Occurrence of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Grown in the USA
Abstract
:1. Introduction
2. Results and Discussions
2.1. Wheat Kernel Quality
| Factors | 2011 | 2012 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | MIN | MAX | Mean | SD | MIN | MAX | |
| Dockage (%) a | 1.1 | 1.4 | 0.0 | 13.4 | 1.1 | 1.2 | 0.0 | 13.3 |
| Shrunken & broken a | 1.6 | 1.4 | 0.0 | 10.0 | 1.2 | 1.1 | 0.0 | 10.1 |
| Damage (%) a | 0.5 | 0.8 | 0.0 | 10.6 | 0.1 | 0.2 | 0.0 | 2.8 |
| Hard and vitreous kernel (%) a | 75.8 | 21.9 | 5.0 | 99.0 | 74.5 | 28.4 | 2.0 | 99.0 |
| Test weight (lbs/bu) a | 60.0 | 2.4 | 52.5 | 65.7 | 60.9 | 1.9 | 53.9 | 65.1 |
| Total defects (%) a | 2.1 | 1.7 | 0.1 | 13.1 | 1.3 | 1.1 | 0.0 | 10.1 |
| Protein (12% mb) b | 14.8 | 1.3 | 10.2 | 18.6 | 14.6 | 1.4 | 10.3 | 20.1 |
| Falling number (s) b | 386.3 | 44.1 | 226.0 | 621.0 | 429.3 | 48.8 | 238.0 | 654.0 |
| 1000 kernel weight (g) b | 26.7 | 4.1 | 16.8 | 40.0 | 29.1 | 4.1 | 16.6 | 44.1 |
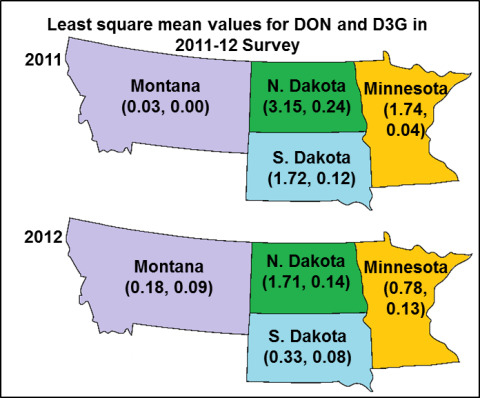
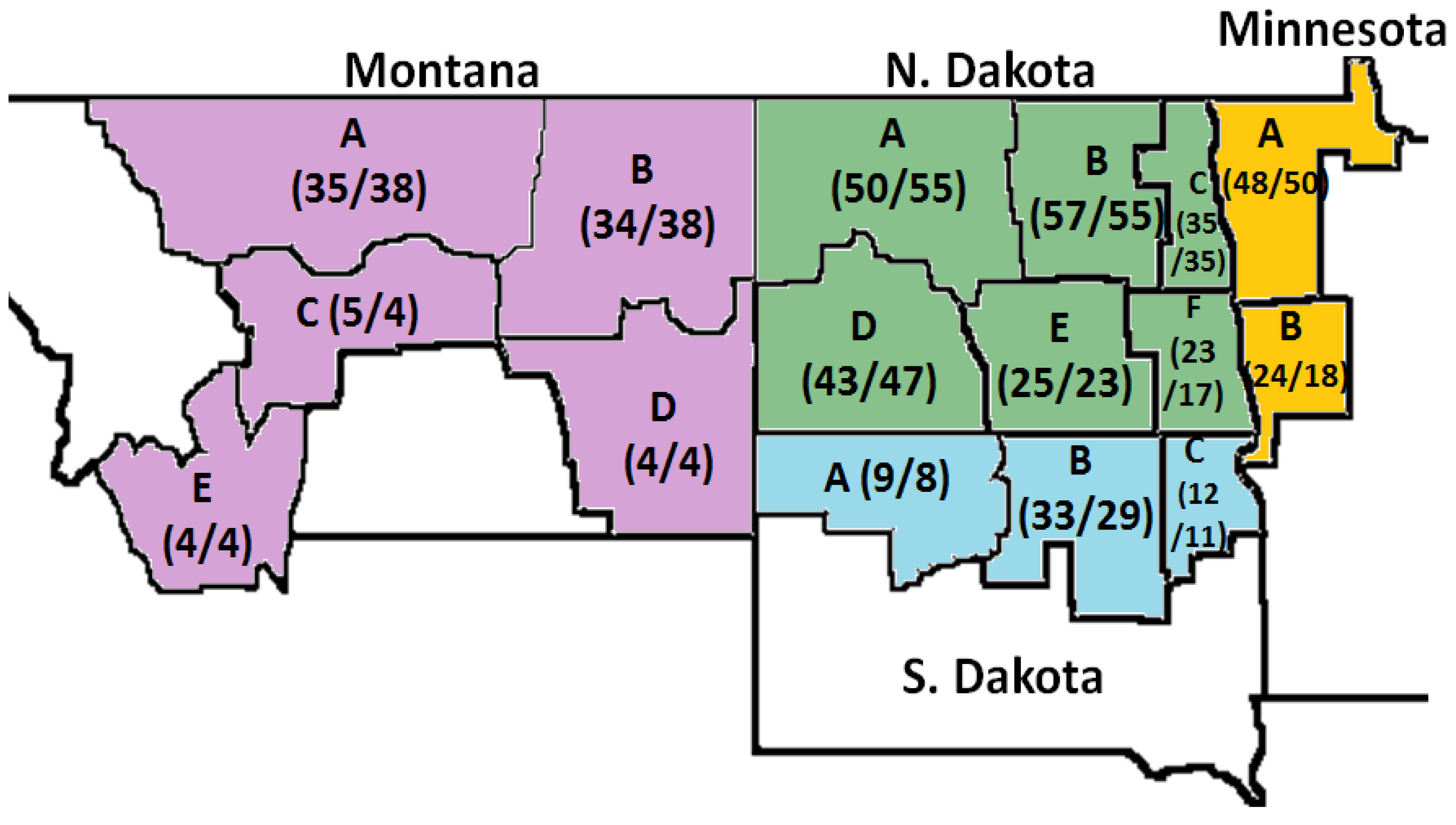
2.2. Effect of the State and Region on DON, D3G and Damage Kernel
| Growing Area | 2011 | 2012 | ||||||
|---|---|---|---|---|---|---|---|---|
| State | GC-DON | LC-DON | D3G | Damage (%) | GC-DON | LC-DON | D3G | Damage (%) |
| MN | 1.35 ** | 1.74 ** | 0.04 | 0.35 ** | 0.89 | 0.78 | 0.128 ** | 0.05 |
| MT | 0.03 | 0.03 | 0.00 | 0.03 | 0.18 | 0.18 | 0.090 | 0.00 |
| ND | 2.80*** | 3.15 *** | 0.24 *** | 0.63 *** | 1.93 *** | 1.71 *** | 0.142 *** | 0.06 *** |
