Role of Fc in Antibody-Mediated Protection from Ricin Toxin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of Intact IgG and Fab/F(ab’)2 for Binding, Neutralization, and in vivo Protection
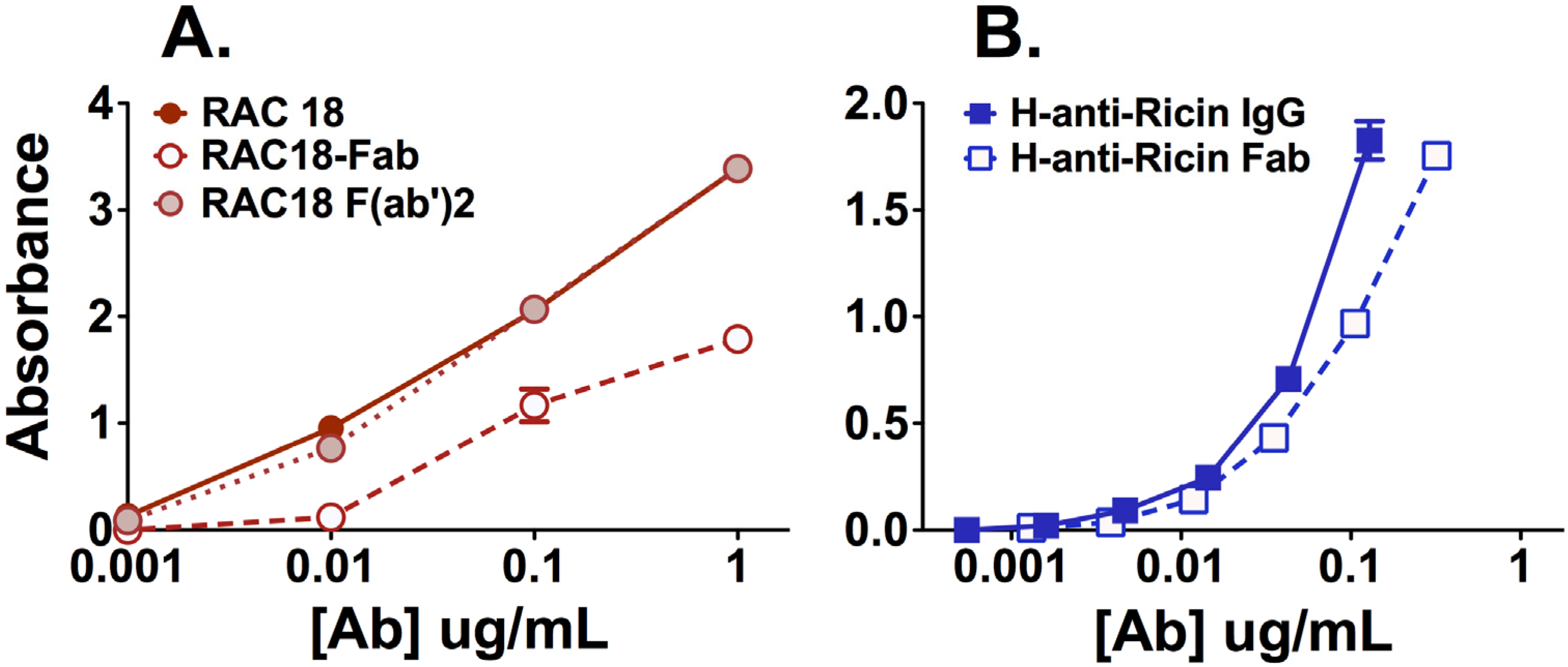
2.1.1. Fab Fragments Bind Ricin Less Avidly than Intact Ab or F(ab’)2
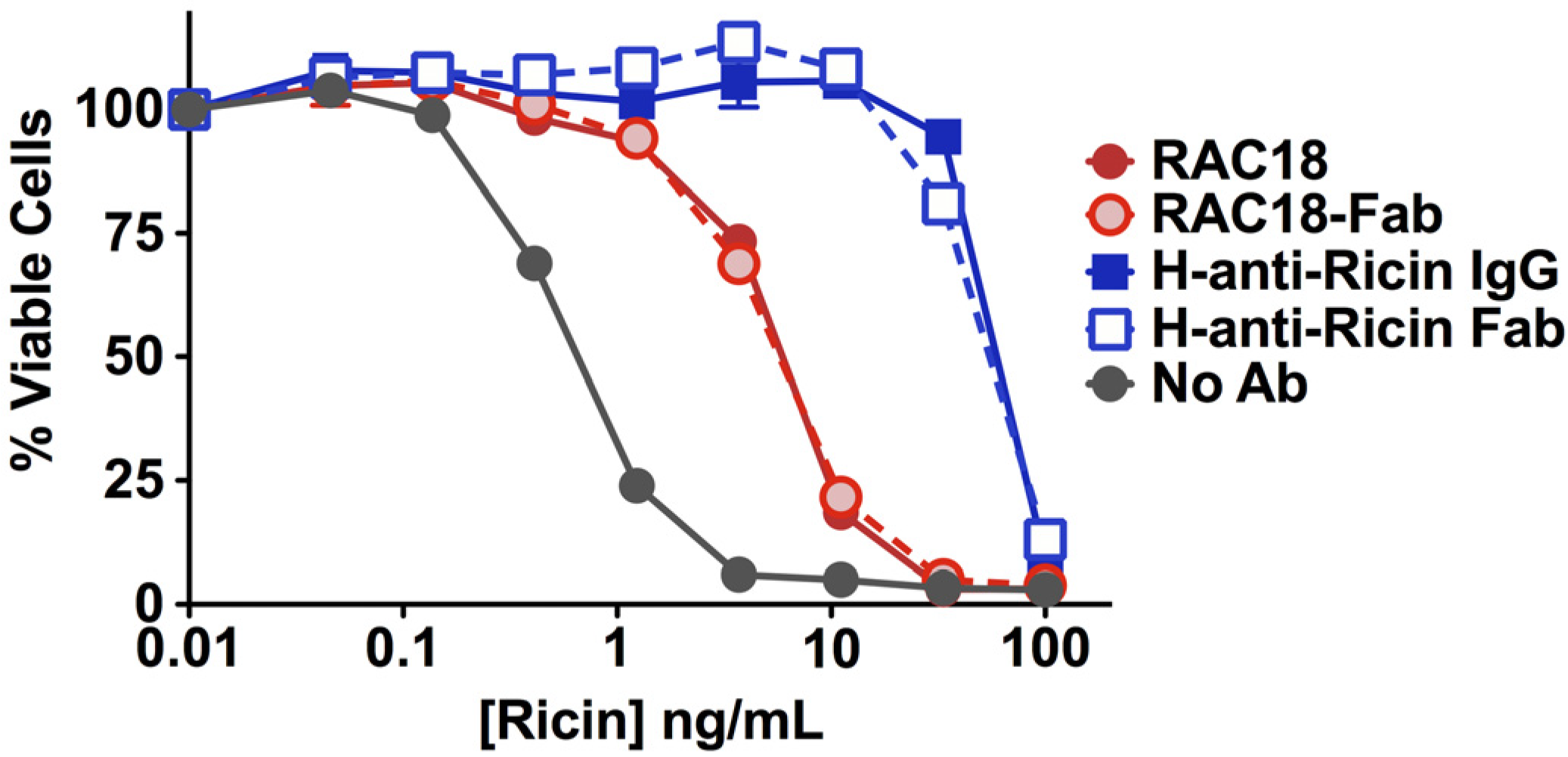
2.1.2. Fab Fragments and Intact IgG Neutralize Ricin Cytotoxicity Equally
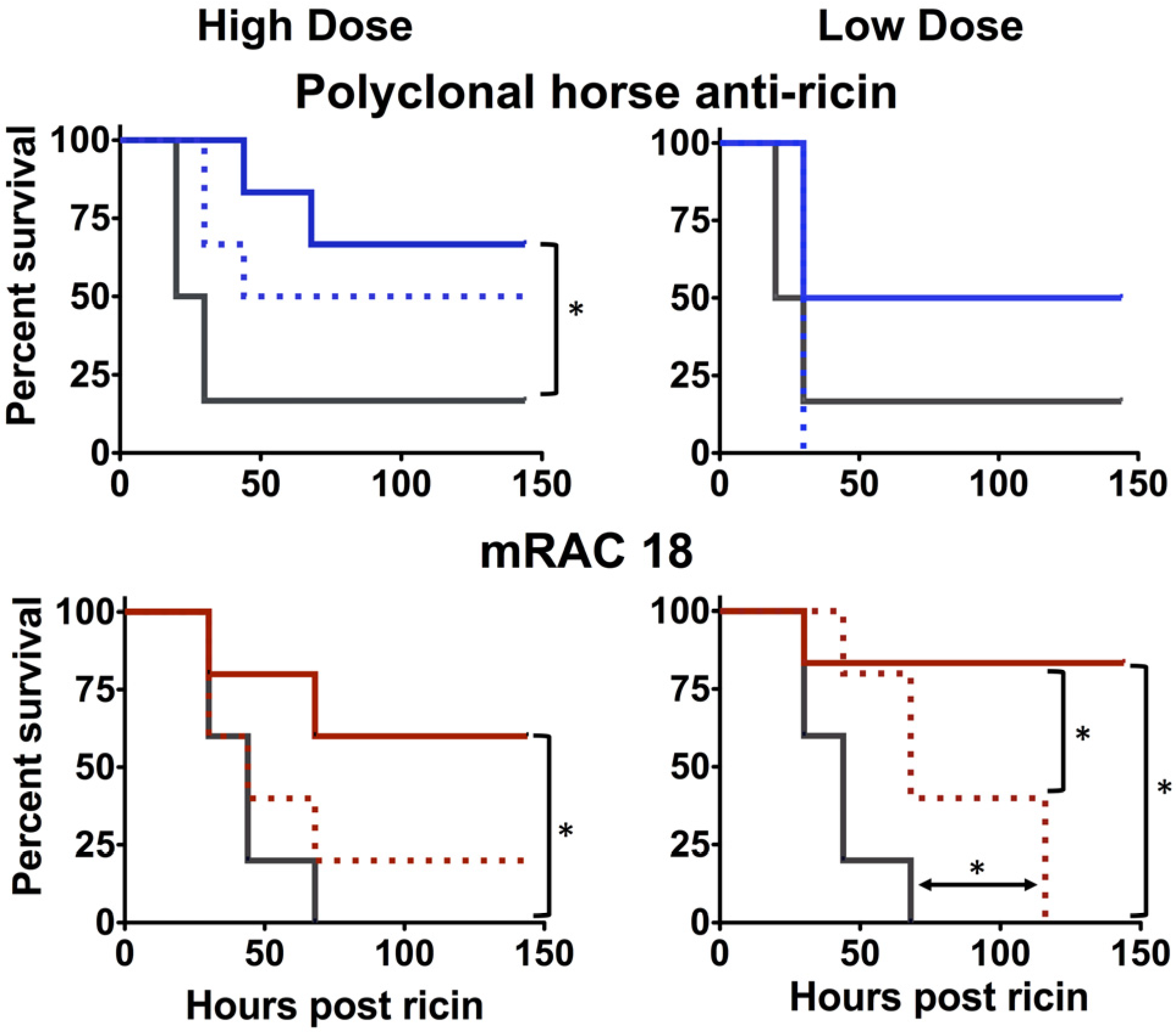
2.1.3. Antibody Fc is Important for in vivo Protection
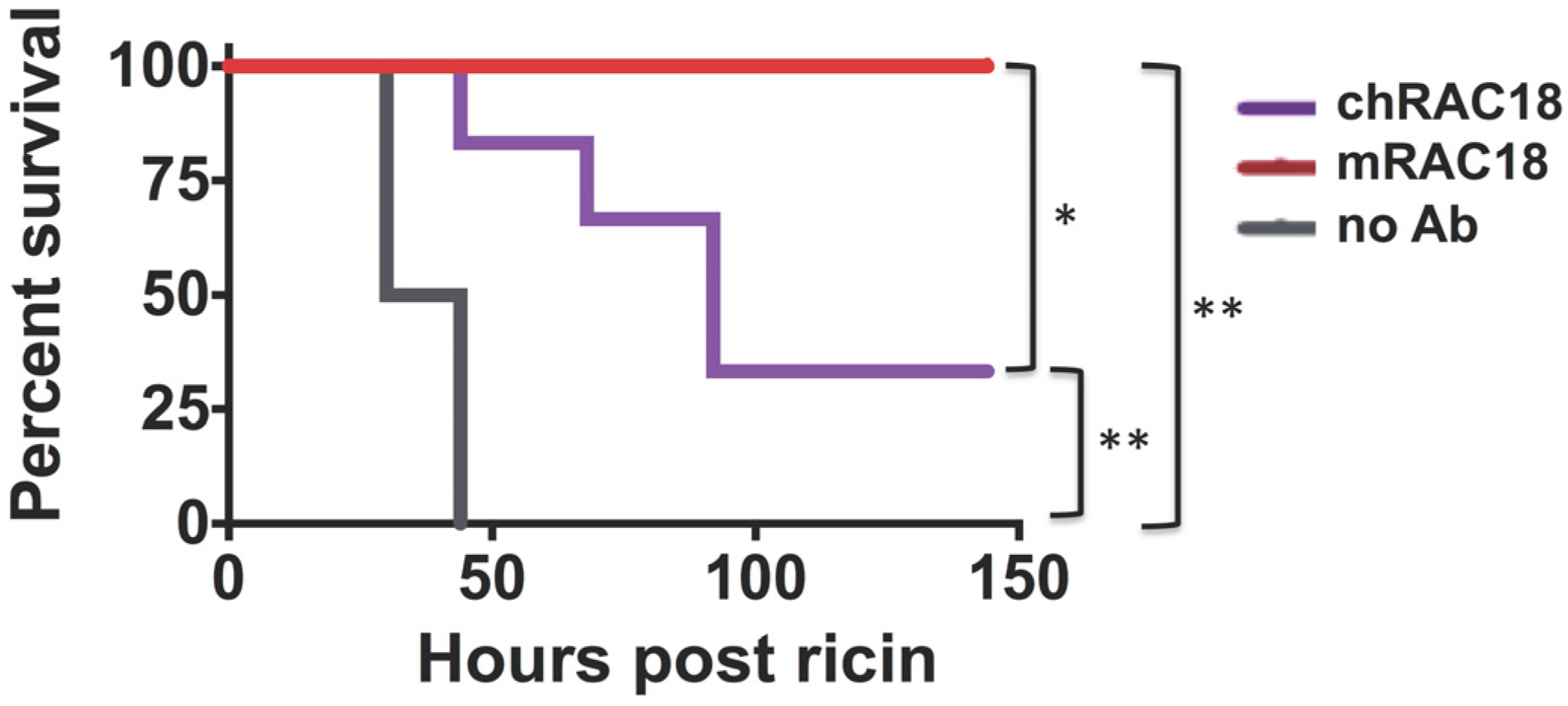
2.2. Fc Receptors Do Not Affect Ab-Mediated Protection from Ricin Cytoxicity
2.2.1. Chimeric RAC18 is Bound by FcRI and FcRIIb Expressed on the Cell Surface

2.2.2. Localization of RAC18 Internalized by FcRγI

2.2.3. Colocalization of Ab Internalized by FcRγI with Ricin

2.2.4. Effect of FcR on Internalization of Ricin and Neutralization by RAC18
2.2.5. Proteasome Inhibitor MG132 Affects neither Ricin Cytotoxicity nor Ab Protection

3. Experimental Section
3.1. Antibodies, Cells and Reagents
3.2. Enzyme Linked ImmunoSorbent Assay (ELISA)
3.3. Neutralization of Ricin Cytotoxicity
3.4. In Vivo Protection from Ricin Toxicity
3.5. Flow Cytometry
3.6. Live Cell Microscopy
4. Conclusions
