Ophiophagus hannah Venom: Proteome, Components Bound by Naja kaouthia Antivenin and Neutralization by N. kaouthia Neurotoxin-Specific Human ScFv
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. O. hannah Venom, Horse-Derived N. kaouthia Antivenin and HuScFv-Specific to N. kaouthia Long Neurotoxin (NkLN-HuScFv)
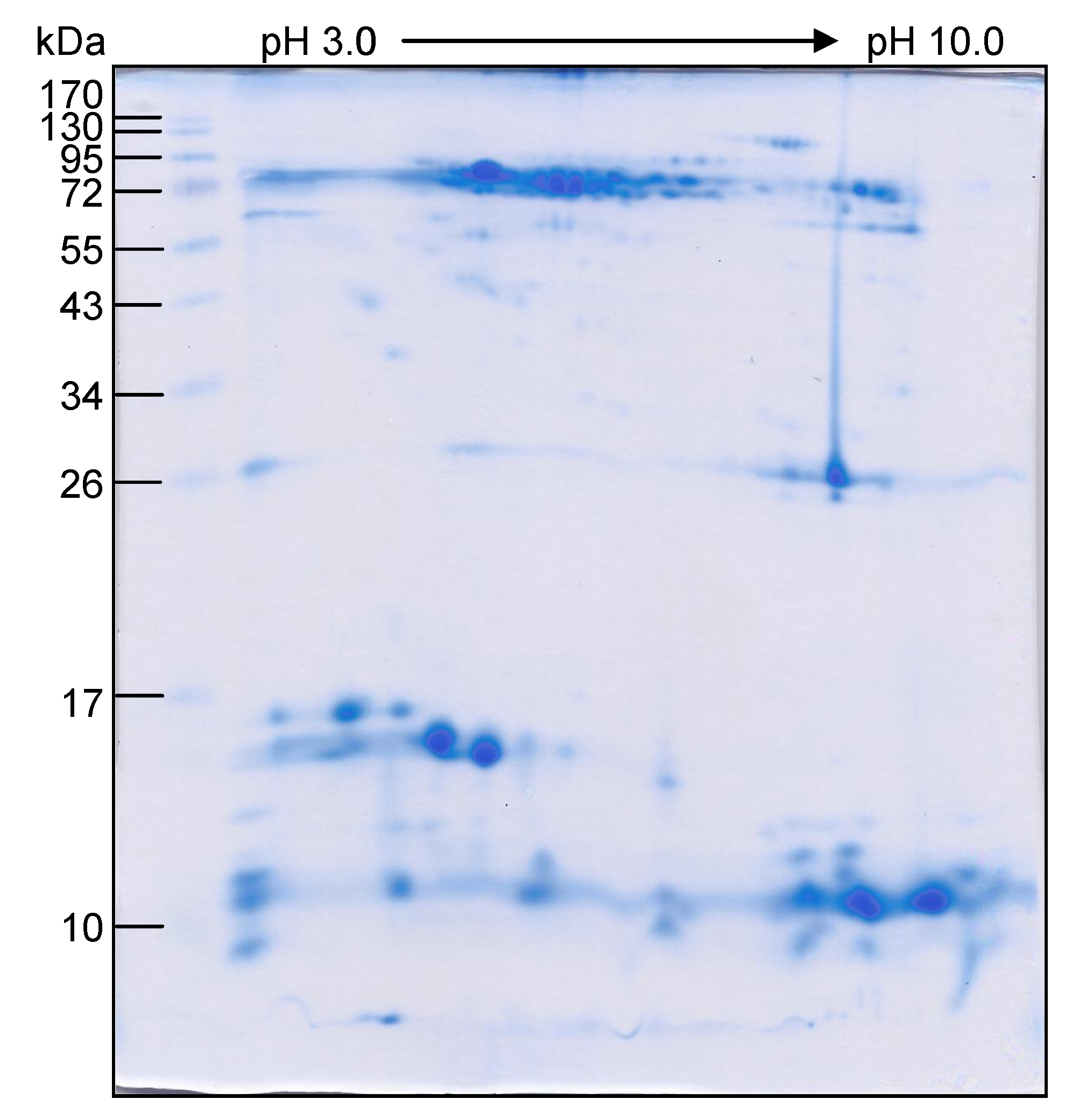
2.3. Characterization of O. hannah Venom Proteome by 1DE-ESI-LC-MS/MS
2.4. Determination of O. hannah Venom Components Cross-Reactive with N. kaouthia Antivenin
2.5. Median Lethal Dose (LD50) of O. hannah Venom in Mice
2.6. Cross-Neutralization of O. hannah Venom by N. kaouthia Antivenin
2.7. Cross-Neutralization of O. hannah Venom by NkLN-HuScFv
2.8. O. hannah Venom Components Cross-Reactive to NkLN-HuScFv
2.9. Computerized Procedure for Determining the Interactions between NkLN-HuScFv and Long Neurotoxins of N. kaouthia and O. hannah
3. Results
3.1. O. hannah Venom Proteome

3.2. O. hannah Venom Components Cross-Reactive to N. kaouthia Antivenin
| Gel piece No. | Protein No. | Accession No. | Protein | Protein score | Mass | m/z | z | Peptide score | Sequence |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | gi|391359389 | Zinc | 911 | 69,003 | 650.38 | 2 | 43.19 | NNLLHFSIWR |
| metalloproteinase- | 799.97 | 2 | 64.70 | TNNVIIPCKPTDVK | |||||
| disintegrin-like | 821.39 | 2 | 102.49 | LYCTGGTENPSEGEK | |||||
| ohanin | 861.43 | 2 | 96.53 | ASYSEIEDIGMVDHR | |||||
| 957.01 | 2 | 136.20 | AMCDVLQSVGIVQDYSK | ||||||
| 1,214.54 | 2 | 126.32 | NECDLPEFCIGQSAECPMDR | ||||||
| 810.39 | 3 | 78.10 | LYCTGGTENPSEGEKISSDPCK | ||||||
| 1,220.67 | 2 | 122.89 | NDNAQLLTGVDLNGYTLGSAYLK | ||||||
| 1,222.53 | 2 | 83.89 | NECDLPEFCIGQSAECPMDR | ||||||
| 860.11 | 3 | 74.59 | SPYLVGAAMAHEIGHNLGMEHDTK | ||||||
| 947.44 | 3 | 65.64 | NECDLPEFCIGQSAECPMDRFHK | ||||||
| 1 | 2 | gi|347595788 | L-amino-acid oxidase | 537 | 55,941 | 690.87 | 2 | 62.68 | QTDENAWYLIK |
| 749.50 | 2 | 69.76 | KIVIVGAGISGLTAAK | ||||||
| 762.92 | 2 | 66.38 | EAGHEVVILEASDR | ||||||
| 776.45 | 2 | 64.94 | IILVCTDKFWEK | ||||||
| 1,056.01 | 2 | 117.64 | MSANNPENFGYQLNPNER | ||||||
| 780.07 | 3 | 63.43 | IYFAGEYTAHPHGWIETSMK | ||||||
| 805.42 | 3 | 91.79 | SASQLFDETLDKVTDDCTLQK | ||||||
| 1 | 3 | gi|22654267 | Acidic phospholipase | 510 | 16,432 | 792.41 | 2 | 101.70 | LPACSSIMDSPYVK |
| A2 | 906.40 | 2 | 86.21 | CCQVHDNCYTQAQK | |||||
| 915.89 | 2 | 94.20 | ADNDECAAFICNCDR | ||||||
| 794.71 | 3 | 129.81 | YADYGCYCGAGGSGTPVDKLDR | ||||||
| 872.10 | 3 | 97.84 | VAAHCFAASPYNNNNYNIDTTTR | ||||||
| 1 | 4 | gi|126035663 | Complement- | 235 | 184,237 | 955.61 | 2 | 124.43 | LILNTPLDTQSLLITVR |
| depleting factor | 999.02 | 2 | 68.09 | TDTEEQILVEAHGDNTPK | |||||
| (O. hannah) | 1,065.65 | 2 | 42.31 | AVPFVIVPLQQGLHDIEVR | |||||
| 1 | 5 | gi|82193162 | Long neurotoxin OH-37 | 183 | 9846 | 670.36 | 2 | 91.89 | KLSFGCAATCPK |
| 1,164.04 | 2 | 91.17 | VNPGIDIECCSTDNCNPHPK | ||||||
| 1 | 6 | gi|338855302 | Phosphodiesterase 1 | 160 | 96,311 | 771.08 | 3 | 95.44 | NPAWWGGQPIWHTATYQGLK |
| 1,216.13 | 2 | 65.03 | NEVTSFENIEVYNLMCDLLK | ||||||
| 1 | 7 | gi|82193155 | Short neurotoxin OH-35 | 126 | 9558 | 1,239.13 | 2 | 126.42 | QYTIFGVTPEICADGQNLCYK |
| 1 | 8 | gi|565306229 | Insulin-like growth factor I (O. hannah) | 115 | 12,329 | 936.45 | 2 | 115.04 | GIVEECCFQSCDLVR |
| 1 | 9 | gi|565293365 | Hypothetical protein L345_17517, partial (O. hannah) | 65 | 25,866 | 777.87 | 2 | 64.55 | GIDSSHWNSYCTK |
| 1 | 10 | gi|391359387 | Zinc metalloproteinase-disintegrin-like mikarin | 63 | 18,426 | 796.44 | 2 | 62.61 | TNTPEQDRYLQVK |
| 2 | 11 | gi|544604740 | Ophiophagus venom | 534 | 183,812 | 606.86 | 2 | 43.11 | AVYVLNDKYK |
| factor | 8,867.00 | 2 | 48.71 | YVLPSFEVHLQPSEK | |||||
| 905.04 | 2 | 58.82 | IKLEGDPGAQVGLVAVDK | ||||||
| 955.61 | 2 | 118.78 | LILNTPLDTQSLLITVR | ||||||
| 999.02 | 2 | 104.68 | TDTEEQILVEAHGDNTPK | ||||||
| 1,010.07 | 2 | 94.74 | YLYGEEVEGVAFVLFGVK | ||||||
| 700.05 | 3 | 67.01 | VPVVSEAIHSEGTTLSDGTAK | ||||||
| 810.39 | 3 | 43.35 | LYCTGGTENPSEGEKISSDPCK | ||||||
| 749.51 | 2 | 96.89 | KVVIIGAGISGLTAAK | ||||||
| 3 | 12 | gi|565315338 | 5~-nucleotidase, partial (O. hannah) | 53 | 28,027 | 958.05 | 2 | 53.17 | VLLPSFLAAGGDGYYMLK |
