Molecular Characterization of Three Novel Phospholipase A2 Proteins from the Venom of Atheris chlorechis, Atheris nitschei and Atheris squamigera
Abstract
:1. Introduction
2. Results
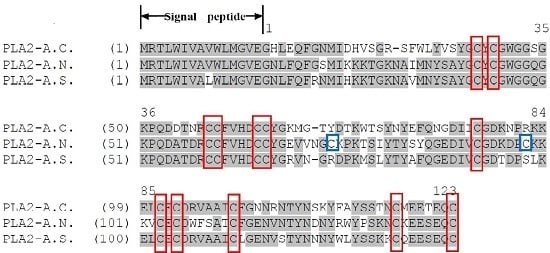
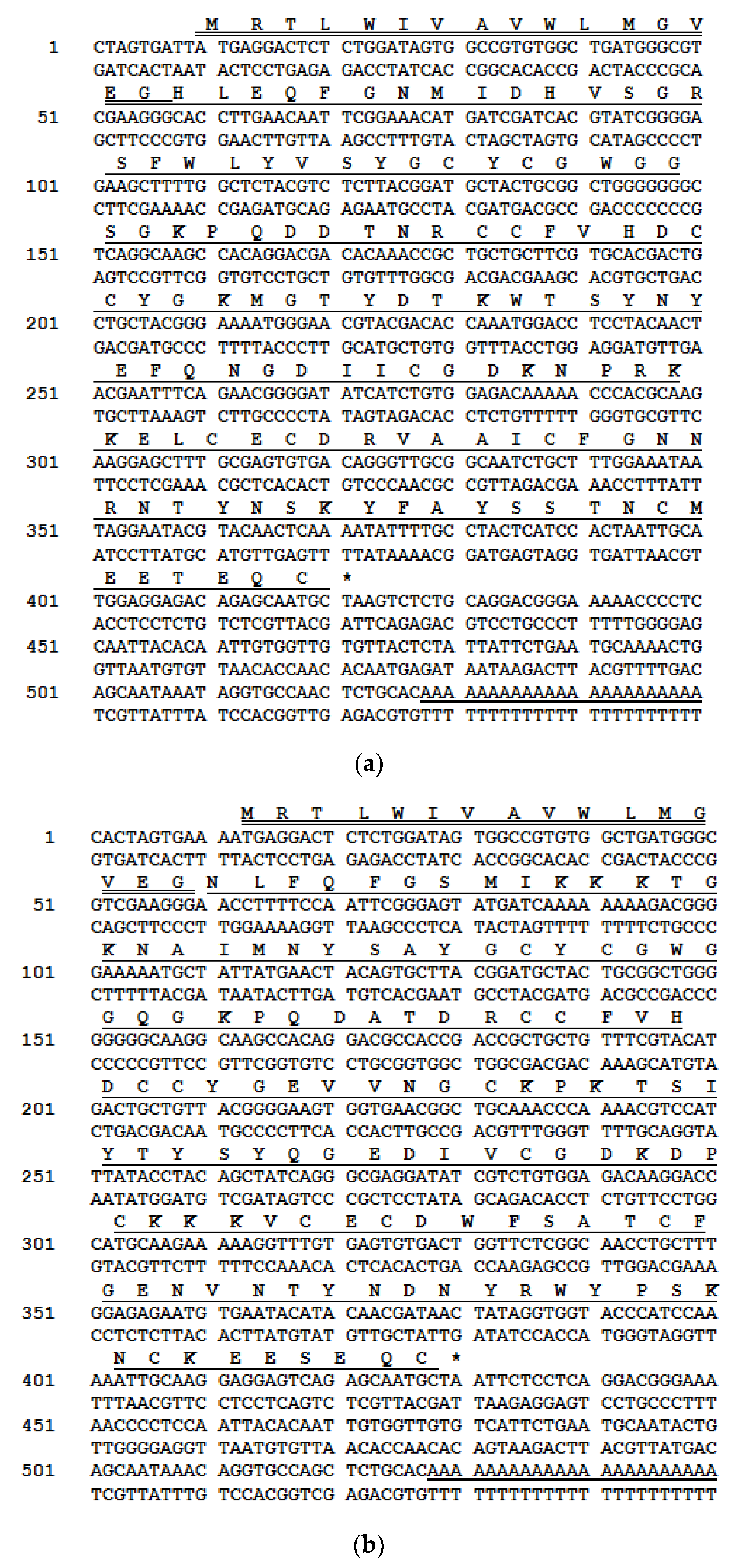
2.1. cDNAs Encoding PLA2 Precursors and Bioinformatic Analyses
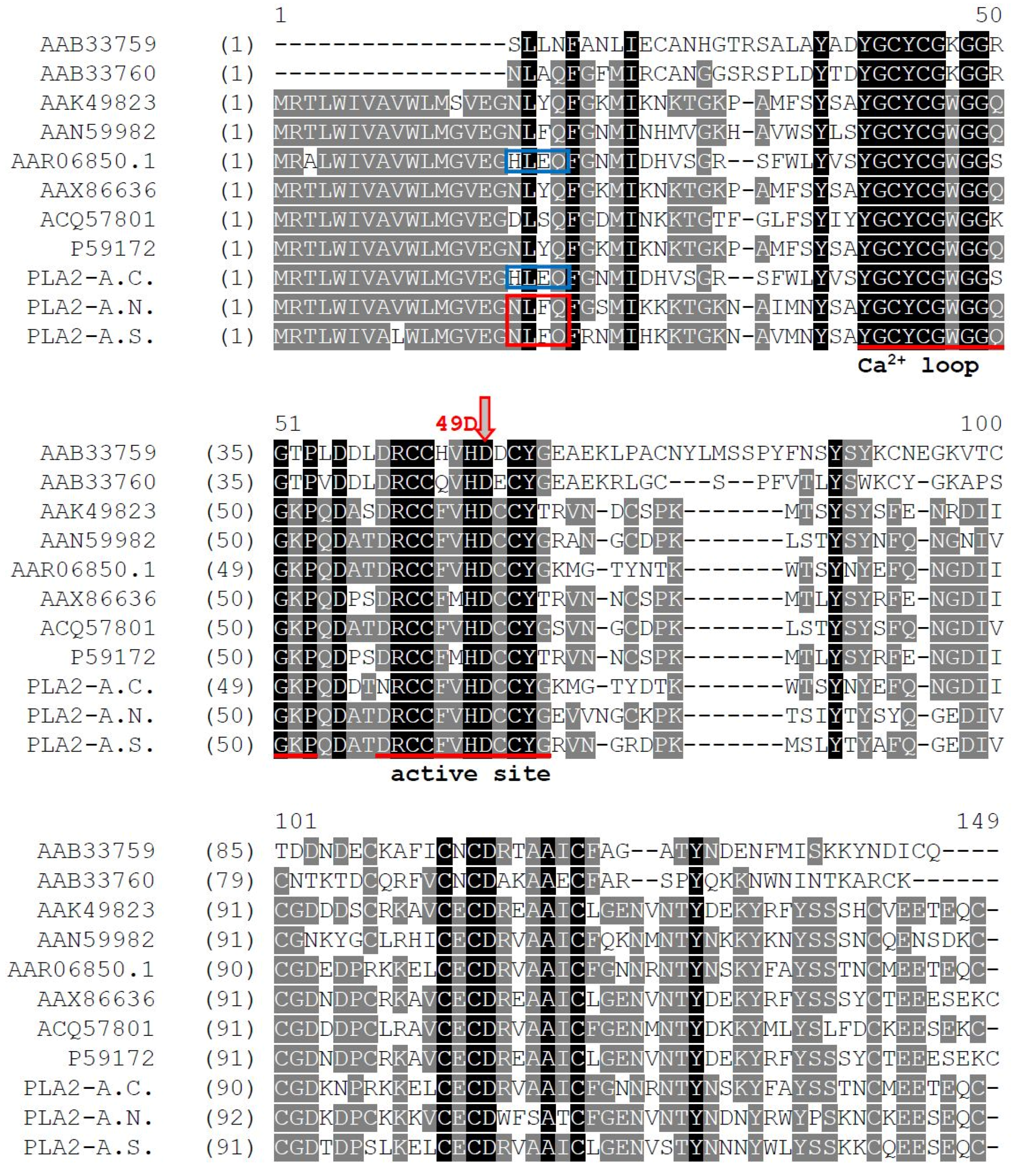
2.2. Identification and Structural Analysis of PLA2 Proteins
3. Discussion
4. Experimental
4.1. Materials
4.2. Molecular Cloning of the Phospholipase A2 Precursor-Encoding cDNAs
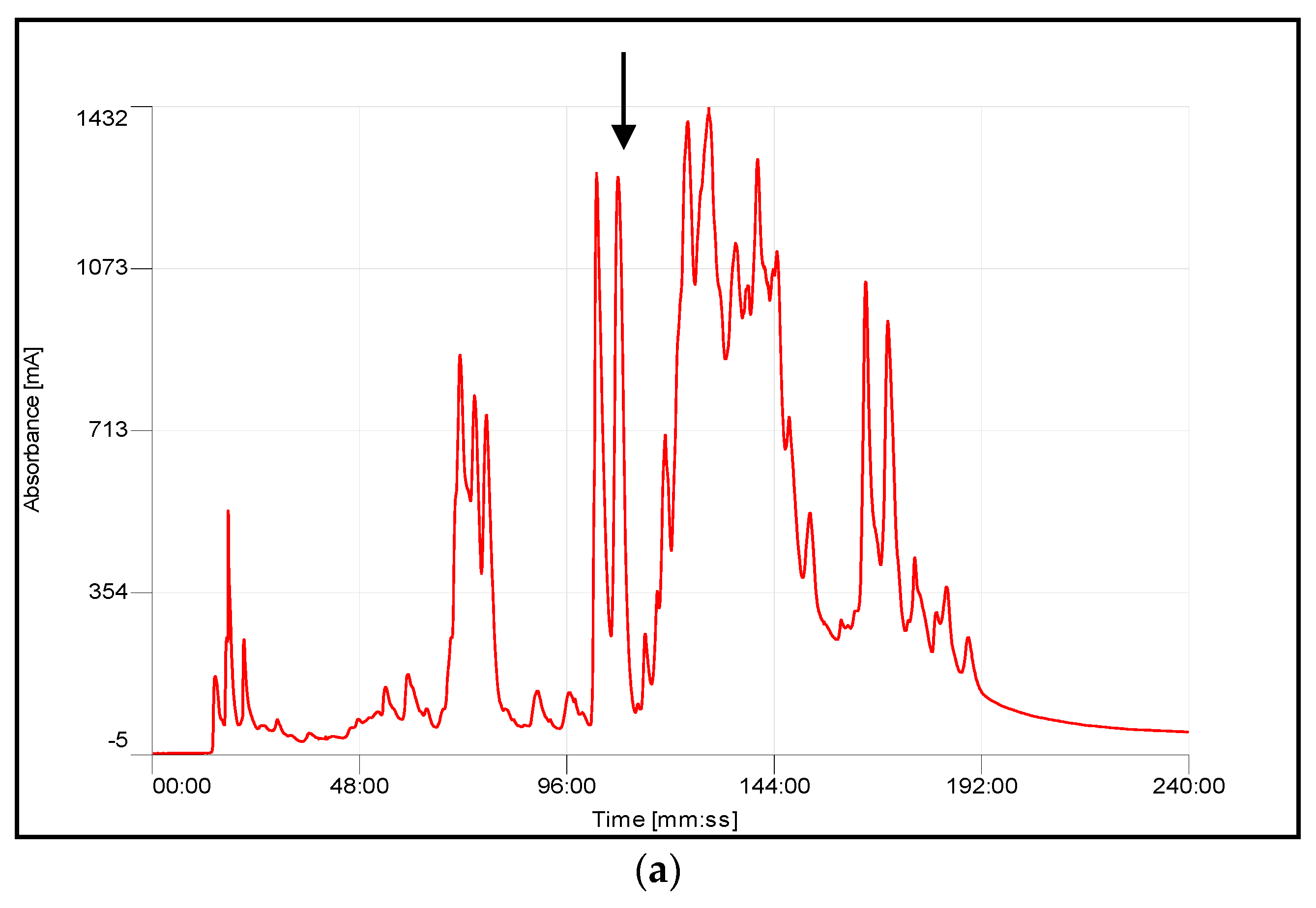
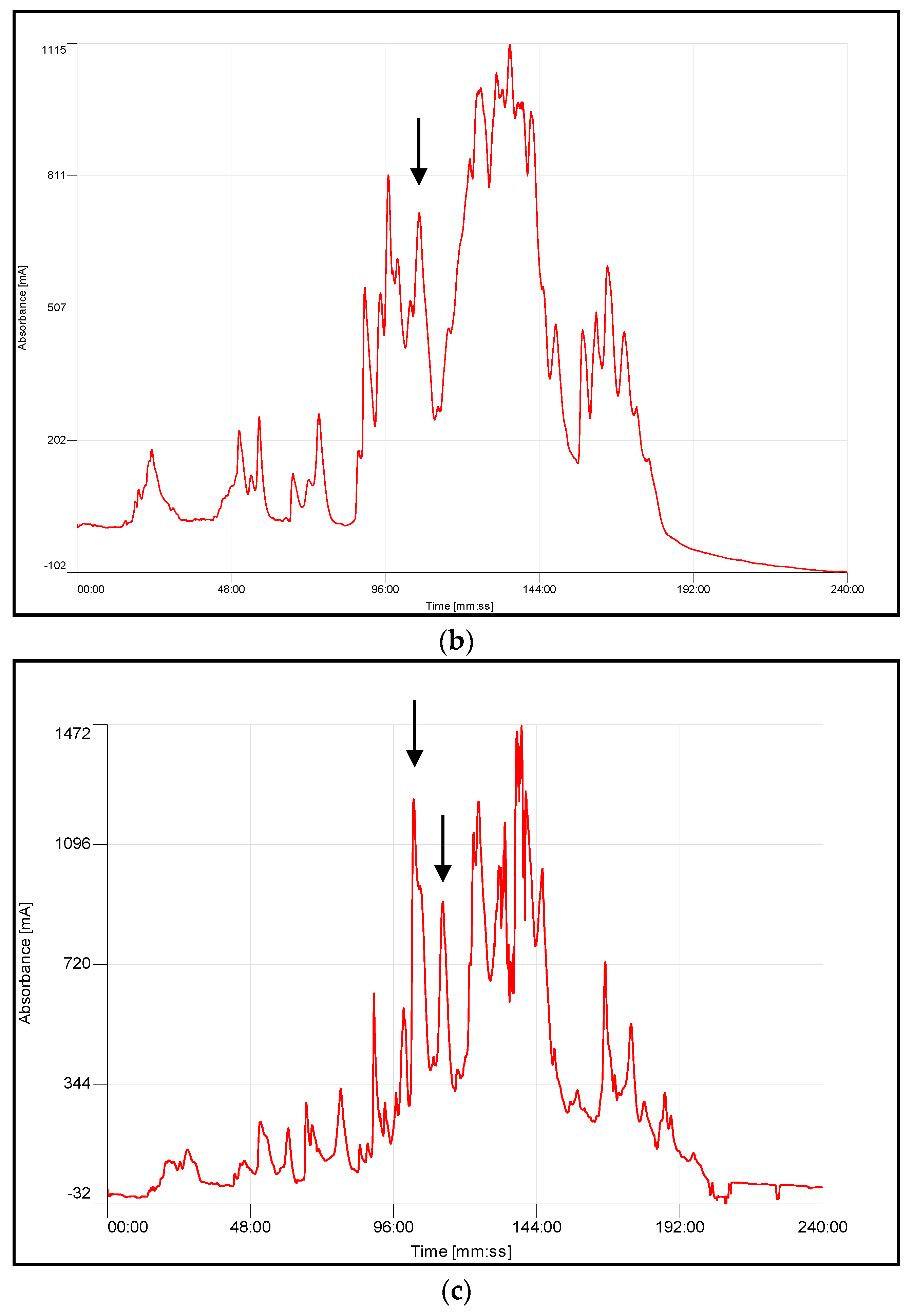
4.3. Chromatographic Fractionation and Activity Determination
4.4. Identification and Structural Investigations
4.5. Ethical Statement
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dennis, E.A. Diversity of group types, regulation, and function of phospholipase A2. J. Biol. Chem. 1994, 269, 13057–13060. [Google Scholar] [PubMed]
- Six, D.A.; Dennis, E.A. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta 2000, 1488, 1–19. [Google Scholar] [CrossRef]