Supplementary Files
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parham, P. The Immune System, 3rd ed.; Garland Publishing: New York, NY, USA, 2009. [Google Scholar]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology. In The Immune System in Health and Disease, 7th ed.; Garland Publishing: New York, NY, USA, 2008. [Google Scholar]
- Maddaloni, M.; Cooke, C.; Wilkinson, R.; Stout, A.V.; Eng, L.; Pincus, S.H. Immunological characteristics associated with protective efficacy of antibodies to ricin. J. Immunol. 2004, 172, 6221–6228. [Google Scholar] [CrossRef]
- Song, K.; Mize, R.R.; Marrero, L.; Corti, M.; Kirk, J.M.; Pincus, S.H. Antibody to ricin a chain hinders intracellular routing of toxin and protects cells even after toxin has been internalized. PLoS ONE 2013, 8, e62417. [Google Scholar]
- Pincus, S.H.; Smallshaw, J.E.; Song, K.; Berry, J.; Vitetta, E.S. Passive and active vaccination strategies to prevent ricin poisoning. Toxins 2011, 3, 1163–1184. [Google Scholar] [CrossRef]
- Krautz-Peterson, G.; Chapman-Bonofiglio, S.; Boisvert, K.; Feng, H.; Herman, I.M.; Tzipori, S.; Sheoran, A.S. Intracellular neutralization of shiga toxin 2 by an a subunit-specific human monoclonal antibody. Infect. Immun. 2008, 76, 1931–1939. [Google Scholar] [CrossRef]