| 4 | 13 | gi|387935404 | Alpha- and beta- | 510 | 28,637 | 573.30 | 2 | 95.85 | GDSGGPLICNR |
| fibrinogenase OHS1 | 812.40 | 2 | 105.35 | IIGGFECNEYEHR | |||||
| 818.40 | 2 | 77.40 | DSCKGDSGGPLICNR | ||||||
| 881.50 | 2 | 102.54 | VMGWGLLTSPEVTFPK | ||||||
| 1,325.22 | 2 | 128.74 | EIQGIVSWGGFPCAQLLEPGVY TK | ||||||
| 4 | 14 | gi|565314693 | Hypothetical protein | 266 | 24,222 | 573.30 | 2 | 95.85 | GDSGGPLICNR |
| L345_07470, partial | 818.40 | 2 | 77.40 | DSCKGDSGGPLICNR | |||||
| (O. hannah) | 1,317.72 | 2 | 92.89 | QIQGVVSWGGFPCAQLLEPGVYT K | |||||
| 4 | 15 | gi|565308117 | hypothetical protein | 206 | 115,185 | 650.38 | 2 | 49.92 | NNLLHFSIWR |
| L345_12124 | 818.40 | 2 | 77.40 | DSCKGDSGGPLICNR | |||||
| (O. hannah) | 871.39 | 2 | 82.22 | NGHSCQNNQGYCFR | |||||
| 957.00 | 2 | 74.28 | AMCDVLQSVGIVQDYSK | ||||||
| 4 | 16 | gi|54035743 | Cobra venom factor | 64 | 184,401 | 841.91 | 2 | 63.74 | VYSYYNLDEKCTK |
| 5 | 17 | gi|565307033 | Hepatocyte growth | 174 | 10,782 | 981.57 | 2 | 104.44 | YSNVVQEALIPIIPDYK |
| factor activator, | 1,161.59 | 2 | 69.52 | FIQPICLPEASMSFPDYYK | |||||
| partial (O. hannah) | 937.51 | 2 | 69.87 | GILDENQWESGLFLPR | |||||
| 5 | 18 | gi|118151738 | Metalloproteinase precursor (Demansia vestigiata) | 62 | 68,267 | 649.30 | 2 | 61.92 | SAECPTDSFQR |
| 5 | 19 | gi|18000318 | Cysteine-rich venom protein (Hydrophis hardwickii) | 58 | 20,109 | 888.95 | 2 | 57.69 | YLYVCQYCPAGNIR |
| 5 | 20 | gi|182705250 | Zinc metalloproteinase/ disintegrin | 53 | 71,170 | 957.00 | 2 | 53.19 | GMCDPKLSVGLVQDYSK |
| 6 | 22 | gi|565292399 | Endonuclease domain- | 234 | 18,064 | 848.51 | 2 | 75.99 | SSTFTLTNIVPQFIK |
| containing 1 protein | 1,055.56 | 2 | 66.13 | GCQQTFAVVGAVPGDTYIAR | |||||
| (O. hannah) | 1,162.10 | 2 | 94.19 | ALQDSQAVLEDYKNLADCNR | |||||
| 888.95 | 2 | 74.76 | YLYVCQYCPAGNIR | ||||||
| 6 | 23 | gi|1584763 | Phospholipase A2 | 137 | 13,447 | 915.89 | 2 | 58.19 | ADNDECAAFICNCDR |
| 794.71 | 3 | 79.24 | YADYGCYCGAGGSGTPVDKLDR | ||||||
| 6 | 24 | gi|565304281 | Endonuclease domain-containing 1 protein, partial (O. hannah) | 126 | 51,035 | 628.36 | 2 | 49.98 | FATLYDKQNR |
| 6 | 26 | gi|565303552 | Phospholipase B-like 1, partial (O. hannah) | 71 | 58,242 | 869.47 | 2 | 73.13 | DLHYATVYWLEAEK |
| 7 | 27 | gi|225547744 | Opharin precursor | 317 | 26,288 | 640.78 | 2 | 43.67 | CPASCFCHNK |
| (O. hannah) | 788.39 | 2 | 78.40 | YKDDFSNCQSLAK | |||||
| 888.95 | 2 | 77.95 | YLYVCQYCPAGNIR | ||||||
| 1,092.02 | 2 | 68.72 | FSCGENLFMSSQPYAWSR | ||||||
| 1,212.51 | 2 | 48.66 | SGPPCGDCPSACDNGLCTNPCK | ||||||
| 8 | 29 | gi|124020977 | PLA2 Hs-1 precursor (Hoplocephalus stephensii) | 61 | 915.88 | 2 | 60.84 | ADNDECKAFICNCDR | |
| 9 | 30 | gi|48428841 | Cysteine-rich venom protein | 123 | 26,229 | 705.39 | 2 | 56.89 | VIQSWYDENKK |
| natrin-2 | 925.46 | 2 | 66.57 | NMLQMEWNSNAAQNAK | |||||
| 9 | 31 | gi|82193154 | Cardiotoxin CTX15 | 104 | 9,346 | 545.84 | 2 | 60.05 | LPSKYDVIR |
| 666.40 | 2 | 43.75 | CLNTPLPLIYK | ||||||
| 738.43 | 2 | 42.00 | FLEQQNQVLQTK | ||||||
| 9 | 32 | gi|32363235 | Thai cobrin | 80 | 12,087 | 757.41 | 3 | 80.32 | ADVTFDSNTAFESLVVSPDKK |
| 9 | 33 | gi|26006829 | Acidic phospholipase | 61 | 15,890 | 847.13 | 3 | 61.15 | VAAICFAGAPYNKENINIDTTTR |
| A2-2 | 792.41 | 2 | 105.12 | LPACSSIMDSPYVK | |||||
| 906.41 | 2 | 77.19 | CCQVHDNCYTQAQK | ||||||
| 915.89 | 2 | 94.44 | ADNDECAAFICNCDR | ||||||
| 794.71 | 3 | 147.85 | YADYGCYCGAGGSGTPVDKLDR | ||||||
| 1,210.54 | 2 | 92.09 | TVTCKADNDECAAFICNCDR | ||||||
| 872.10 | 3 | 101.16 | VAAHCFAASPYNNNNYNIDTTTR | ||||||
| 925.45 | 3 | 109.09 | VAAHCFAASPYNNNNYNIDTTTRC | ||||||
| 10 | 34 | gi|82199673 | Cytotoxin-27 | 251 | 9,305 | 799.38 | 2 | 67.56 | SSADVEVLCCDTNK |
| (ß Cytotoxin) | 1,102.61 | 2 | 115.40 | CLNTPLPLIYTTCPIGQDK | |||||
| 1,138.12 | 2 | 68.32 | KCLNTPLPLIYTTCPIGQDK | ||||||
| 10 | 35 | gi|82175774 | Short neurotoxin | 247 | 8,886 | 560.25 | 2 | 47.13 | YSVCCSTDK |
| OH-46 | 761.34 | 2 | 62.70 | YSVCCSTDKCNK | |||||
| 929.47 | 3 | 137.57 | TTMFFPNHPVLLMGCTYNCPTER | ||||||
| 10 | 36 | gi|123916245 | Cardiotoxin CTX21 | 242 | 9,249 | 978.48 | 2 | 58.44 | SSADVVVVCCDTNKCNK |
| 1,102.61 | 2 | 115.4 | CLNTPLPLIYTTCPIGQDK | ||||||
| 1,138.12 | 2 | 68.32 | KCLNTPLPLIYTTCPIGQDK | ||||||
