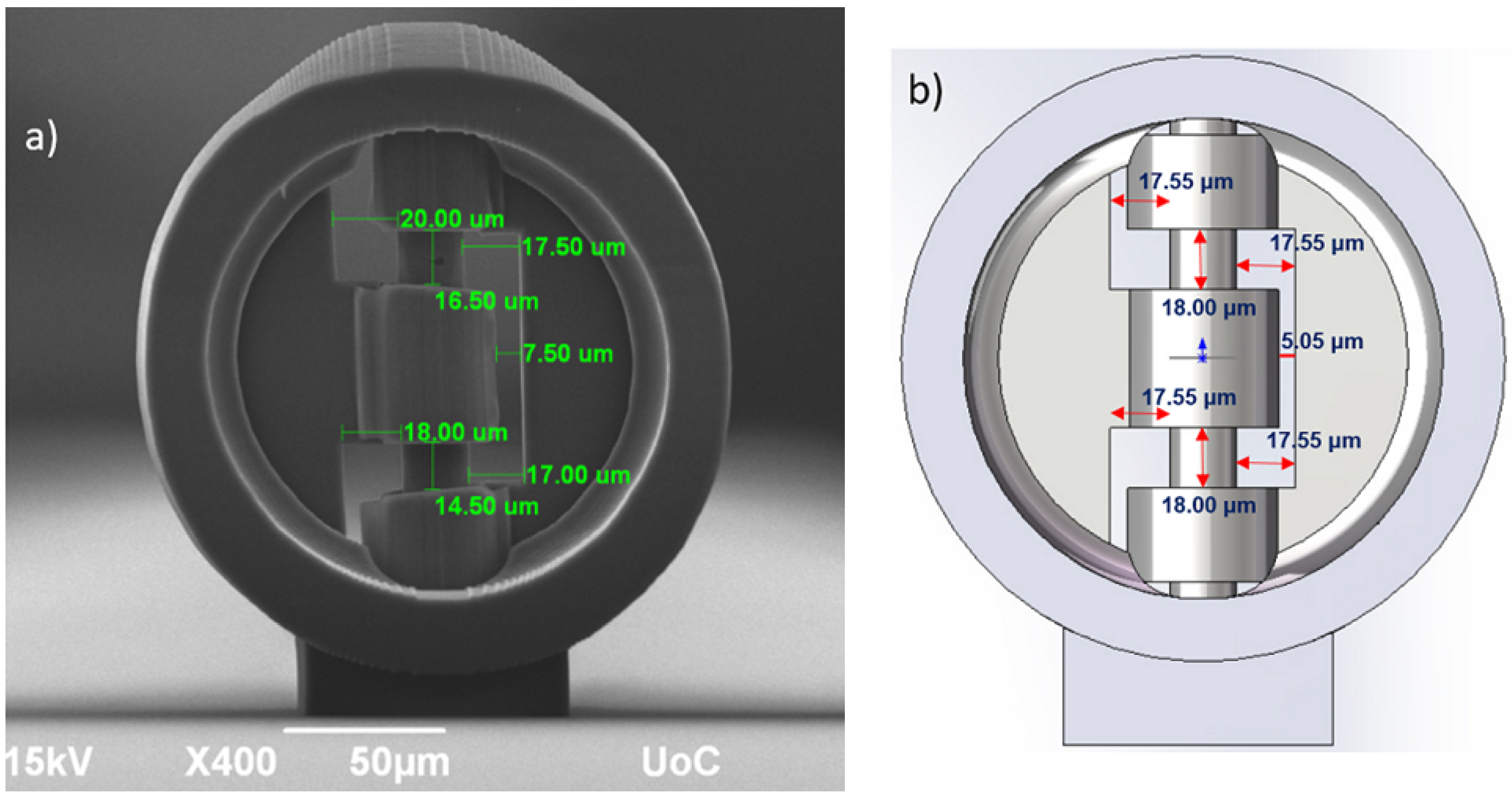
3. Flow Performance Simulations
 n−1
n−1
 is the shear rate and n = 0.785 the non-Newtonian index [14]. At the inlet of the domain, a fully developed velocity profile of 1 cm/s was considered. At the outlet, a free flow outlet condition with null pressure was considered, while no-slip condition imposed at the solid surfaces. In order to avoid the influence of the boundary conditions, the computational domain extended for a length of 3 and 4 valve diameters downstream and upstream, respectively. The results are shown in Figure 4, Figure 5 and Figure 6. On the left hand side of each figure, simulations of the computer-designed micro-valve are shown while on the right hand side simulations of the fabricated one.
