A Contact Angle Study of the Interaction between Embedded Amphiphilic Molecules and the PDMS Matrix in an Aqueous Environment
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Master Preparation and PDMS Replica
2.3. Nuclear Magnetic Resonance Spectroscopy
2.4. Contact Angle Measurement
3. Results and Discussion
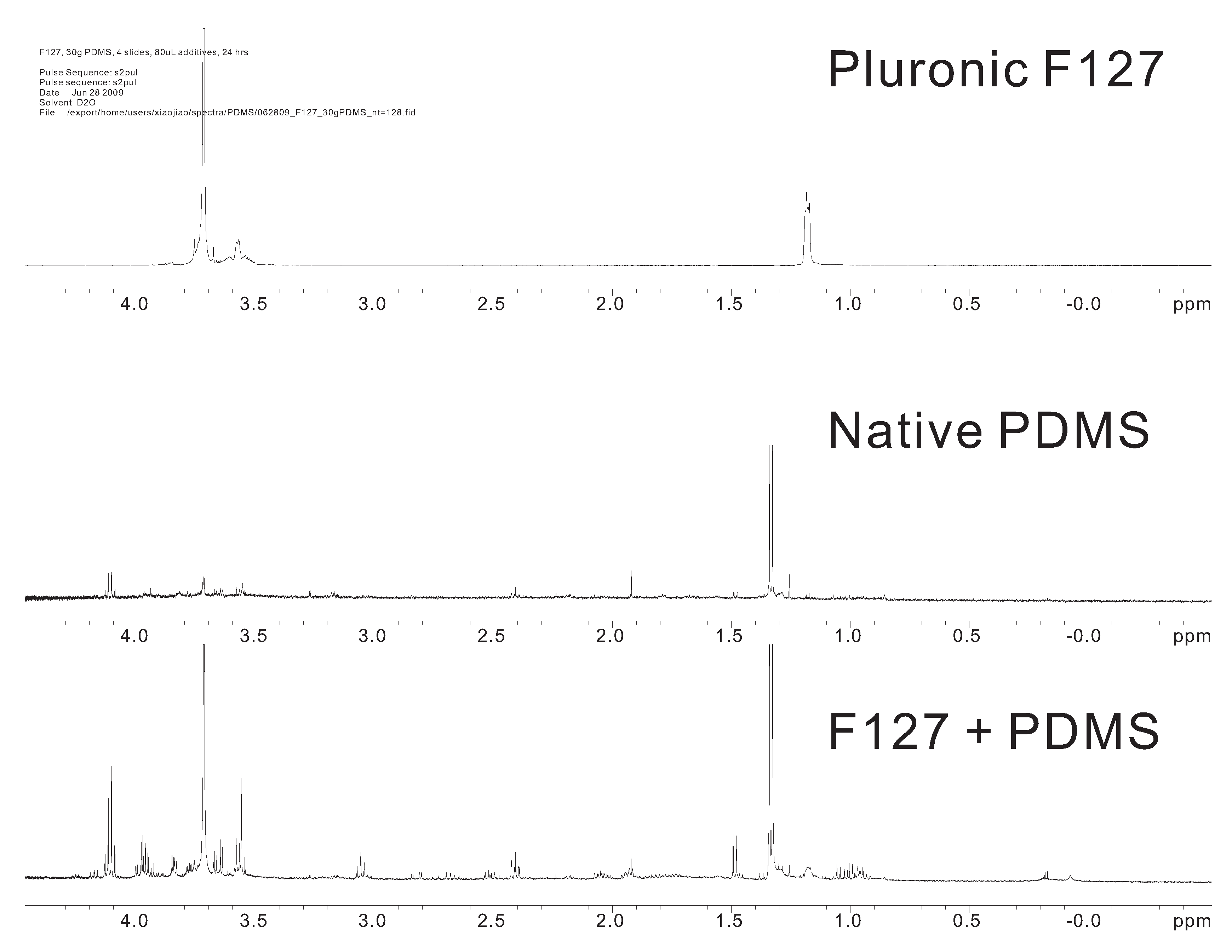
3.1. NMR Study of the Extracted Substance in the Immersed Water
3.2. Master Preparation and PDMS Replica
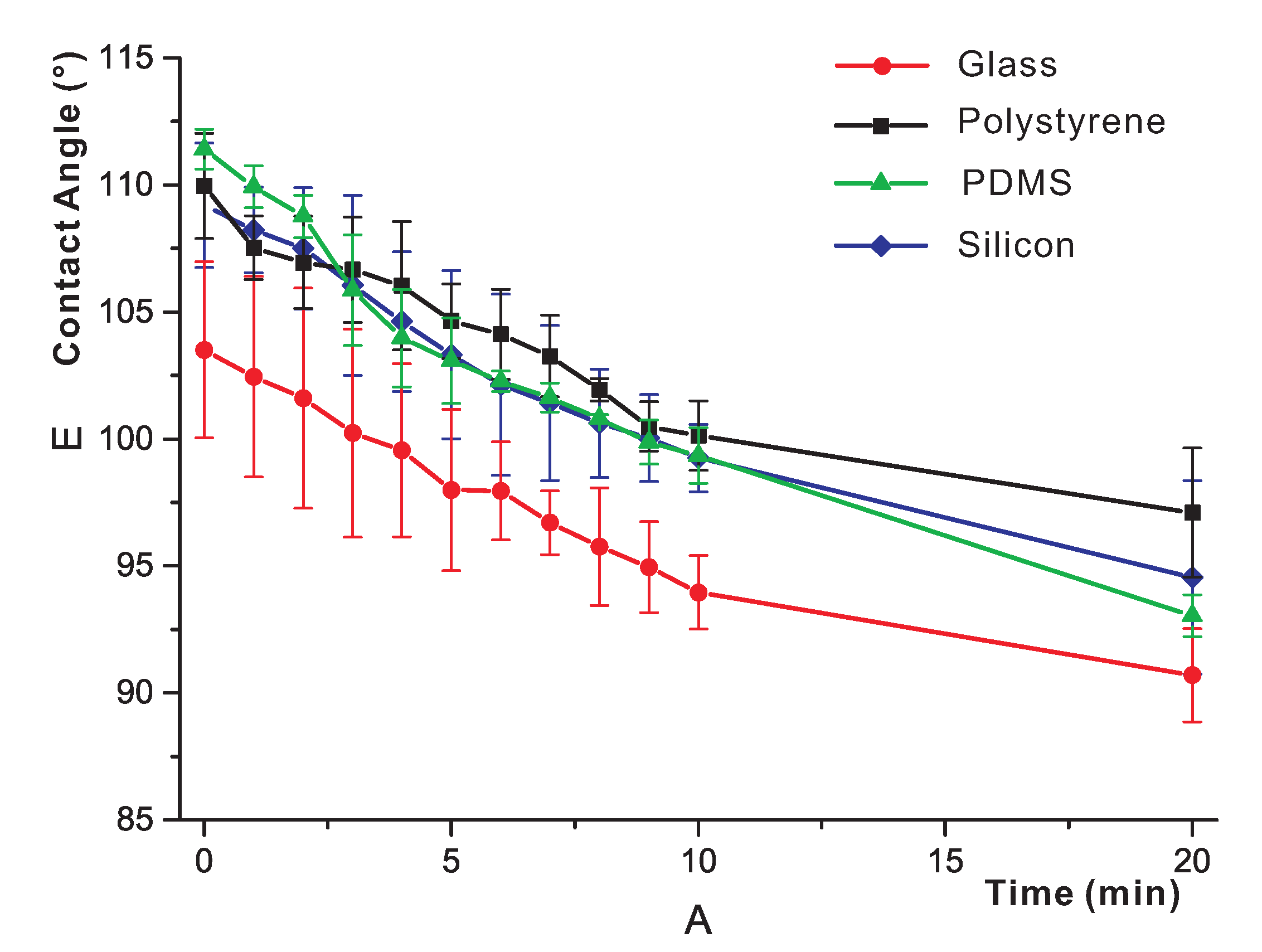
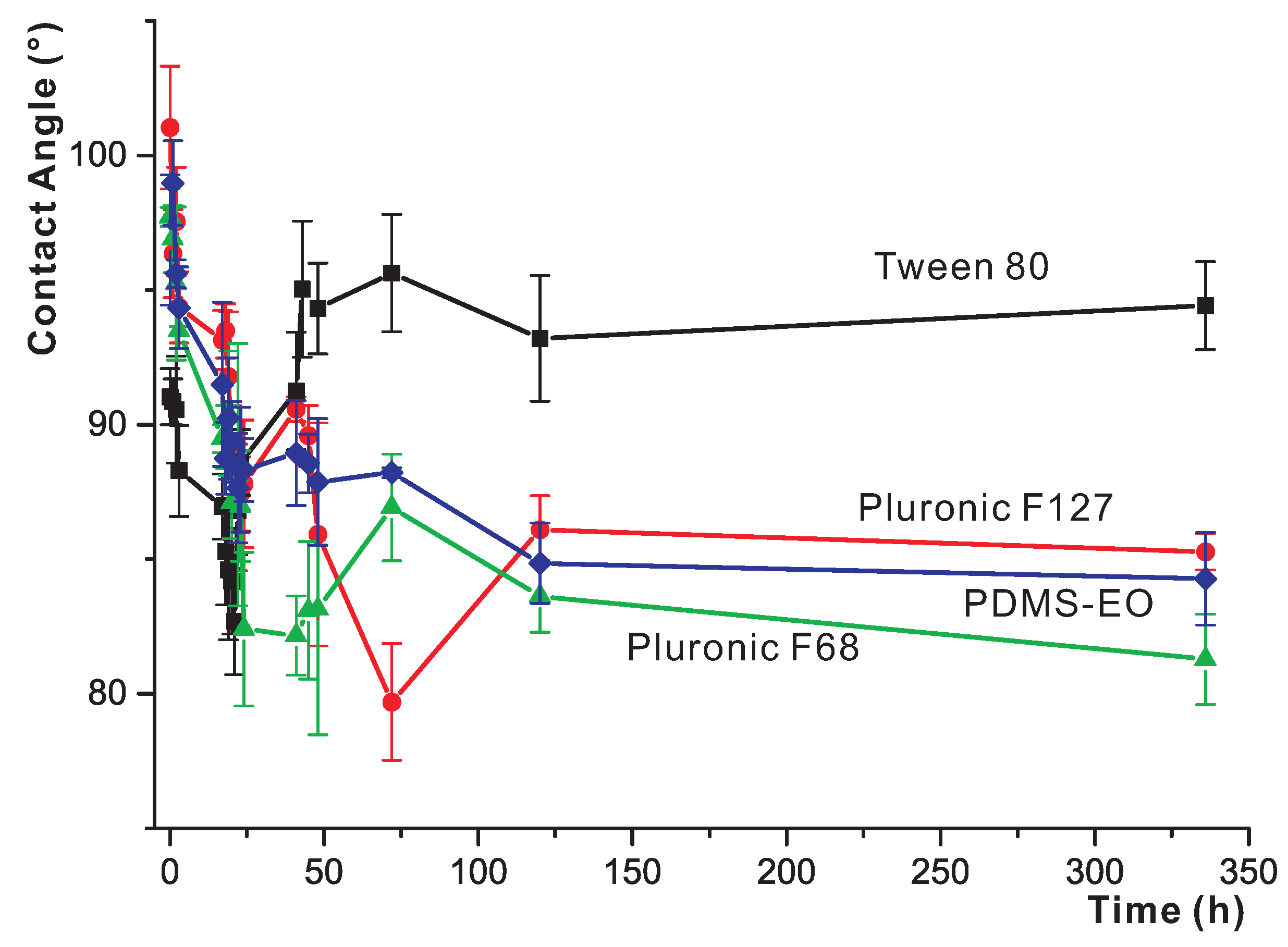
3.3. Short-Term Study of the Effects of Water Exposure
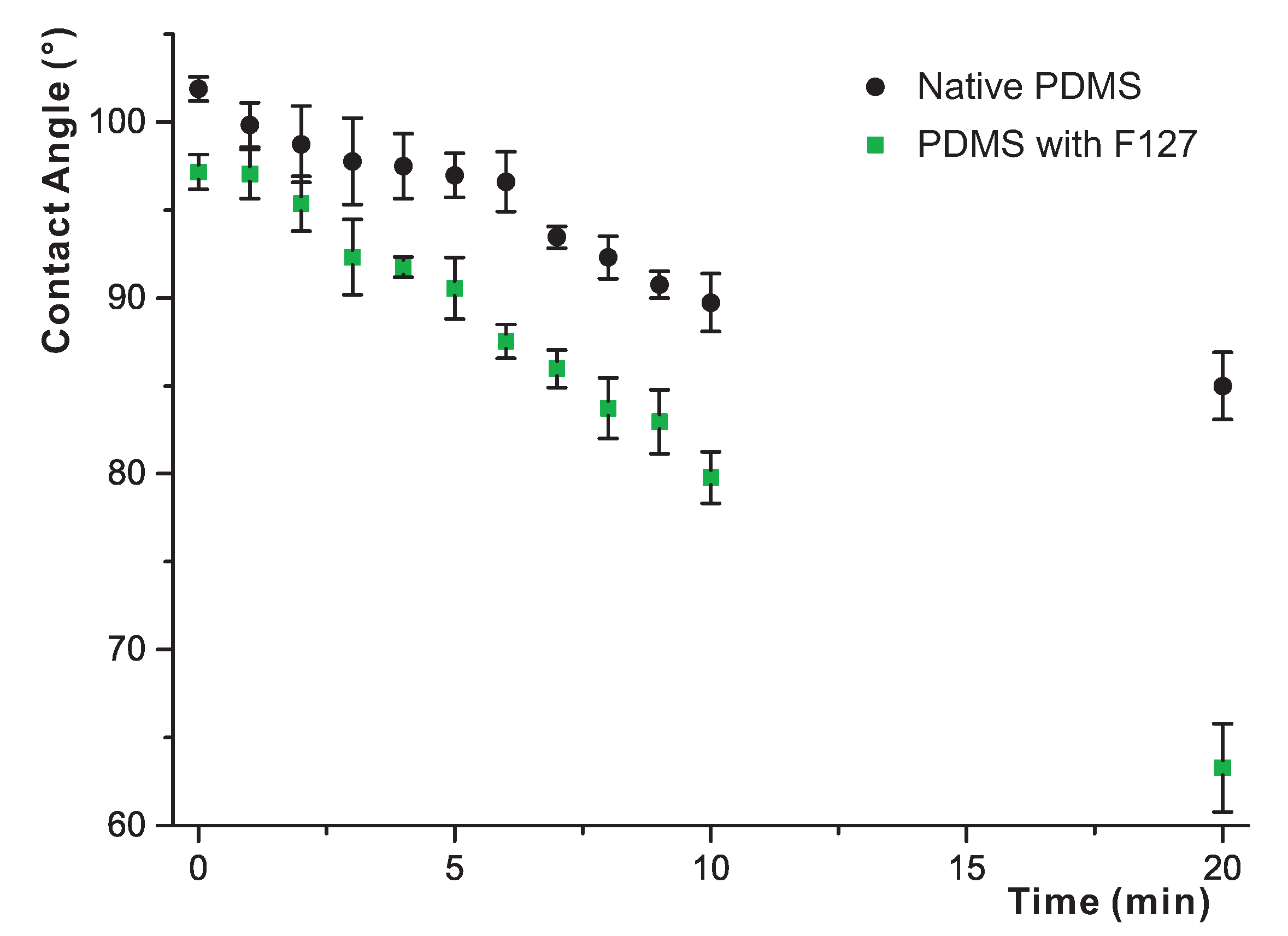
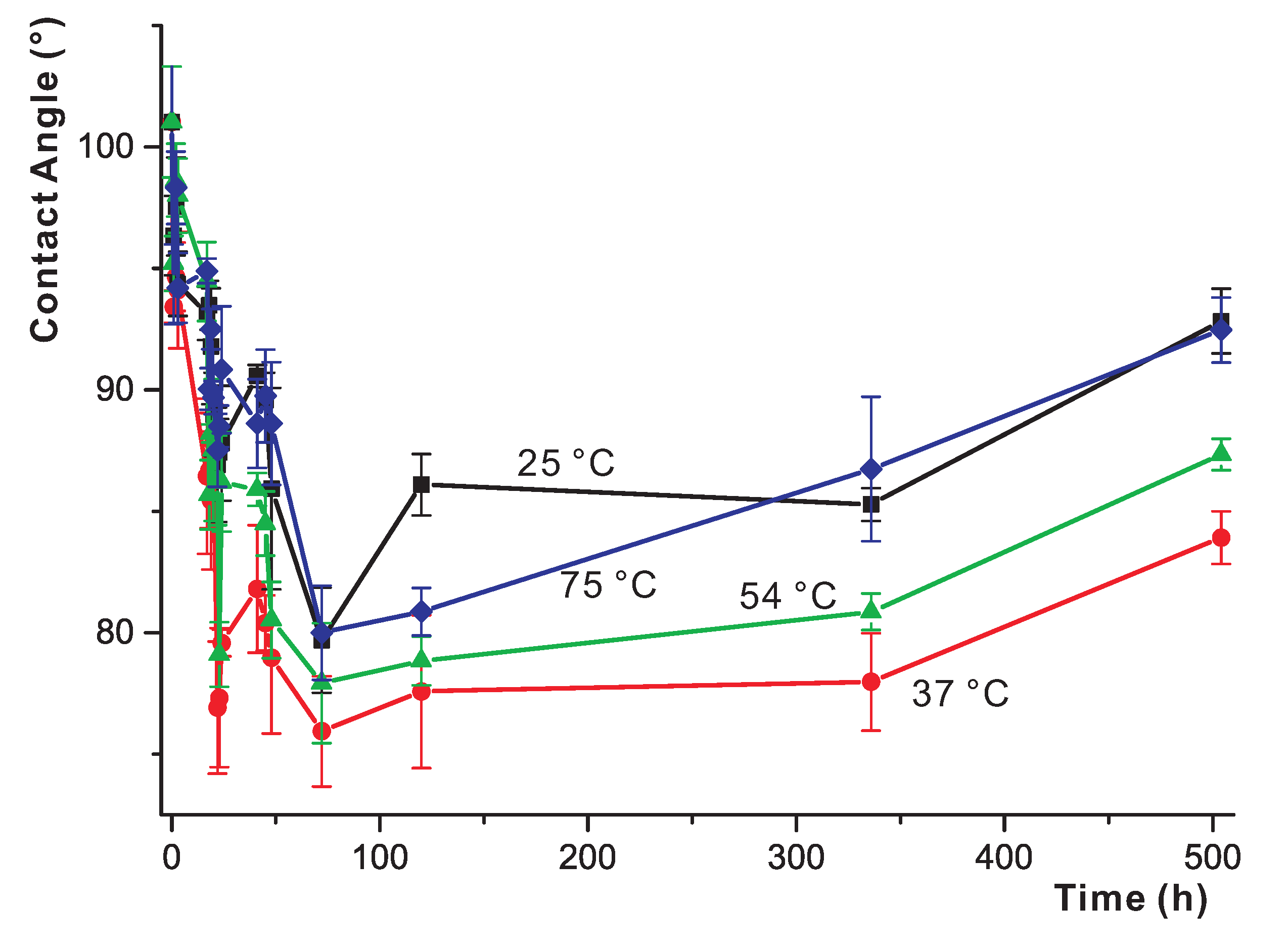
3.4. Long-Term Study of the Effects of Water Exposure

| Amphiphilic Molecules | Chemical Structure | Molecular Weight (Mw) |
|---|---|---|