- Dufton, M.J.; Hider, R.C. Classification of phospholipases A2 according to sequence. Evolutionary and pharmacological implications. Eur. J. Biochem. 1983, 137, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Botes, D.P.; Viljoen, C.C. Bitis gabonica venom. The amino acid sequence of phospholipase A. J. Biol. Chem. 1974, 249, 3827–3835. [Google Scholar] [PubMed]
- Buckland, A.G.; Wilton, D.C. The antibacterial properties of secreted phospholipases A2. Biochim. Biophys. Acta 2000, 1488, 71–82. [Google Scholar] [CrossRef]
- Jan, V.M.; Guillemin, I.; Robbe-Vincent, A.; Choumet, V. Phospholipase A2 diversity and polymorphism in European viper venoms: Paradoxical molecular evolution in Viperinae. Toxicon 2007, 50, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Leiguez, E.; Giannotti, K.C.; Moreira, V.; Matsubara, M.H.; Gutiérrez, J.M.; Lomonte, B.; Rodríguez, J.P.; Balsinde, J.; Teixeira, C. Critical role of TLR2 and Myd88 for functional response of macrophages to a group IIa-secreted phospholipase A2 from snake venom. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.; Mukherjee, A.K. Anticoagulant and membrane damaging properties of snake venom phospholipase A2 enzymes. Toxinology 2015. [Google Scholar] [CrossRef]
- Sudarshan, S.; Dhananjaya, B.L. The antimicrobial activity of an acidic phospholipase A2 (NN-XIa-PLA2) from the venom of Naja naja naja (Indian Cobra). Appl. Biochem. Biotechnol. 2015, 176, 2027–2038. [Google Scholar] [CrossRef] [PubMed]