| SD | 1.35 ** | 1.72 * | 0.12 | 0.36 * | 0.37 | 0.33 | 0.085 | 0.03 |
| Region | ||||||||
| MN-A | 1.07 | 1.21 | 0.01 | 0.23 | 0.82 | 0.72 | 0.124 * | 0.08 * |
| MN-B | 1.63 * | 2.27 ** | 0.06 | 0.47 * | 0.96 | 0.84 | 0.133 | 0.02 |
| MT-A | 0.00 | 0.00 | 0.00 | 0.01 | 0.09 | 0.12 | 0.067 | 0.01 |
| MT-B | 0.00 | 0.00 | 0.00 | 0.05 | 0.04 | 0.05 | 0.176 ** | 0.00 |
| MT-C | 0.13 | 0.13 | 0.00 | 0.03 | 0.11 | 0.20 | 0.105 | 0.00 |
| MT-D | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.08 | 0.015 | 0.00 |
| MT-E | 0.04 | 0.00 | 0.00 | 0.00 | 0.65 | 0.47 | 0.088 | 0.00 |
| ND-A | 3.89 *** | 4.26 *** | 0.31 *** | 0.75 *** | 6.91 *** | 5.97 *** | 0.295 *** | 0.15 *** |
| ND-B | 3.77 *** | 4.32 *** | 0.30 *** | 0.69 *** | 2.48 *** | 2.22 *** | 0.174 *** | 0.12 *** |
| ND-C | 2.91 *** | 3.36 *** | 0.30 *** | 0.61 *** | 0.66 | 0.60 | 0.060 | 0.06 |
| ND-D | 1.08 | 1.10 | 0.02 | 0.61 *** | 0.34 | 0.35 | 0.094 | 0.01 |
| ND-E | 2.19 ** | 2.54 ** | 0.09 | 0.66 *** | 0.71 | 0.72 | 0.096 | 0.02 |
| ND-F | 3.00 ** | 3.34 *** | 0.39 *** | 0.49 ** | 0.47 | 0.38 | 0.133 | 0.01 |
| SD-A | 0.21 | 0.15 | 0.00 | 0.13 | 0.49 | 0.42 | 0.094 | 0.00 |
| SD-B | 2.41 *** | 3.20 *** | 0.29 ** | 0.57 ** | 0.22 | 0.23 | 0.097 | 0.00 |
| SD-C | 1.42 | 1.82 | 0.06 | 0.38 | 0.41 | 0.34 | 0.066 | 0.09 |
| Year | Source | Degrees of freedom | Mean square | |||
|---|---|---|---|---|---|---|
| GC-DON | LC-DON | D3G | Damage | |||
| 2011 | ST | 3 | 221.3 *** | 282.8 *** | 2.15 *** | 10.19 *** |