| Pluronic F127 | (PEO)100–(PPO)65–(PEO)100 | ~12,600 |
| Pluronic F68 | (PEO)78–(PPO)30–(PEO)78 | ~8,400 |
| PDMS-EO (AB111108) | CH3[Si(CH3)2O]n–(C2H5O)mH | 5,000–6,000 |
| Tween80 | 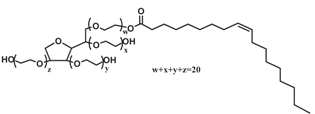 | 1,310 |

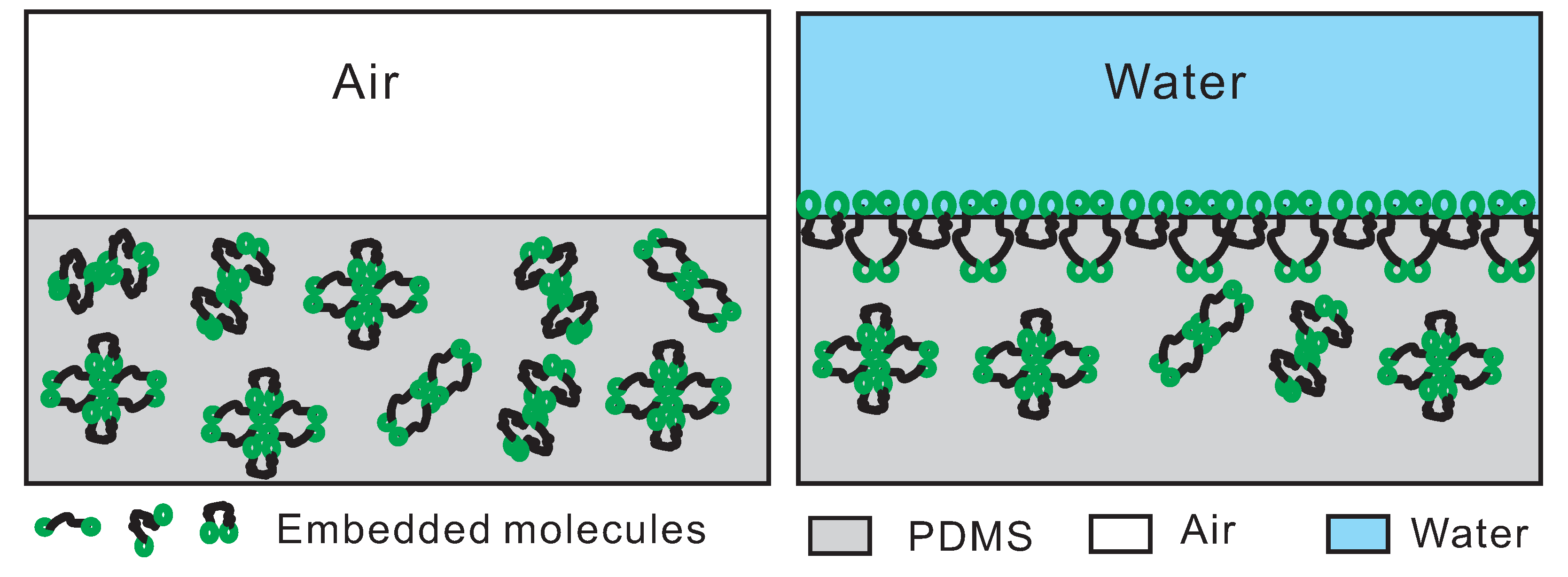
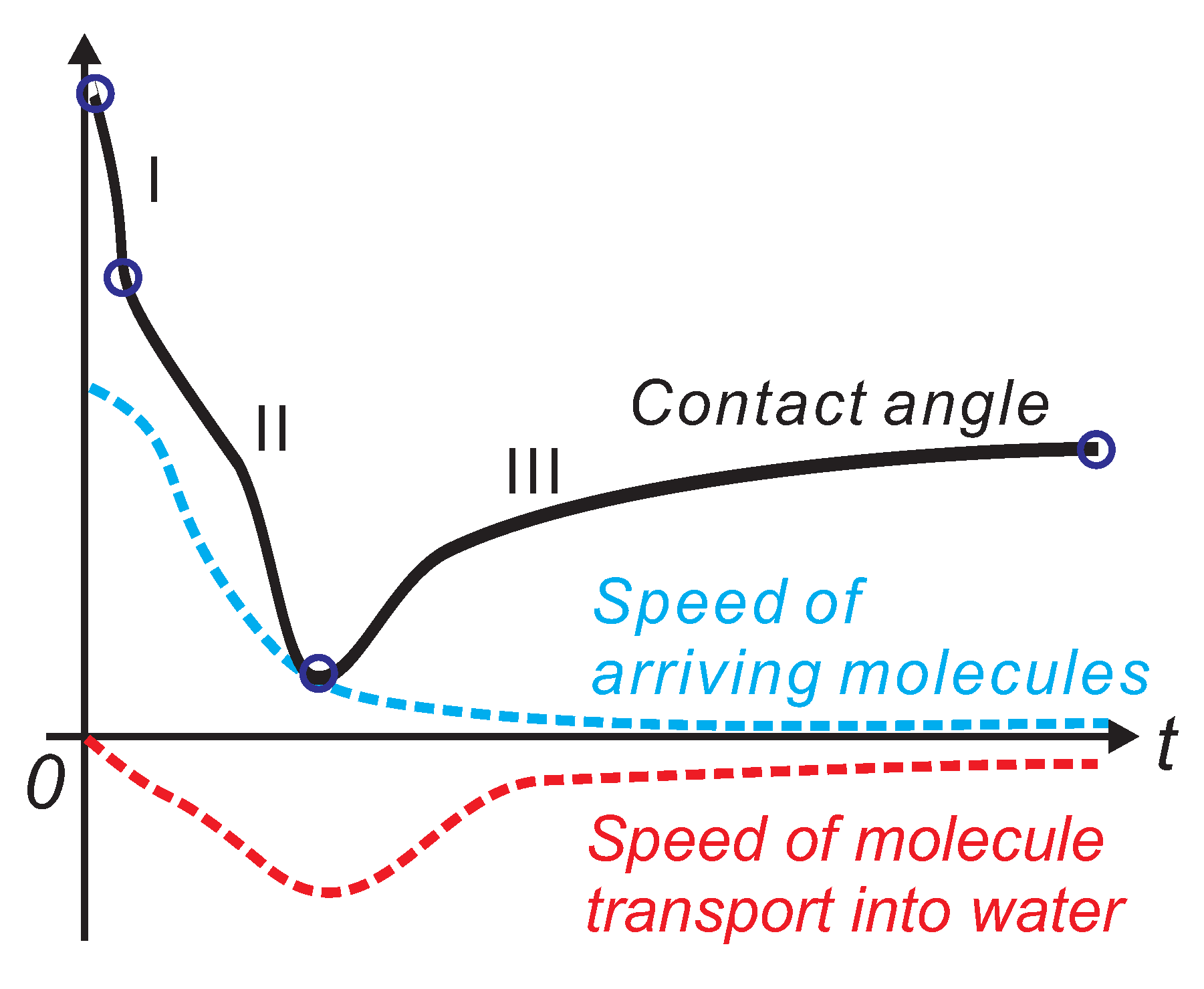
3.5. Interpretation of the Interactions between the Embedded Amphiphilic Molecules and PDMS Matrix
4. Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Makamba, H.; Kim, J.H.; Lim, K.; Park, N.; Hahn, J.H. Surface modification of poly(dimethylsiloxane) microchannels. Electrophoresis 2003, 24, 3607–3619. [Google Scholar] [CrossRef]
- Wong, I.; Ho, C.H. Surface molecular property modifications for poly(dimethylsiloxane) (PDMS) based microfluidic devices. Microfluid. Nanofluidics 2009, 7, 291–306. [Google Scholar] [CrossRef]