is the shear rate and n = 0.785 the non-Newtonian index [14]. At the inlet of the domain, a fully developed velocity profile of 1 cm/s was considered. At the outlet, a free flow outlet condition with null pressure was considered, while no-slip condition imposed at the solid surfaces. In order to avoid the influence of the boundary conditions, the computational domain extended for a length of 3 and 4 valve diameters downstream and upstream, respectively. The results are shown in Figure 4, Figure 5 and Figure 6. On the left hand side of each figure, simulations of the computer-designed micro-valve are shown while on the right hand side simulations of the fabricated one.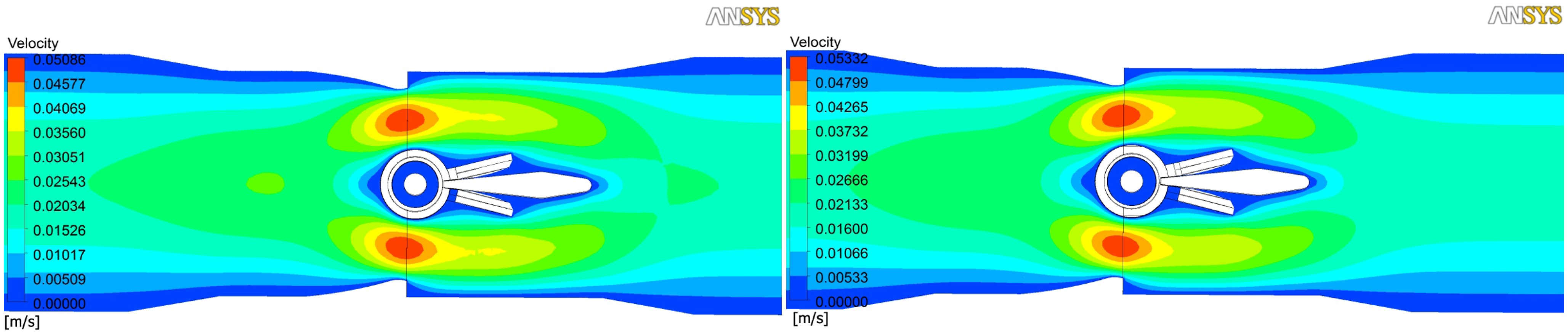


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malinauskas, M.; Farsari, M.; Piskarskas, A.; Juodkazis, S. Ultrafast laser nanostructuring of photopolymers: A decade of advances. Phys. Rep. 2013, 533, 1–31. [Google Scholar] [CrossRef]
- Xu, B.B.; Zhang, Y.L.; Xia, H.; Dong, W.F.; Ding, H.; Sun, H.B. Fabrication and multifunction integration of microfluidic chips by femtosecond laser direct writing. Lab Chip 2013, 13, 1677–1690. [Google Scholar] [CrossRef]
- Fischer, J.; Wegener, M. Three-dimensional optical laser lithography beyond the diffraction limit. Laser Photonics Rev. 2013, 7, 22–44. [Google Scholar] [CrossRef]
- Vasilantonakis, N.; Terzaki, K.; Sakellari, I.; Purlys, V.; Gray, D.; Soukoulis, C. M.; Vamvakaki, M.; Kafesaki, M.; Farsari, M. Three-Dimensional Metallic Photonic Crystals with Optical Bandgaps. Adv. Mater. 2012, 24, 1101–1105. [Google Scholar] [CrossRef]
- Olsen, M.H.; Hjorto, G.M.; Hansen, M.; Met, O.; Svane, I.M.; Larsen, N.B. In-chip fabrication of free-form 3D constructs for directed cell migration analysis. Lab Chip 2013, 13, 4800–4809. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Mironov, V.; Stampfl, J.; Liska, R. Engineering 3D cell-culture matrices: Multiphoton processing technologies for biological and tissue engineering applications. Expert Rev. Med. Devices 2012, 9, 613–633. [Google Scholar] [CrossRef]
- Raimondi, M.T.; Eaton, S.M.; Lagana, M.; Aprile, V.; Nava, M.M.; Cerullo, G.; Osellame, R. Three-dimensional structural niches engineered via two-photon laser polymerization promote stem cell homing. Acta Biomater. 2013, 9, 4579–4584. [Google Scholar] [CrossRef]
- Terzaki, K.; Kalloudi, E.; Mossou, E.; Mitchell, E.P.; Forsyth, V.T.; Rosseeva, E.; Simon, P.; Vamvakaki, M.; Chatzinikolaidou, M.; Mitraki, A.; et al. Mineralized self-assembled peptides on 3D laser-made scaffolds: A new route toward ʻscaffold on scaffoldʼ hard tissue engineering. Biofabrication 2013, 5, 045002. [Google Scholar] [CrossRef]
- Groen, M.S.; Brouwer, D.M.; Wiegerink, R.J.; Lotters, J.C. Design Considerations for a Micromachined Proportional Control Valve. Micromachines 2012, 3, 396–412. [Google Scholar] [CrossRef]