- Akiyoshi, D.E.; Sheoran, A.S.; Rich, C.M.; Richard, L.; Chapman-Bonofiglio, S.; Tzipori, S. Evaluation of fab and F(ab’)2 fragments and isotype variants of a recombinant human monoclonal antibody against shiga toxin 2. Infect. Immun. 2010, 78, 1376–1382. [Google Scholar] [CrossRef]
- Pelat, T.; Hust, M.; Hale, M.; Lefranc, M.; Dubel, S.; Thullier, P. Isolation of a human-like antibody fragment (scFv) that neutralizes ricin biological activity. BMC Biotechnol. 2009, 9, 60. [Google Scholar] [CrossRef]
- Chow, S.-K.; Casadevall, A. Monoclonal antibodies and toxins—A perspective on function and isotype. Toxins 2012, 4, 430–454. [Google Scholar] [CrossRef]
- Abboud, N.; Chow, S.-K.; Saylor, C.; Janda, A.; Ravetch, J.V.; Scharff, M.D.; Casadevall, A. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J. Exp. Med. 2010, 207, 2395–2405. [Google Scholar] [CrossRef]
- Pincus, S.H.; Eng, L.; Cooke, C.L.; Maddaloni, M. Identification of hypoglycemia in mice as a surrogate marker of ricin toxicosis. Comp. Med. 2002, 52, 530–533. [Google Scholar]
- Perez, L.G.; Costa, M.R.; Todd, C.A.; Haynes, B.F.; Montefiori, D.C. Utilization of IGG Fc receptors by human immunodeficiency virus type 1: A specific role for antibodies against the membrane proximal external region of gp41. J. Virol. 2009, 83, 7397–7410. [Google Scholar] [CrossRef]