| Rga (ST) | 12 | 28.0 ** | 40.4 *** | 0.43 * | 0.45 | |
| CTY (Rga × ST) | 100 | 8.9 | 13.2 | 0.19 | 0.44 | |
| Residual | 320 | 9.7 | 14.0 | 0.24 | 0.70 | |
| 2012 | ST | 3 | 151.2 *** | 111.2 *** | 0.15 *** | 0.22 ** |
| Rga (ST) | 12 | 104.3 *** | 72.8 *** | 0.12 | 0.12 *** | |
| CTY (Rga × ST) | 100 | 14.5 | 11.3 | 0.07 | 0.04 | |
| Residual | 320 | 12.0 | 10.9 | 0.07 | 0.04 | |
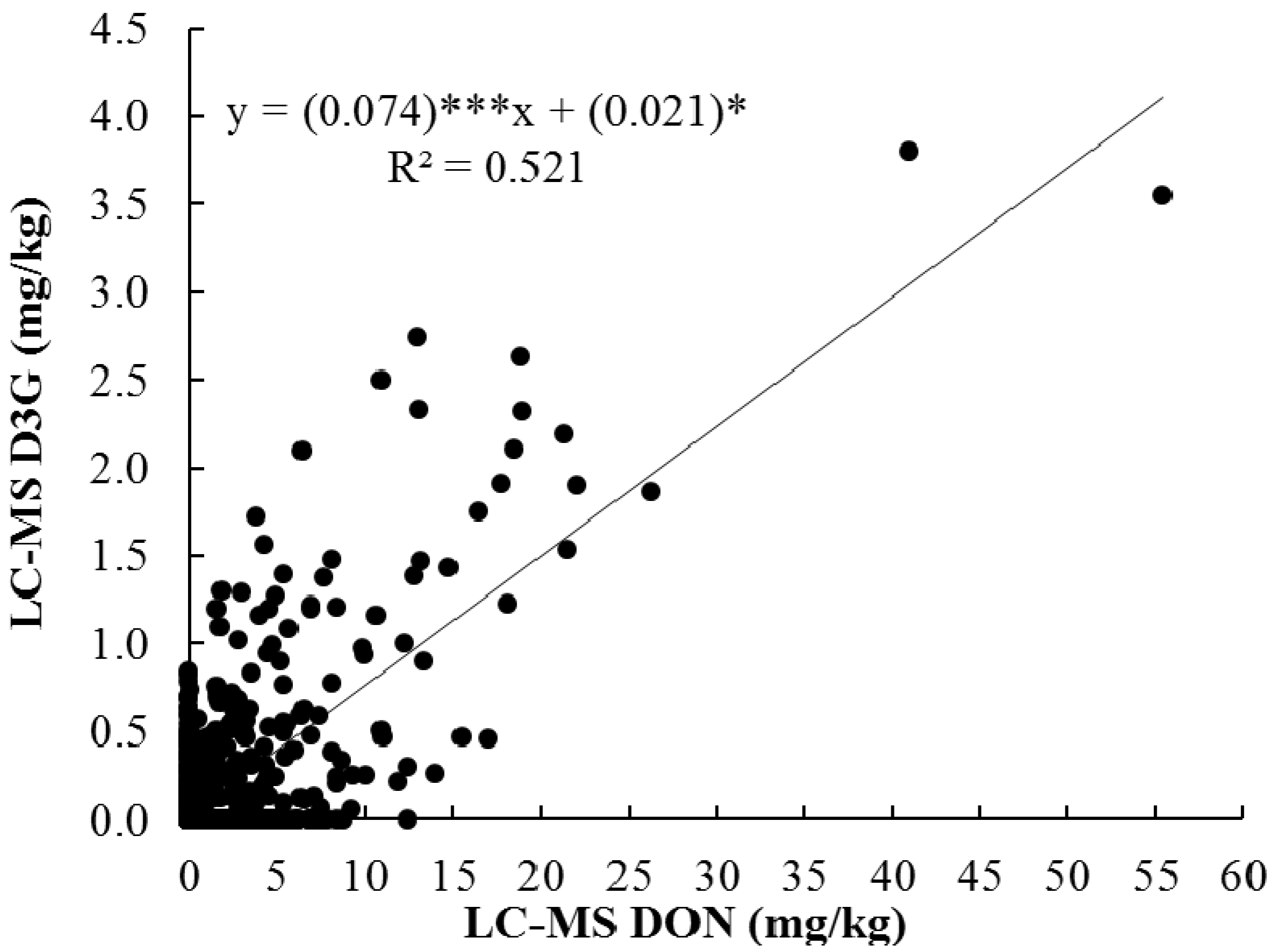
2.3. Correlation among DON, D3G with Percent Damaged Kernels

| Year | Variables | GC-DON | LC-DON | D3G | Damage |
|---|---|---|---|---|---|
| Linear correlation | |||||
| 2011 | GC-DON | - | 0.99 *** | 0.91 *** | 0.91 *** |
| LC-DON | 0.99 *** | - | 0.91 *** | 0.91 *** | |
| D3G | 0.94 *** | 0.92 *** | - | 0.74 ** | |
| Damage | 0.89 *** | 0.89 *** | 0.87 *** | - | |
| Rank correlation | |||||
| Linear correlation | |||||
| 2012 | GC-DON | - | 1.00 *** | 0.83 *** | 0.79 *** |
| LC-DON | 0.99 *** | - | 0.83 *** | 0.79 *** | |
| D3G | 0.41NS | 0.37NS | - | 0.60 * | |
| Damage | 0.72 ** | 0.69 ** | 0.28NS | - | |
| Rank correlation | |||||

3. Experimental Section
3.1. Standards and Chemicals
3.2. Samples
3.3. Wheat Kernel Quality
3.4. Sample Preparation for GC–ECD and LC-MS
3.4.1. Free DON Analysis with GC–ECD
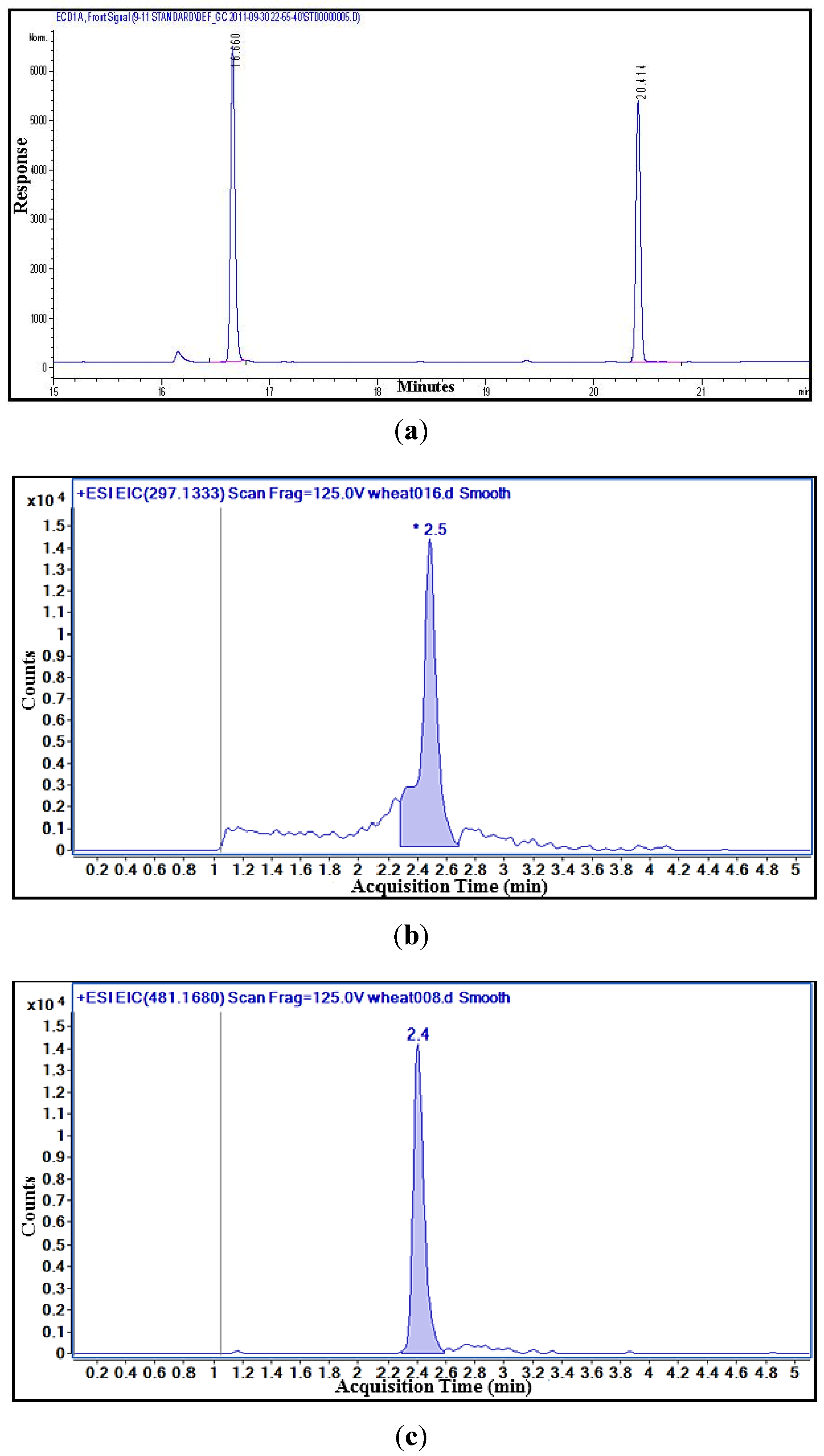
3.4.2. Free DON and D3G Analysis with LC-MS
3.4.3. LC-MS Instrumentation and Methodology
3.5. Statistics Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wegulo, S. Factors influencing deoxinivalenol accumulation in small grain cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef]