- Zhou, J.W.; Ellis, A.V.; Voelcker, N.H. Surface modification for PDMS-based microfluidic devices. Electrophoresis 2010, 33, 89–104. [Google Scholar]
- Mukhopadhyay, R. When PDMS isn’t the best. Anal. Chem. 2007, 79, 3248–3253. [Google Scholar] [CrossRef]
- Hillborg, H.; Ankner, J.F.; Gedde, U.W.; Smith, G.D.; Yasuda, H.K.; Wikstrom, K. Crosslinked polydimethylsiloxane exposed to oxygen plasma studied by neutron reflectometry and other surface specific techniques. Polymer 2000, 41, 6851–6863. [Google Scholar]
- Wu, Z.G.; Hjort, K.; Wicher, G.; Svenningsen, A.F. Microfluidic high viability neural cell separation using viscoelastically tuned hydrodynamic spreading. Biomed. Microdevices 2008, 10, 631–638. [Google Scholar] [CrossRef]
- Hedlund, E.; Pruszak, J.; Ferree, A.; Vinuela, A.; Hong, S.; Isacson, O.; Kim, K.S. Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells 2007, 25, 1126–1135. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly(dimethylsiloxane) based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly(ethylene oxide) poly(propylene oxide) poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modelling. Colloids Surface 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Singh-Joy, S.D.; McLain, V.C. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int. J. Toxicol. 2008, 27, 93–128. [Google Scholar] [CrossRef]
- Li, J.T.; Carlsson, J.; Huang, S.C.; Caldwell, K.D. Adsorption of Poly(ethylene oxide)-Containing Block Copolymers a Route to Protein Resistance. In Hydrophilic Polymers; Glass, J.E., Ed.; American Chemical Society: Washington, DC, USA, 1996; pp. 61–78. [Google