| 10 | 37 | gi|116242842 | Ohanin | 177 | 21,161 | 749.40 | 2 | 88.66 | FSSSPCVLGSPGFR |
| 1,135.61 | 2 | 88.46 | ADVTFDSNTAFESLVVSPDKK | ||||||
| 1,239.13 | 2 | 140.55 | QYTIFGVTPEICADGQNLCYK | ||||||
| 666.40 | 2 | 59.72 | CLNTPLPLIYK | ||||||
| 799.38 | 2 | 67.56 | SSADVEVLCCDTNK | ||||||
| 1,022.51 | 2 | 64.29 | VSETIEICPDGQNFCFK | ||||||
| 10 | 40 | gi|239977312 | Protease inhibitor TCI | 105 | 8,976 | 998.00 | 2 | 104.67 | AYIPSFYYNPDASACQK |
| 10 | 41 | gi|123913366 | Weak neurotoxin WNTX-34 | 98 | 9,808 | 826.35 | 2 | 97.90 | EIVQCCSTDECNH |
| 10 | 42 | gi|82193157 | Long neurotoxin OH-17 | 88 | 8,032 | 893.90 | 2 | 88.18 | CCSTDNCNPFTPWK |
| 10 | 43 | gi|82193161 | Long neurotoxin OH-55 | 82 | 10,203 | 1,013.59 | 2 | 81.91 | VNLGCAATCPIVKPGVEIK |
| 10 | 44 | gi|128951 | Long neurotoxin-4 | 75 | 8,009 | 941.47 | 2 | 75.32 | IISEACPPGQDLCYMK |
| 10 | 45 | gi|83305935 | Neurotoxin-like protein 1 | 74 | 7,037 | 625.83 | 2 | 74.01 | KDEVIQCCAK |
| 10 | 46 | gi|182705233 | Weak toxin DE-1 | 74 | 9,297 | 650.87 | 2 | 73.67 | VNPPISIICCK |
| 10 | 47 | gi|239938646 | Venom chymotrypsin inhibitor | 52 | 9,130 | 830.42 | 2 | 52.00 | FCELPPEPGLCNAR |
| 10 | 48 | gi|123907650 | Cardiotoxin CTX-23 | 146 | 9,290 | 459.74 | 2 | 33.85 | TCPIGQDK |
| 666.40 | 2 | 58.07 | CLNTPLPLIYK | ||||||
| 978.48 | 2 | 54.36 | SSADVVVVCCDTNKCNK | ||||||
| 10 | 49 | gi|123913365 | Muscarinic toxin-38 | 48 | 9,678 | 756.38 | 2 | 48.13 | TCPDGQNLCYKR |
| 10 | 50 | gi|128944 | Long neurotoxin-2 | 70 | 893.90 | 2 | 69.94 | CCSTDNCNPFPTWK |
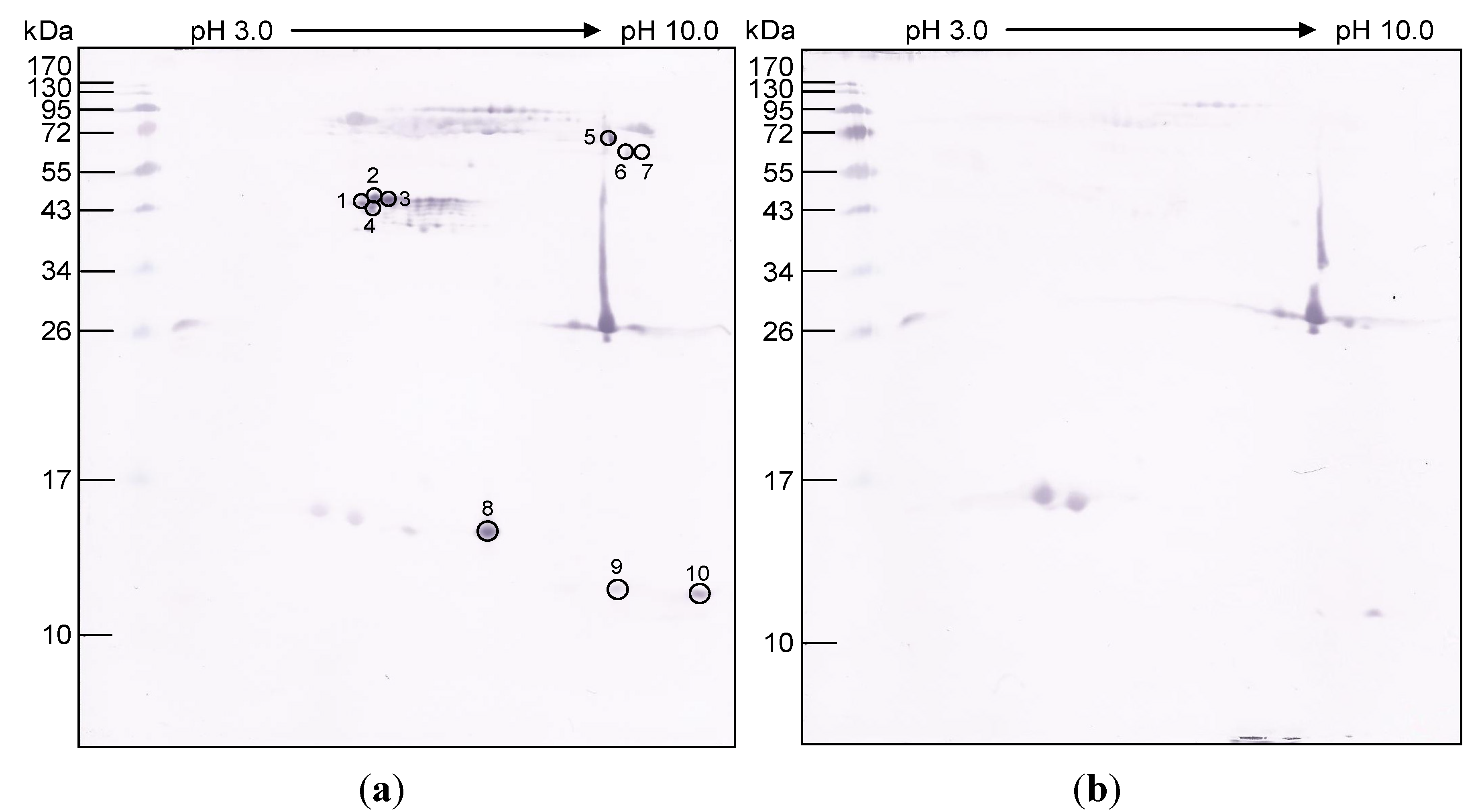
| Gel piece No. | Accession No. | Protein | Protein score | Mass | m/z | z | Peptide score | Sequence |
|---|---|---|---|---|---|---|---|---|
| 1 | gi|544604740 | O. hannah venom factor | 54 | 183,812 | 428.26 | 2 | 29.96 | VVPEGVQK |
| 710.74 | 3 | 26.31 | AVPFVIVPLQQGLHDIEVR | |||||
| 2 | gi|38049474 | Cardiotoxin-like protein | 187 | 9,818 | 481.73 | 2 | 38.14 | GCIDICPK |
| (O. hannah) | 9,818 | 545.82 | 3 | 27.06 | LPSKYDVIR | |||
| 9,818 | 799.36 | 2 | 65.14 | SSADVEVLCCDTNK | ||||
| 9,818 | 735.39 | 3 | 64.75 | CLNTPLPLIYTTCPIGQDK | ||||
| 3 | gi|387165368 | OVF precursor protein (O. hannah) | 52 | 185,408 | 482.26 | 3 | 27.04 | HFEVGFIQPGSVK |
| 52 | 185,408 | 710.74 | 3 | 25.54 | AVPFVIVPLQQGLHDIEVR | |||
| 4 | No matched protein in the database | |||||||
| 5 | gi|28972961 | Ophanin (O. hannah) | 82 | 27,764 | 567.79 | 2 | 29.55 | SVSPTASNMLK |
| 27,764 | 640.78 | 2 | 54.73 | QSSCQDEWIK | ||||
| 6 | gi|225547744 | Opharin precursor | 46 | 27,200 | 418.73 | 2 | 26.72 | GSIATPYK |
| (O. hannah) | 27,200 | 525.91 | 2 | 20.06 | YKDDFSNCQSLAK | |||
| 7 | gi|82570105 | Weak neurotoxin precursor | 53 | 10,492 | 484.23 | 2 | 28.00 | NGENVCFK |
| (O. hannah) | 10,492 | 551.21 | 3 | 25.30 | EIVQCCSTDECNH | |||
| 8 | gi|82570073 | Long chain neurotoxin precursor (O. hannah) | 25 | 11,155 | 644.28 | 2 | 24.64 | TWCDVFCGSR |
| 9 | gi|82570073 | Long chain neurotoxin | 60 | 11,155 | 644.20 | 2 | 27.10 | TWCDVFCGSR |
| precursor (O. hannah) | 956.40 | 2 | 32.50 | IISETCPPGQDLCYMK | ||||
| gi|51105381 | Long chain neurotoxin | 123 | 8,602 | 603.20 | 2 | 49.40 | IDLGCAATCPK | |
| precursor (O. hannah) | 659.20 | 2 | 50.12 | SWCDVFCTSR | ||||
| 837.30 | 2 | 23.54 | SETCPDGENICYTK | |||||
| gi|51105369 | Long chain neurotoxin | 60 | 10,477 | 606.20 | 2 | 27.95 | LSFGCAATCPK | |
| precursor (O. hannah) | 642.70 | 2 | 32.33 | SWCDAWCGSR | ||||
| 9 | gi|51105375 | Long chain neurotoxin | 38 | 10,538 | 595.70 | 2 | 37.91 | VNLGCAATCPK |
| precursor (O. hannah) | ||||||||
| gi|82570079 | Long chain neurotoxin | 74 | 10,944 | 491.20 | 2 | 41.32 | CYVTPDVK | |
| precursor (O. hannah) | 837.30 | 2 | 32.65 | SETCPDGENICYTK | ||||
| gi|128944 | Long neurotoxin-2 | 72 | 8,602 | 603.20 | 2 | 43.12 | IDLGCAATCPK | |
| (Neurotoxin B) | 937.00 | 3 | 28.64 | TKCYVTPDATSQTCPDGQDICYTK | ||||
| gi|51105373 | Long chain neurotoxin | 77 | 10,830 | 491.20 | 2 | 31.89 | CYVTPDVK | |
| precursor (O. hannah) | 676.00 | 3 | 45.21 | VNLGCAATCPIVKPGVEIK | ||||
| gi|51105391 | Short chain alpha neurotoxin precursor (O. hannah) | 36 | 10,071 | 826.30 | 3 | 35.67 | QYTIFGVTPEICADGQNLCYK | |