- Simsek, S.; Burgess, K.; Whitney, K.L.; Gu, Y.; Qian, S.Y. Analysis of Deoxynivalenol and deoxynivalenol-3-glucoside in wheat. Food Control 2012, 26, 287–292. [Google Scholar] [CrossRef]
- Paul, P.A.; Lipps, P.E.; Madden, L.V. Relationship between visual estimates of fusarium head blight intensity and deoxynivalenol accumulation in harvested wheat grain: A meta-analysis. Phytopathology 2005, 95, 1225–1236. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium species. J. R. Soc. Interface 2011, 8, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F-graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef]
- Muhovski, Y.; Batoko, H.; Jacquemin, J.M. Identification, characterization and mapping of differentially expressed genes in a winter wheat cultivar (Centenaire) resistant to Fusarium graminearum infection. Mol. Biol. Reports 2012, 39, 9583–9600. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Arunachalam, C.; Doohan, F.M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar] [CrossRef]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef]
- Desmarchelier, A.; Seefelder, W. Survey of deoxynivalenol and deoxynivalenol-3-glucoside in cereal-based products by liquid chromatography electrospray ionization tandem mass spectrometry. World Mycotoxin J. 2011, 4, 29–35. [Google Scholar] [CrossRef]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glossl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef]