- Sandvig, K.; van Deurs, B. Endocytosis, intracellular transport, and cytotoxic action of shiga toxin and ricin. Physiol. Rev. 1996, 76, 949–966. [Google Scholar]
- Van Deurs, B.; Sandvig, K.; Petersen, O.W.; Olsnes, S.; Simons, K.; Griffiths, G. Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J. Cell Biol 1988, 106, 253–267. [Google Scholar] [CrossRef]
- Sandvig, K.; Torgersen, M.L.; Engedal, N.; Skotland, T.; Iversen, T.-G. Protein toxins from plants and bacteria: Probes for intracellular transport and tools in medicine. FEBS Lett. 2010, 584, 2626–2634. [Google Scholar] [CrossRef]
- Schmidt, A.C. Response to dengue fever—The good, the bad, and the ugly? Engl. J. Med. 2010, 363, 484–487. [Google Scholar] [CrossRef]
- Mallery, D.L.; McEwan, W.A.; Bidgood, S.R.; Towers, G.J.; Johnson, C.M.; James, L.C. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc. Natl. Acad. Sci. USA 2010, 107, 19985–19990. [Google Scholar]
- Vaysburd, M.; Watkinson, R.E.; Cooper, H.; Reed, M.; O’Connell, K.; Smith, J.; Cruickshanks, J.; James, L.C. Intracellular antibody receptor TRIM21 prevents fatal viral infection. Proc. Natl. Acad. Sci. USA 2013, 110, 12397–12401. [Google Scholar] [CrossRef]