| 10 | gi|51105397 | Short chain alpha | 114 | 9,859 | 481.70 | 2 | 29.87 | GCIDICPK |
| neurotoxin precursor | 666.30 | 2 | 36.34 | CLNTPLPLIYK | ||||
| (O. hannah) | 667.20 | 3 | 47.91 | SSADVEVLCCDTNKCNK | ||||
| gi|82570105 | Weak neurotoxin | 46 | 10,492 | 826.20 | 2 | 46.23 | EIVQCCSTDECNH | |
| precursor (O. hannah) | 799.30 | 2 | 41.31 | SSADVEVLCCDTNK | ||||
| 667.20 | 3 | 27.16 | SSADVEVLCCDTNKCNK | |||||
| gi|82570079 | Long chain neurotoxin | 146 | 10,944 | 491.20 | 2 | 38.11 | CYVTPDVK | |
| precursor (O. hannah) | 837.30 | 2 | 36.70 | SETCPDGENICYTK | ||||
| 596.20 | 3 | 27.89 | CCSTDNCNPFTPWK | |||||
| 648.20 | 3 | 43.67 | CCSTDNCNPFTPWKR | |||||
| gi|128944 | Long neurotoxin 2 | 167 | 8,602 | 603.20 | 2 | 27.85 | IDLGCAATCPK | |
| (Neurotoxin B) | 596.20 | 3 | 33.17 | CCSTDNCNPFPTWK | ||||
| Group of mice | Treatment (injected i.p. with) | Ratio of venom : HuScFv (w/w) | Mean ± SD of mouse dead time ( min) | % Survival |
|---|---|---|---|---|
| 1 | Venom-NkLN-HuScFv mixture | 1:10 | 111 ± 16.5 a | 0 |
| 2 | Venom-irrelevant HuScFv mixture | 1:10 | 88 ± 7.2 b | 0 |
| 3 | Venom-NkLN-HuScFv mixture | 1:50 | 155 ± 58.7 a | 0 |
| 4 | Venom-irrelevant HuScFv mixture | 1:50 | 93 ± 6.8 b | 0 |
| 5 | Venom in buffer | NA | 78 ± 7.3 b | 0 |
| 6 | NKLN-HuScFv alone | NA | All mice survived at the end of experiments | 100 |
| 7 | Venom-NkLN-HuScFv mixture and followed 10 min later with NkLN-HuScFv alone | 1:50 | 220 a | 80 |
| 8 | Venom-irrelevant HuScFv mixture and followed 10 min later with irrelevant HuScFv alone | 1:50 | 95 ± 24.4 b | 0 |
3.3. Median Lethal Dose (LD50) of O. hannah Venom and the Venom Cross-Neutralization by the Monospecific N. kaouthia Antivenin
3.4. Cross-Neutralization of O. hannah Venom by HuScFv Specific to N. kaouthia Neurotoxin
3.5. O. hannah Venom Components Cross-Reactive to NkLN-HuScFv
3.6. Computerized Models of Interactions between N. kaouthia and O. hannah Long Neurotoxins and NkLN-HuScFv

| Neurotoxin | NkLN-HuScFv | |
|---|---|---|
| Residue(s) | Domain/Subdomain | |
| CBTX (P01391) | ||
| W25 | D55 | VH/CDR2 |
| C26 | Y52 | VH/CDR2 |
| S31 | Q104 | VH/CDR3 |
| R36 | D55, D57 | VH /CDR2 |
| K49 | E74 | VH/FR3 |
| T50 | N28, S31 | VH/CDR1 |
| OH-LNTX (Q2VBP4) | ||
| C26 | Y52 | VH/CDR2 |
| W29 | D57 | VH/CDR2 |
| G31 | Q104 | VH/CDR3 |
| R33 | D57 | VH/CDR2 |
| K36 | D54, D55 | VH/CDR2 |
| R47 | N28 | VH/CDR1 |
| N49 | S31 | VH/CDR1 |

4. Discussion
5. Conclusions
Appendixes
| Protein type/family | Protein subtype | Protein name(s) | Function(s) |
|---|---|---|---|
| 1. Three-finger toxin | Long neurotoxin | OH-2, OH-4, OH-17, OH-37,OH-55 and LNTX37 | These three-finger toxins bind to muscular and neuronal nicotinic acetylcholine receptors (α-7-9) and block neuromuscular transmission at the postsynaptic site causing paralysis [44,45]. |
| Short neurotoxin | OH-35, | This neurotoxin binds to muscarinic acetylcholine receptor and blocks synaptic nerve transmission [46]. | |
| OH-46 | This three-finger toxin binds and inhibits the nicotinic acetylcholine receptor [47]. | ||
| Cardiotoxin/Cytotoxin | CTX-15 (OH-84), CTX-21 and CTX-23 | These three-finger toxins are cytotoxic and hemolytic [48]. | |
| CTX-27 (ß Cardiotoxin) | Acts as a beta-blocker by binding to beta-1 and beta-2 adrenergic receptors. It dose-dependently decreases the heart rate. At 100 mg/kg, intraperitoneal injection into mice mediate labored breathing, impaired locomotion, lack of response to external stimuli, and death (after 30 min) [49]. | ||
| Weak neurotoxin | WNTX-34 | This three-finger toxin binds to muscular and neuronal (α-7) nicotinic acetylcholine receptors with low affinity and very low affinity, respectively [46]. | |
| Neurotoxin-like | Neurotoxin-like protein 1 | Unknown | |
| Weak toxin | OH-DE-1 | Binds to the muscular nicotinic acetylcholine receptor causes paralysis by blocking neuromuscular transmission at the postsynaptic site [38,50]. | |
| 2. Phospholipase | OH-PLA2, OH-acidic -1 PLA2 and OH-acidic-2 PLA2 PLA2 Hs-1 precursor | These PLAs recognize and hydrolyze the sn-2 acyl bonds of phospholipids releasing arachidonic acid and lysophospholipids. They have neurotoxicity, anti-coagulating activity, cardiotoxicity, myonecrotic/myotoxic activity, anti-platelet activity and edema-inducing activity [35,51]. | |