- Adamich, M.; Dennis, E.A. Specificity reversal in phospholipase A2 hydrolysis of lipid mixtures. Biochem. Biophys. Res. Commun. 1987, 80, 424–428. [Google Scholar] [CrossRef]
- Berger, M.; Santi, L.; Beysdasilva-da-Silva, W.O.; Oliveira, F.M.S.; Caliari, M.V.; Yates, J.R.Y., III; Ribeiro Vieira, M.A.; Guimarães, J.A. Mechanisms of acute kidney injury induced by experimental Lonomia obliqua envenomation. Arch. Toxicol. 2015, 89, 459–483. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A. South African Snake Venoms and Antivenoms; South African Institute for Medical Research: Johannesburg, South African, 1955. [Google Scholar]
- Branch, W.R.; Haagner, G.V.; Morgan, D.R.; Lanoie, L.O. Venoms and snakebite. J. Herpetol. Assoc. Afr. 1991, 39, 28–29. [Google Scholar] [CrossRef]
- Favreau, P.; Chenval, O.; Menin, L.; Michalet, S.; Gerner, H.; Principaud, F.; Thai, R.; Stöcklin, R. The venom of the snake genus Atheris contains a new class of peptides with clusters of histidine and glycine residues. Rapid 2007, 21, 406–412. [Google Scholar]