- McEwan, W.A.; Tam, J.C.H.; Watkinson, R.E.; Bidgood, S.R.; Mallery, D.L.; James, L.C. Intracellular antibody-bound pathogens stimulate immune signaling via the fc receptor TRIM21. Nat. Immunol. 2013, 14, 327–336. [Google Scholar]
- Pietroni, P.; Vasisht, N.; Cook, J.P.; Roberts, D.M.; Lord, J.M.; Hartmann Petersen, R.; Roberts, L.M.; Spooner, R.A. The proteasome cap RPT5/Rpt5p subunit prevents aggregation of unfolded ricin a chain. Biochem. J. 2013, 453, 435–445. [Google Scholar] [CrossRef]
- Zhao, Z.; Worthylake, D.; LeCour, L.; Maresh, G.A.; Pincus, S.H. Crystal structure and computational modeling of the fab fragment from a protective anti-ricin monoclonal antibody. PLoS One 2012, 7, e52613. [Google Scholar]
- Richalet-Sécordel, P.M.; Rauffer-Bruyère, N.; Christensen, L.L.; Ofenloch-Haehnle, B.; Seidel, C.; van Regenmortel, M.H. Concentration measurement of unpurified proteins using biosensor technology under conditions of partial mass transport limitation. Anal. Biochem. 1997, 249, 165–173. [Google Scholar] [CrossRef]