| Phospholipase B-like 1, partial | Unknown | ||
| 3. Cysteine-rich secretory protein (CRiSP) | O. hannah opharin precursor | Binds to voltage-gated calcium channels on smooth muscle and causes high potassium-induced depolarization which weakly blocks smooth muscle contraction [52]. | |
| Cysteine-rich venom protein-2 (Natrin-2) | This protein acts as an inflammatory modulator that interferes with wound-healing process of the bitten victim by regulating adhesion molecule expression on endothelial cells [53] and also blocks muscle contraction evoked by potassium [54]. | ||
| Cysteine-rich venom protein | Blocks contraction of smooth muscle elicited by high potassium-induced depolarization and target voltage-gated calcium channels (Cav) on smooth muscle [55]. | ||
| 4. Cobra venom factor/ complement C3 | O. hannah complement-depleting factor | Anti-complement activity [56]. | |
| 5. Muscarinic toxin, type A | O. hannah muscarinic toxin-38 | This toxin binds to muscarinic acetylcholine receptor and blocks synaptic nerve transmission [57]. | |
| 6. L- amino acid oxidase | L amino acid oxidase | These enzymes catalyse oxidative deamination of L-amino acid to form α-keto acid, NH4 and H2O2. The enzymatic activity on platelet aggregation is controversial: inhibit platelet aggregation induced by ADP and the thromboxane analog U46619 versus induce platelet aggregation through the hydrogen peroxide formation. The H2O2 also causes edema and human cell apoptosis [58,59,60] | |
| 7. Hypothetical protein | Hypothetical protein L345_17517 Hypothetical protein L345_07470 Hypothetical protein L345_12124 Hypothetical protein L345_15308 | Unknown function | |
| 8. Low cysteine protein | Thai cobrin | Thai cobrin and ohanin (protein type/family 13, Vespryn) are classified in the same low cysteine protein family [61]. | |
| 9. Phosphodiesterase-1 | Phosphodiesterase-1 | Phosphodiesterase catalyzes the hydrolysis of phosphodiester bonds of double-stranded and single-stranded DNA, rRNA, and tRNAs resulting in 5' mononucleotide formation and subsequent release of free adenosines which are multitoxic [62]. | |
| 10. Proteases | Metalloproteinase | Protobothrops jerdonii disintegrin | It inhibits ADP-induced human platelet aggregation. It binds the receptor GPIIb/GPIIIa on the platelet surface resulting in hemorrhage [63]. |
| Zinc metalloproteinase-disintegrin-like ohanin | Snake venom zinc metalloproteinase that has hemorrhagic activity. Inhibits ADP-, TMVA- and stejnulxin-induced platelet aggregation in a dose-dependent manner (on washed platelet, but not on platelet rich plasm). Also specifically degrades alpha-chain of fibrinogen (FGA) [64] | ||
| Zinc metalloproteinase-disintegrin-like mikarin | Snake venom zinc metalloproteinase that calcium-independently catalyzes the conversion of prothrombin (F2) to alpha-thrombin through the formation of a thrombin intermediate.[65] | ||
| Metalloproteinase-precursor | unknown | ||
| Kallikrein enzyme | OH fibrinogenases -α and -ß | These O. hannah venom serine proteases mediate fibrinogenolysis on both alpha and beta-chains of fibrin and amidolytic activity [66]. | |
| 11. Vespryn toxin | Ohanin | Ohanin is a low cysteine protein. This neurotoxin causes dose-dependent hypolocomotion and hyperalgesia in mice. It may directly act on the central nervous system [61]. | |
| 12. Kunitz | Protease inhibitor | O. hannah serine protease inhibitor; Protease inhibitor TCI Venom chymotrypsin inhibitor | Strongly inhibit trypsin and chymotrypsin [67]. |
| 13. Growth factor activator | Hepatocyte growth factor activator, partial Insulin-like growth factor I | Unknown function | |
| 14. Others | Coagulation factor XII, partial Endonuclease domain-containing 1 5’ nucleotidase, partial (OH) | Unknown function |
| Spot No(s). | Protein type/family | Protein name |
|---|---|---|
| 1 and 3 | Cobra venom factor/complement C3 | OVF precursor |
| 2 | Three finger toxin | Cardiotoxin-like protein |
| 5 and 6 | Cysteine rich secretory protein (CRiSP) | Ophanin/opharin and opharin precursor |
| 7 and 10 | Three finger toxin which has ancestral ten cysteine arrangements; potent in birds/reptiles [61] | Weak neurotoxin precursor |