- Mebs, D.; Holada, K.; Kornalik, F.; Simak, J. Severe coagulopathy after a bite of a green bush viper (Atheris squamiger): Severe coagulopathy after a bite case report and biochemical analysis of the venom. Toxicon 1998, 36, 1333–1340. [Google Scholar] [CrossRef]
- Top, L.J.; Tulleken, J.E.; Ligtenberg, J.J.; Meertens, J.H.; van de Werf, T.S.; Zijlstra, J.G. Serious envenomation after a snakebite by a Western bush viper (Atheris chlorechis) in The Netherlands: A case report. Neth. J. Med. 2006, 64, 153–156. [Google Scholar] [PubMed]
- Valenta, J.; Stach, Z.; Fricova, D.; Zak, J.; Balik, M. Envenoming by the viperid snake Proatheris superciliaris: A case report. Toxicon 2008, 52, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D.; Fach, A.; Herrmann, H.W. Enzyme analysis of Atheris snake venom. Toxicon 1997, 35, 813. [Google Scholar] [CrossRef]
- He, W.; Chen, X.; Lei, W.; Wei, C.; Mei, Z.; Chen, T.; Shaw, C. Cloning and characterisation of three novel disintegrin precursors from the venoms of three Atheris species: Atheris chlorechis, Atheris nitschei and Atheris squamigera. Toxicon Off. J. Int. Soc. Toxinol. 2013, 71, 31–40. [Google Scholar]