- Folks, T.; Benn, S.; Rabson, A.; Theodore, T.; Hoggan, M.D.; Martin, M.; Lightfoote, M.; Sell, K. Characterization of a continuous t-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (aids)-associated retrovirus. Proc. Natl. Acad. Sci. USA 1985, 82, 4539–4543. [Google Scholar] [CrossRef]
- Derdeyn, C.A.; Decker, J.M.; Sfakianos, J.N.; Wu, X.; O’Brien, W.A.; Ratner, L.; Kappes, J.C.; Shaw, G.M.; Hunter, E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 2000, 74, 8358–8367. [Google Scholar] [CrossRef]
- Wei, X.; Decker, J.M.; Liu, H.; Zhang, Z.; Arani, R.B.; Kilby, J.M.; Saag, M.S.; Wu, X.; Shaw, G.M.; Kappes, J.C. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 2002, 46, 1896–1905. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pincus, S.H.; Das, A.; Song, K.; Maresh, G.A.; Corti, M.; Berry, J. Role of Fc in Antibody-Mediated Protection from Ricin Toxin. Toxins 2014, 6, 1512-1525. https://doi.org/10.3390/toxins6051512
Pincus SH, Das A, Song K, Maresh GA, Corti M, Berry J. Role of Fc in Antibody-Mediated Protection from Ricin Toxin. Toxins. 2014; 6(5):1512-1525. https://doi.org/10.3390/toxins6051512
Chicago/Turabian StylePincus, Seth. H., Anushka Das, Kejing Song, Grace A. Maresh, Miriam Corti, and Jody Berry. 2014. "Role of Fc in Antibody-Mediated Protection from Ricin Toxin" Toxins 6, no. 5: 1512-1525. https://doi.org/10.3390/toxins6051512