| 8, 9 and 10 | Three finger toxin | Long chain neurotoxins |
| 9 and 10 | Three finger toxin | short chain alpha neurotoxin |
| 4 | No match | No match |
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- World Health Organization. Neglected tropical diseases; snakebites 2011. Available online: http://www.who.int/neglected_diseases/diseases/snakebites/en/ (accessed on 23 February 2013).
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, J. The global burden of snakebites: A literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5. [Google Scholar] [CrossRef]
- Chippaux, J.P. The development and use of immunotherapy in Africa. Toxicon 1998, 36, 1503–1506. [Google Scholar] [CrossRef]
- Rojnuckarin, P.; Suteparak, S.; Sibunruang, S. Diagnosis and management of venomous snakebites in Southeast Asia. Asian Biomed. 2012, 6, 795–805. [Google Scholar]
- Warrell, D.A. WHO/SEARO guidelines for the clinical management of snakebites in the southeast Asian region. Southeast Asian J. Trop. Med. Public Health 1999, 30, 1–85. [Google Scholar]
- Theakston, R.D.; Warrell, D.A.; Griffiths, E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon 2003, 41, 541–557. [Google Scholar] [CrossRef]
- Vejayan, J.; Tang, S.M.; Halijah, I. The role of conventional two-dimensional electrophoresis (2DE) and its newer applications in the study of snake venoms. In Biochemistry, Genetics and Molecular Biology, “Proteomic Applications in Biology”; Heazelwood, J.L., Petzold, C.J., Eds.; InTech: Kuala Lumpur, Malaysia, 2012; pp. 1–29, ISBN 978-953-307-613-3. [Google Scholar]
- Ahmed, S.M.; Ahmed, M.; Nadeem, A.; Mahajan, J.; Choudhary, A.; Pal, J. Emergency treatment of a snake bite: Pearls from literature. J. Emerg. Trauma Shock 2008, 1, 97–105. [Google Scholar] [CrossRef]
- Kulkeaw, K.; Sakolvaree, Y.; Srimanote, P.; Tongtawe, P.; Maneewatch, S.; Sookrung, N.; Tungtrongchitr, A.; Tapchaisri, P.; Kurazono, H.; Chaicumpa, W. Human monoclonal ScFv neutralize lethal Thai cobra, Naja kaouthia, neurotoxin. J. Proteomics 2009, 72, 270–282. [Google Scholar] [CrossRef]
- Chavanayarn, C.; Thanongsaksrikul, J.; Thueng-in, K.; Bangphoomi, K.; Sookrung, N.; Chaicumpa, W. Humanized-single domain antibodies (VH/VHH) that bound specifically to Naja kaouthia phospholipase A2 and neutralized the enzymatic activity. Toxins 2012, 4, 554–567. [Google Scholar] [CrossRef]
- The National Center for Biotechnology Information (NCBI) Database. Available online: www. ncbi.nlm.nih.gov/ (accessed on 16 March 2014).
- NCBI/BLAST Home Page. Standard Protein BLAST Tool. Available online: http://www.ncbi.nlm.nih.gov/BLAST (accessed on 26 March 2014).
- Vonk, F.J.; Casewell, N.R.; Henkel, C.V.; Heimberg, A.M.; Jansen, H.J.; McCleary, R.J.R.; Kerkkamp, H.M.E.; Vos, R.A.; Guerreiro, I.; Calvete, J.J.; et al. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl. Acad. Sci. USA 2013, 110, 20651–20656. [Google Scholar] [CrossRef]
- Sakolvaree, Y.; Maneewatch, S.; Jiemsap, S.; Klaysing, B.; Tongtawe, P.; Srimanote, P.; Saengjaruk, P.; Banyen, S.; Tapchaisri, P.; Chongsa-nguan, M.; et al. Proteome and immunome of pathogenic Leptospira spp. revealed by 2DE and 2DE-immunoblotting with immune serum. Asian Pac. J. Allergy Immunol. 2008, 25, 53–73. [Google Scholar]
- Meier, J.; Theakston, R.D. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon 1986, 24, 395–401. [Google Scholar] [CrossRef]
- Wisniewski, M.S.; Hill, R.E.; Havey, J.M.; Bogdan, G.M.; Dart, R.C. Australiantigersnake (Notechis scutatus) and mexican coral snake (Micruris species) antivenoms prevent death from United States coral snake (Micrurus fulvius fulvius) venom in a mouse model. J. Toxicol. Clin. Toxicol. 2003, 41, 7–10. [Google Scholar] [CrossRef]
- Richardson, W.H., 3rd; Tanen, D.A.; Tong, T.C.; Betten, D.P.; Carstairs, S.D.; Williams, S.R.; Cantrell, F.L.; Clark, R.F. Crotalidae polyvalent immune Fab (ovine) antivenom is effective in the neutralization of South American Viperidae venoms in a murine model. Ann. Emerg. Med. 2005, 45, 598–602. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar]
- UniPort Home Page. Core Data: UniProtKB. Available online: http://www.uniprot.org (accessed on 26 March 2014).