- Berthiller, F.; Schuhmacher, R.; Adam, G.; Krska, R. Formation, determination and significance of masked and other conjugated mycotoxins. Anal. Bioanal. Chem. 2009, 395, 1243–1252. [Google Scholar] [CrossRef]
- Rasmussen, P.H.; Nielsen, K.F.; Ghorbani, F.; Spliid, N.H.; Nielsen, G.C.; Jørgensen, L.N. Occurrence of different trichothecenes and deoxynivalenol-3-b-d-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin Res. 2012, 28, 181–190. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall'Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterhazy, A.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for fusarium head blight resistance in wheat. Mol. Plant-Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef]
- Nagl, V.; Schwartz, H.; Krska, R.; Moll, W.D.; Knasmuller, S.; Ritzmann, M.; Adam, G.; Berthiller, F. Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol. Lett. 2012, 213, 367–373. [Google Scholar] [CrossRef]
- European Food Safety Authority. Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379–3435. [Google Scholar]
- U.S. Hard Red Spring Wheat 2012 Regional Quality Report. Available online: http://www.uswheat.org/cropQuality/doc/1B247A99F59BC22785257C15006659F3/$File/HRS2012.pdf?OpenElement# (accessed on 16 December 2013).
- Serranti, S.; Cesare, D.; Bonifazi, G. The development of a hyperspectral imaging method for the detection of Fusarium-damaged, yellow berry and vitreous Italian durum wheat kernels. Biosyst. Eng. 2013, 115, 20–30. [Google Scholar] [CrossRef]