- Francischetti, I.M.B.; My-Pham, V.; Harrison, J.; Garfield, M.K.; Ribeiro, J.M.C. Bitis gabonica (gaboon viper) snake venom gland: Toward a catalog for the full-length transcripts (cDNA) and proteins. Gene 2004, 337, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, N.A.; Ghulikyan, L.; Kishmiryan, A.; Andreeva, T.V.; Utkin, Y.N.; Tsetlin, V.I.; Lomonte, B.; Ayvazyan, N.M. Phospholipases a2 from Viperidae snakes: Differences in membranotropic activity between enzymatically active toxin and its inactive isoforms. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Valentin, E.; Lambeau, G. What can venom phospholipases A2 tell us about the functional diversity of mammalian secreted phospholipases A2? Biochimie 2000, 82, 815–831. [Google Scholar] [CrossRef]


| Protein | Accession Number | Organism | Identities | Group |
|---|---|---|---|---|
| PLA2-A.C. | AAR06850.1 | Bitis gabonica | 96% | GIIB |
| PLA2-A.N. | AAK49823.1 | Echis coloratus | 71% | GIIA |
| ACQ57801.1 | Macrovipera lebetina | 71% | GIIA | |
| PLA2-A.S. | AAK49822 | Echis coloratus | 73% | GIIA |
| ACQ57801.1 | Macrovipera lebetina | 72% | GIIA |
| Species | Retention Time (min) | Average Mass Observation | Average Mass Calculation | MS/MS-Derived Sequence |
|---|---|---|---|---|
| A. chlorechis | 103–104 | 13,960.5 Da | 13,964 Da | HLEQFGNMIDHVSGR |
| CCFVHDCCYGK | ||||
| MGTYDTK | ||||
| ELCECDR | ||||