- Protein Model Portal Home Page. Available online: http://www.proteinmodelportal.org (accessed on 26 March 2014).
- Research Collaboratory for Structural Bioinformatics Protein Data Bank Home Page. Available online: http://www.rcsb.org (accessed on 26 March 2014).
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9. [Google Scholar] [CrossRef]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protocols 2010, 5, 725–738. [Google Scholar] [CrossRef]
- Yang Zhang’s Research Group Home Page. Online Service: I-TASSER. Available online: http://zhanglab.ccmb.med.umich.edu/I-TASSER/ (accessed on 26 March 2014).
- Yang Zhang’s Research Group Home Page. Online Service: ModRefiner. Available online: http://zhanglab.ccmb.med.umich.edu/ModRefiner/ (accessed on 26 March 2014).
- Xu, D.; Zhang, Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys. J. 2011, 101, 2525–2534. [Google Scholar] [CrossRef]
- Yang Zhang’s Research Group Home Page. Online Service FG-MD. Available online: http://zhanglab.ccmb.med.umich.edu/FG-MD/ (accessed on 26 March 2014).
- Zhang, J.; Liang, Y.; Zhang, Y. Atomic-level protein structure refinement using fragment-guided molecular dynamics conformation sampling. Structure 2011, 19, 1784–1795. [Google Scholar] [CrossRef]
- Comeau, S.R.; Gatchell, D.W.; Vajda, S.; Camacho, C.J. ClusPro: An automated docking and discrimination method for the prediction of protein complexes. Bioinformatics 2004, 20, 45–50. [Google Scholar] [CrossRef]
- Comeau, S.R.; Gatchell, D.W.; Vajda, S.; Camacho, C.J. ClusPro: A fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004, 32, W96–W99. [Google Scholar] [CrossRef]
- Kozakov, D.; Brenke, R.; Comeau, S.R.; Vajda, S. PIPER: An FFT-based protein docking program with pairwise potentials. Proteins 2006, 65, 392–406. [Google Scholar] [CrossRef]
- Brenke, R.; Hall, D.R.; Chuang, G.Y.; Comeau, S.R.; Bohnuud, T.; Beglov, D.; Schueler-Furman, O.; Vajda, S.; Kozakov, D. Application of asymmetric statistical potentials to antibody-protein docking. Bioinformatics 2012, 28, 2608–2614. [Google Scholar] [CrossRef]
- Kozakov, D.; Beglov, D.; Bohnuud, T.; Mottarella, S.; Xia, B.; Hall, D.R.; Vajda, S. How good is automated protein docking? Prot. Struct. Funct. Bioinforma. 2013, 81, 2159–2166. [Google Scholar] [CrossRef]
- Huang, M.Z.; Gopalakrishnakone, P.; Chung, C.M.; Kini, R.M. Complete amino acid sequence of an acidic, cardiotoxic phospholipase A2 from the venom of Ophiophagus hannah (king cobra): A novel cobra venom enzyme with “pancreatic loop”. Arch. Biochem. Biophys. 1997, 338, 150–156. [Google Scholar] [CrossRef]
- Vejayan, J.; Shin, Y.L.; Ponnudurai, G.; Ambu, S.; Ibrahim, I. Protein profile analysis of Malaysian snake venoms by two-dimensional gel electrophoresis. J. Venom. Anim. Toxins.Trop. Dis. 2010, 16, 623–630. [Google Scholar] [CrossRef]
- Wej, J.E.; Lu, Q.M.; Lin, Y.; Li, D.S.; Xiong, Y.L.; Wang, W.Y. Alpha-neurotoxins of Naja atra and Naja kaouthia snakes in different regions. Acta Biochim. Biophy. Sin. 2003, 35, 683–688. [Google Scholar]
- Nawarak, J.; Sinchaikul, S.; Wu, C.Y.; Liau, M.Y.; Phutrakul, S.; Chen, S.T. Proteomics of snake venoms from Elapidae and Viperidae families by multidimensional chromatographic methods. Electrophoresis 2003, 24, 2838–2854. [Google Scholar] [CrossRef]
- Vonk, F.J. Snake evolution and prospecting of snake venom. Ph.D. Thesis., Faculty of Science, Leiden University, Leiden, The Netherlands, 6 September 2012. [Google Scholar]
- Chang, H.C.; Tsai, T.S.; Tsai, I.H. Functional proteomic approach to discover geographic variations of king cobra venoms from Southeast Asia and China. J. Proteomics 2013, 26, 141–153. [Google Scholar] [CrossRef]
- Kulkeaw, K.; Chaicumpa, W.; Sakolvaree, Y.; Tongtawe, P.; Tapchaisri, P. Proteome and immunome of the venom of the Thai cobra, Naja kaouthia. Toxicon 2007, 49, 1026–1041. [Google Scholar] [CrossRef]
- Chanhome, L.; Cox, M.J.; Vasaruchapong, T.; Chaiyabutr, N.; Sitprija, V. Characterization of venomous snakes of Thailand. Asian Biomedicin. 2011, 5, 311–328. [Google Scholar]
- Leong, P.K.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of Afro-Asian cobra and Asian krait venoms by a Thai polyvalent snake antivenom (Neuro Polyvalent Snake Antivenom). PLoS Negl. Trop. Dis. 2012, 6. [Google Scholar] [CrossRef]
- Peng, S.S.; Kumar, T.K.; Jayaraman, G.; Chang, C.C.; Yu, C. Solution structure of toxin B, a long neurotoxin from the venom of the king cobra (Ophiophagus hannah). J. Biol. Chem. 1997, 272, 7817–7823. [Google Scholar] [CrossRef]
- Chang, L.S.; Liou, J.C.; Lin, S.R.; Huang, H.B. Purification and characterization of a neurotoxin from the venom of Ophiophagus hannah (king cobra). Biochem. Biophys. Res. Commun. 2002, 294, 574–578. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Liu, J.; Xu, K. Novelgenes encoding six kinds of three finger toxins in Ophiophagus hannah (king cobra) and function characterization of two recombinant long-chain neurotoxins. Biochem. J. 2006, 398, 233–242. [Google Scholar] [CrossRef]
- He, Y.Y.; Lee, W.H.; Zhang, Y. Cloning and purification of alpha-neurotoxins from king cobra (Ophiophagus hannah). Toxicon 2004, 44, 295–303. [Google Scholar] [CrossRef]
- Zhang, H.L.; Xu, S.J.; Wang, Q.Y.; Song, S.Y.; Shu, Y.Y.; Lin, Z.J. Structure of a cardiotoxic phospholipase A(2) from Ophiophagus hannah with the “pancreatic loop”. J. Struct. Biol. 2002, 138, 207–215. [Google Scholar] [CrossRef]