- Schizas, C.; Melissinaki, V.; Gaidukeviciute, A.; Reinhardt, C.; Ohrt, C.; Dedoussis, V.; Chichkov, B.N.; Fotakis, C.; Farsari, M.; Karalekas, D. On the design and fabrication by two-photon polymerization of a readily assembled micro-valve. Int. J. Adv. Manuf. Technol. 2010, 48, 435–441. [Google Scholar] [CrossRef]
- Danilevicius, P.; Rekstyte, S.; Balciunas, E.; Kraniauskas, A.; Jarasiene, R.; Sirmenis, R.; Baltriukiene, D.; Bukelskiene, V.; Gadonas, R.; Malinauskas, M. Micro-structured polymer scaffolds fabricated by direct laser writing for tissue engineering. J. Biomed. Opt. 2012, 17, 081405-1. [Google Scholar] [CrossRef]
- Seidlits, S.K.; Schmidt, C.E.; Shear, J.B. High-resolution patterning of hydrogels in three dimensions using direct-write photofabrication for cell guidance. Adv. Funct. Mater. 2009, 19, 3543–3551. [Google Scholar] [CrossRef]
- Sakellari, I.; Kabouraki, E.; Gray, D.; Purlys, V.; Fotakis, C.; Pikulin, A.; Bityurin, N.; Vamvakaki, M.; Farsari, M. Diffusion-Assisted High Resolution Direct Femtosecond Laser Writing. ACS Nano 2012, 6, 2302–2311. [Google Scholar] [CrossRef]
- Qing, X.; Damodaran, M. Computational modelling of non-Newtonian effects on flow in channels with moving wall indentations. Int. J. Comput. Fluid Dyn. 2001, 15, 329–336. [Google Scholar] [CrossRef]
- Juodkazis, S.; Mizeikis, V.; Seet, K.K.; Misawa, H.; Wegst, U.G.K. Mechanical properties and tuning of three-dimensional polymeric photonic crystals. Appl. Phys. Lett. 2007, 91, 241904. [Google Scholar] [CrossRef]
- Skarmoutsou, A.; Lolas, G.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M.; Farsari, M. Nanomechanical properties of hybrid coatings for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2013, 25, 48–62. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Galanopoulos, S.; Chatzidai, N.; Melissinaki, V.; Selimis, A.; Schizas, C.; Farsari, M.; Karalekas, D. Design, Fabrication and Computational Characterization of a 3D Micro-Valve Built by Multi-Photon Polymerization. Micromachines 2014, 5, 505-514. https://doi.org/10.3390/mi5030505
Galanopoulos S, Chatzidai N, Melissinaki V, Selimis A, Schizas C, Farsari M, Karalekas D. Design, Fabrication and Computational Characterization of a 3D Micro-Valve Built by Multi-Photon Polymerization. Micromachines. 2014; 5(3):505-514. https://doi.org/10.3390/mi5030505
Chicago/Turabian StyleGalanopoulos, Stratos, Nikoleta Chatzidai, Vasileia Melissinaki, Alexandros Selimis, Charalampos Schizas, Maria Farsari, and Dimitris Karalekas. 2014. "Design, Fabrication and Computational Characterization of a 3D Micro-Valve Built by Multi-Photon Polymerization" Micromachines 5, no. 3: 505-514. https://doi.org/10.3390/mi5030505