- Garrido-Lestache, E.; Lopez-Bellido, R.J.; Lopez-Bellido, L. Effect of N rate, timing and splitting and N type on bread-making quality in hard red spring wheat under rainfed Mediterranean conditions. Field Crops Res. 2004, 85, 213–236. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists International (AACCI). Approved Methods of the American Association of Cereal Chemists; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Tacke, B.K.; Casper, H.H. Determination of deoxynivalenol in wheat, barley, and malt by column cleanup and gas chromatography with electron capture detection. J. AOAC Int. 1996, 79, 472–475. [Google Scholar]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC-MS-MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Simsek, S.; Ovando-Martínez, M.; Ozsisli, B.; Whitney, K.; Ohm, J.-B. Occurrence of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Grown in the USA. Toxins 2013, 5, 2656-2670. https://doi.org/10.3390/toxins5122656
Simsek S, Ovando-Martínez M, Ozsisli B, Whitney K, Ohm J-B. Occurrence of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Grown in the USA. Toxins. 2013; 5(12):2656-2670. https://doi.org/10.3390/toxins5122656
Chicago/Turabian StyleSimsek, Senay, Maribel Ovando-Martínez, Bahri Ozsisli, Kristin Whitney, and Jae-Bom Ohm. 2013. "Occurrence of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Grown in the USA" Toxins 5, no. 12: 2656-2670. https://doi.org/10.3390/toxins5122656