- Rajagopalan, N.; Pung, Y.F.; Zhu, Y.Z.; Wong, P.T.; Kumar, P.P.; Kini, R.M. Beta-cardiotoxin: A new three-finger toxin from Ophiophagus hannah (king cobra) venom with beta-blocker activity. FASEB J. 2007, 21, 3685–3695. [Google Scholar] [CrossRef]
- Joubert, F.J. Snake venoms. The amino-acid sequence of polypeptide DE-1 from Ophiophagus hannah (king cobra) venom. Hoppe Seylers Z. Physiol. Chem. 1977, 358, 565–574. [Google Scholar] [CrossRef]
- Tsai, H.I. Phospholipase A2 of Asian snake venoms. J. Toxicol. Toxin Rev. 1997, 16, 79–114. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef]
- Wang, L.Y.; Kuo, H.J.; Lee, C.H. Cobra CRISP functions as an inflammatory modulator via a novel Zn2+- and heparan sulfate-dependent transcriptional regulation of endothelial cell adhesion molecules. J. Biol. Chem. 2010, 285, 37872–37883. [Google Scholar] [CrossRef]
- Wang, J.; Shen, B.; Guo, M.; Lou, X.; Duan, Y.; Cheng, X.P.; Teng, M.; Niu, L.; Liu, Q.; Huang, Q.; et al. lockingeffect and crystalstructure of natrintoxin, a cysteine-richsecretoryprotein from Naja atra venom that targets the BKCa channel. Biochemistry 2005, 44, 10145–10152. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Koike, H.; Sugiyama, Y.; Motoyoshi, K.; Wada, T.; Hishinuma, S.; Mita, M.; Morita, T. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur. J. Biochem. 2002, 269, 2708–2715. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, Q.Y.; Jin, Y.; Zhang, Y.; Lee, W.H.; Zhang, Y. Molecularcloning and characterization of a complement-depletingfactor from kingcobra, Ophiophagus hannah. Toxicon 2012, 60, 290–301. [Google Scholar] [CrossRef]
- Jerusalinsky, D.; Kornisiuk, E.; Alfaro, P. Muscarinictoxins: Novelpharmacologicaltools for the muscariniccholinergic system. Toxicon 2000, 38, 747–761. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, W.H.; Zeng, L.; Zhang, Y. Molecular characterization of L-amino acid oxidase from king cobra venom. Toxicon 2007, 50, 479–489. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yu, T.F.; Lian, E.C. Purification and characterization of L-amino acid oxidase from king cobra (Ophiophagus hannah) venom and its effects on human platelet aggregation. Toxicon 1994, 32, 1349–1358. [Google Scholar] [CrossRef]
- Tan, N.H.; Fung, S.Y. Snake venom L-amino acid oxidases and their potential biomedical applications. Malaysian J. Biochem. Mol. Biol. 2008, 16, 1–10. [Google Scholar]
- Pung, Y.F.; Wong, P.T.; Kumar, P.P.; Hodgson, W.C.; Kini, R.M. Ohanin, a novel protein from king cobra venom, induces hypolocomotion and hyperalgesia in mice. J. Biol. Chem. 2005, 280, 13137–13147. [Google Scholar]
- Rokyta, R.D.; Wray, P.K.; Lemmon, R.A.; Lemmon, E.M.; Caudle, S.B. A high-throughput venom-gland transcriptome for the eastern diamond back rattle snake (Crotalus adamanteus) and evidence for pervasive positive selection across toxin classes. Toxicon 2000, 38, 747–761. [Google Scholar] [CrossRef]
- Chen, R.Q.; Jin, Y.; Wu, J.B.; Zhou, X.D.; Lu, Q.M.; Wang, W.Y.; Xiong, Y.L. A new protein structure of P-II class snake venom metalloproteinases: It comprises metalloproteinase and disintegrin domains. Biochem. Biophys. Res. Commun. 2003, 310, 182–187. [Google Scholar] [CrossRef]
- Guo, X.X.; Zeng, L.; Lee, W.H.; Zhang, Y.; Jin, Y. Isolation and cloning of a metalloproteinase from king cobra snake venom. Toxicon 2007, 49, 954–965. [Google Scholar] [CrossRef]
- Gao, R.; Manjunatha, K.R.; Gopalakrishnakone, P. A novel prothrombin activator from the venom of Micropechis ikaheka: Isolation and characterization. Arch. Biochem. Biophys. 2002, 408, 87–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, W.H.; Xiong, Y.L.; Wang, W.Y.; Zu, S.W. Characterization of OhS1, an arginine/lysine amidase from the venom of king cobra (Ophiophagus hannah). Toxicon 1994, 32, 615–623. [Google Scholar] [CrossRef]
- He, Y.Y.; Liu, S.B.; Lee, W.H. Isolation, expression and characterization of a novel dual serine protease inhibitor, OH-TCI, from king cobra venom. Peptides 2008, 29, 1692–1699. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Danpaiboon, W.; Reamtong, O.; Sookrung, N.; Seesuay, W.; Sakolvaree, Y.; Thanongsaksrikul, J.; Dong-din-on, F.; Srimanote, P.; Thueng-in, K.; Chaicumpa, W. Ophiophagus hannah Venom: Proteome, Components Bound by Naja kaouthia Antivenin and Neutralization by N. kaouthia Neurotoxin-Specific Human ScFv. Toxins 2014, 6, 1526-1558. https://doi.org/10.3390/toxins6051526
Danpaiboon W, Reamtong O, Sookrung N, Seesuay W, Sakolvaree Y, Thanongsaksrikul J, Dong-din-on F, Srimanote P, Thueng-in K, Chaicumpa W. Ophiophagus hannah Venom: Proteome, Components Bound by Naja kaouthia Antivenin and Neutralization by N. kaouthia Neurotoxin-Specific Human ScFv. Toxins. 2014; 6(5):1526-1558. https://doi.org/10.3390/toxins6051526
Chicago/Turabian StyleDanpaiboon, Witchuda, Onrapak Reamtong, Nitat Sookrung, Watee Seesuay, Yuwaporn Sakolvaree, Jeeraphong Thanongsaksrikul, Fonthip Dong-din-on, Potjanee Srimanote, Kanyarat Thueng-in, and Wanpen Chaicumpa. 2014. "Ophiophagus hannah Venom: Proteome, Components Bound by Naja kaouthia Antivenin and Neutralization by N. kaouthia Neurotoxin-Specific Human ScFv" Toxins 6, no. 5: 1526-1558. https://doi.org/10.3390/toxins6051526
APA StyleDanpaiboon, W., Reamtong, O., Sookrung, N., Seesuay, W., Sakolvaree, Y., Thanongsaksrikul, J., Dong-din-on, F., Srimanote, P., Thueng-in, K., & Chaicumpa, W. (2014). Ophiophagus hannah Venom: Proteome, Components Bound by Naja kaouthia Antivenin and Neutralization by N. kaouthia Neurotoxin-Specific Human ScFv. Toxins, 6(5), 1526-1558. https://doi.org/10.3390/toxins6051526