Scholar]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; de Gennes, P.G. Protein—Surface interactions in the presence of polyethylene oxide: I. Simplified theory. J. Colloid Interface Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Jeon, S.I.; Andrade, J.D. Protein—Surface interactions in the presence of polyethylene oxide: II. Effect of protein size. J. Colloid Interface Sci. 1991, 142, 159–166. [Google Scholar] [CrossRef]
- Ottenbrite, R. Frontiers in Biomedical Polymer Applications; Technomic Publishing Co.: Lancaster, UK, 1999. [Google Scholar]
- Kabanova, A.V.; Alakhovb, V.Y. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J. Control. Release 2002, 82, 189–212. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Alakhovb, V.Y. Pluronic block copolymers evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 2009, 130, 98–106. [Google Scholar] [CrossRef]
- Nagant, C.; Savage, P.B.; Dehaye, J.P. Effect of pluronic acid F-127 on the toxicity towards eukaryotic cells of CSA-13, a cationic steroid analogue of antimicrobial peptides. J. Appl. Microbiol. 2012, 112, 1173–1183. [Google Scholar] [CrossRef]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a cell encapsulation material: utilization of membrane-stabilizing agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef]
- Luk, V.N.; Mo, G.C.H.; Wheeler, A.R. Pluronic additives: A solution to sticky problems in digital microfluidics. Langmuir 2008, 24, 6382–6389. [Google Scholar] [CrossRef]
- Wu, Z.G.; Hjort, K. Surface modification of PDMS by gradient-induced migration of embedded Pluronic. Lab Chip 2009, 9, 1500–1503. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, G.; Gao, Y.; An, Y. Surface wettability of (3-aminopropyl)triethoxysilane self-assembled monolayers. J. Phys. Chem. B 2010, 115, 450–454. [Google Scholar]
- Hjerten, S. High-performance electrophoresis: Elimination of electroendosmosis and solute adsorption. J. Chromatogr. A 1985, 347, 191–198. [Google Scholar] [CrossRef]
- Li, J.T.; Caldwell, K.D.; Rapoport, N. Surface properties of pluronic-coated polymeric colloids. Langmuir 1994, 10, 4475–4482. [Google Scholar] [CrossRef]
- Thompson, D.B.; Fawcett, A.S.; Brook, M.A. Simple Strategies to Manipulate Hydrophilic Domains in Silicones. In Silicon Based Polymers Advances in Synthesis and Supramolecular Organization; Ganachaud, F., Boileau, S., Bourt, B., Eds.; Springer: Amsterdam, The Nethrelands, 2008; pp. 29–38. [Google Scholar]
- Myers, D. Surfactant Science and Technology, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qiu, W.; Sun, X.; Wu, C.; Hjort, K.; Wu, Z. A Contact Angle Study of the Interaction between Embedded Amphiphilic Molecules and the PDMS Matrix in an Aqueous Environment. Micromachines 2014, 5, 515-527. https://doi.org/10.3390/mi5030515
Qiu W, Sun X, Wu C, Hjort K, Wu Z. A Contact Angle Study of the Interaction between Embedded Amphiphilic Molecules and the PDMS Matrix in an Aqueous Environment. Micromachines. 2014; 5(3):515-527. https://doi.org/10.3390/mi5030515
Chicago/Turabian StyleQiu, Wenjun, Xiaojiao Sun, Chaoqun Wu, Klas Hjort, and Zhigang Wu. 2014. "A Contact Angle Study of the Interaction between Embedded Amphiphilic Molecules and the PDMS Matrix in an Aqueous Environment" Micromachines 5, no. 3: 515-527. https://doi.org/10.3390/mi5030515