| VAAICFGNNR | ||||
| NTYNSK | ||||
| A. nitschei | 106–109 | 13,975 Da | 13,979 Da | NLFQFGSMIK |
| NAIMNYSAYGCYCGWGGQGKPQDATDR | ||||
| DKDPCK | ||||
| VNTYNDNYR | ||||
| WYPSK | ||||
| A. squamigera | 105–107 | 13,840 Da | 13,841 Da | NLFQFR |
| NMIHK | ||||
| NAVMNYSAYGCYCGWGGQGKPQDATDR | ||||
| 113 | 13,847 Da | NLFQFR CCFVHDCCYGR | ||
| ELCECDR | ||||
| CQEESEQC |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chen, X.; Zhou, M.; Wang, L.; Chen, T.; Shaw, C. Molecular Characterization of Three Novel Phospholipase A2 Proteins from the Venom of Atheris chlorechis, Atheris nitschei and Atheris squamigera. Toxins 2016, 8, 168. https://doi.org/10.3390/toxins8060168
Wang H, Chen X, Zhou M, Wang L, Chen T, Shaw C. Molecular Characterization of Three Novel Phospholipase A2 Proteins from the Venom of Atheris chlorechis, Atheris nitschei and Atheris squamigera. Toxins. 2016; 8(6):168. https://doi.org/10.3390/toxins8060168
Chicago/Turabian StyleWang, He, Xiaole Chen, Mei Zhou, Lei Wang, Tianbao Chen, and Chris Shaw. 2016. "Molecular Characterization of Three Novel Phospholipase A2 Proteins from the Venom of Atheris chlorechis, Atheris nitschei and Atheris squamigera" Toxins 8, no. 6: 168. https://doi.org/10.3390/toxins8060168