Vixapatin (VP12), a C-Type Lectin-Protein from Vipera xantina palestinae Venom: Characterization as a Novel Anti-angiogenic Compound
Abstract
:1. Introduction
2. Results and Discussion
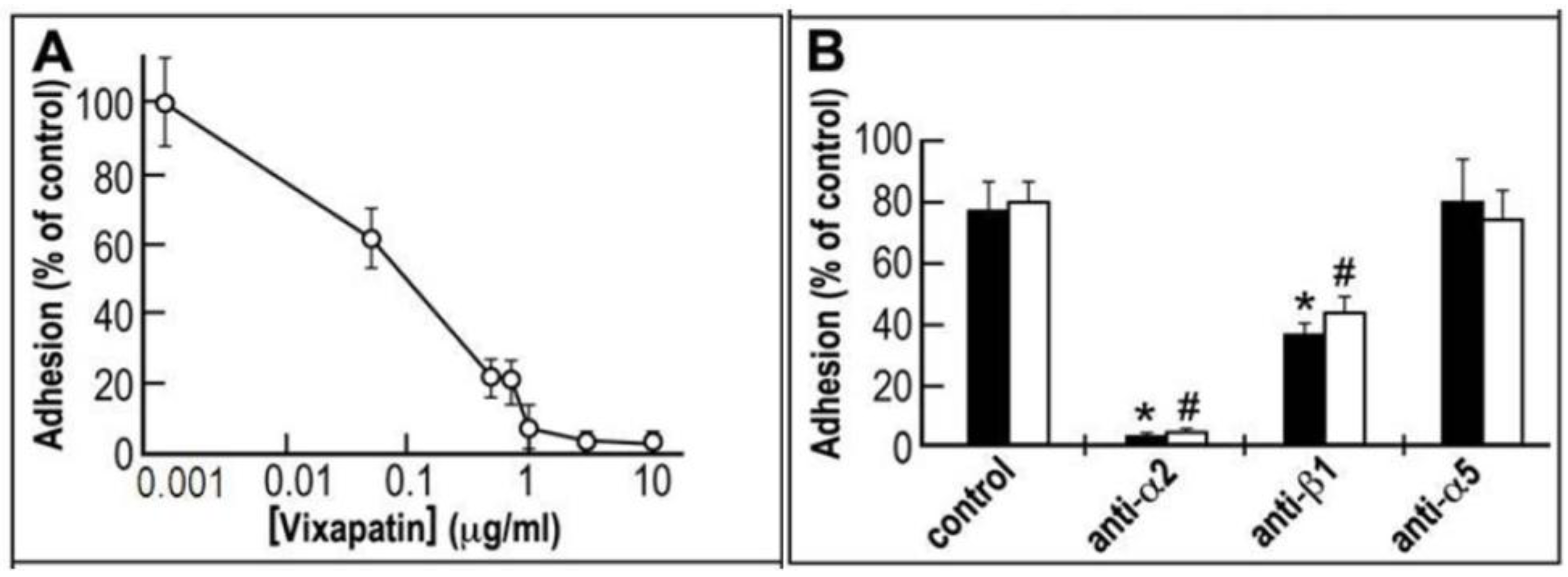
2.1. Anti-adhesive Properties of Vixapatin
2.2. Effect of Vixapatin on Proliferation of HDMEC
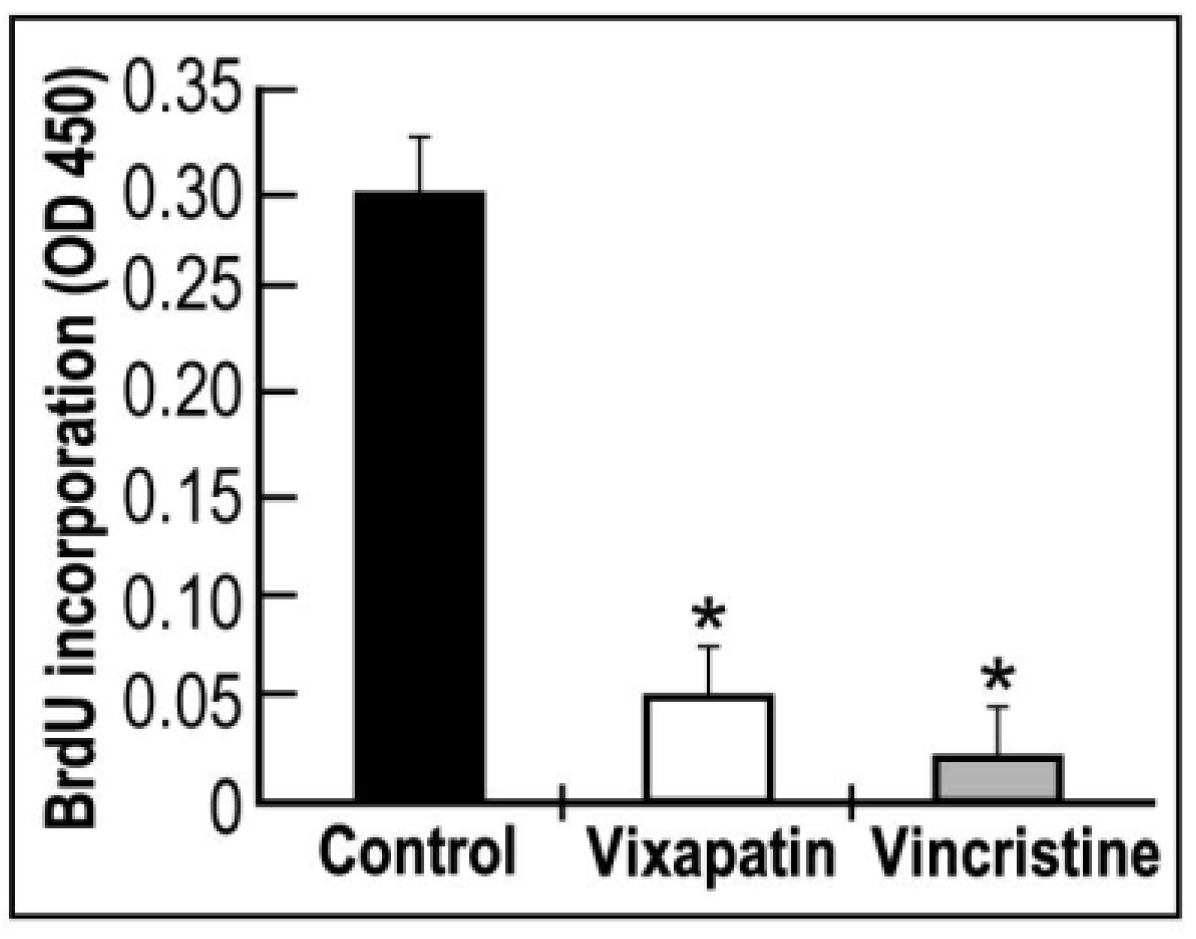
2.3. Effect of Vixapatin on Cell Migration

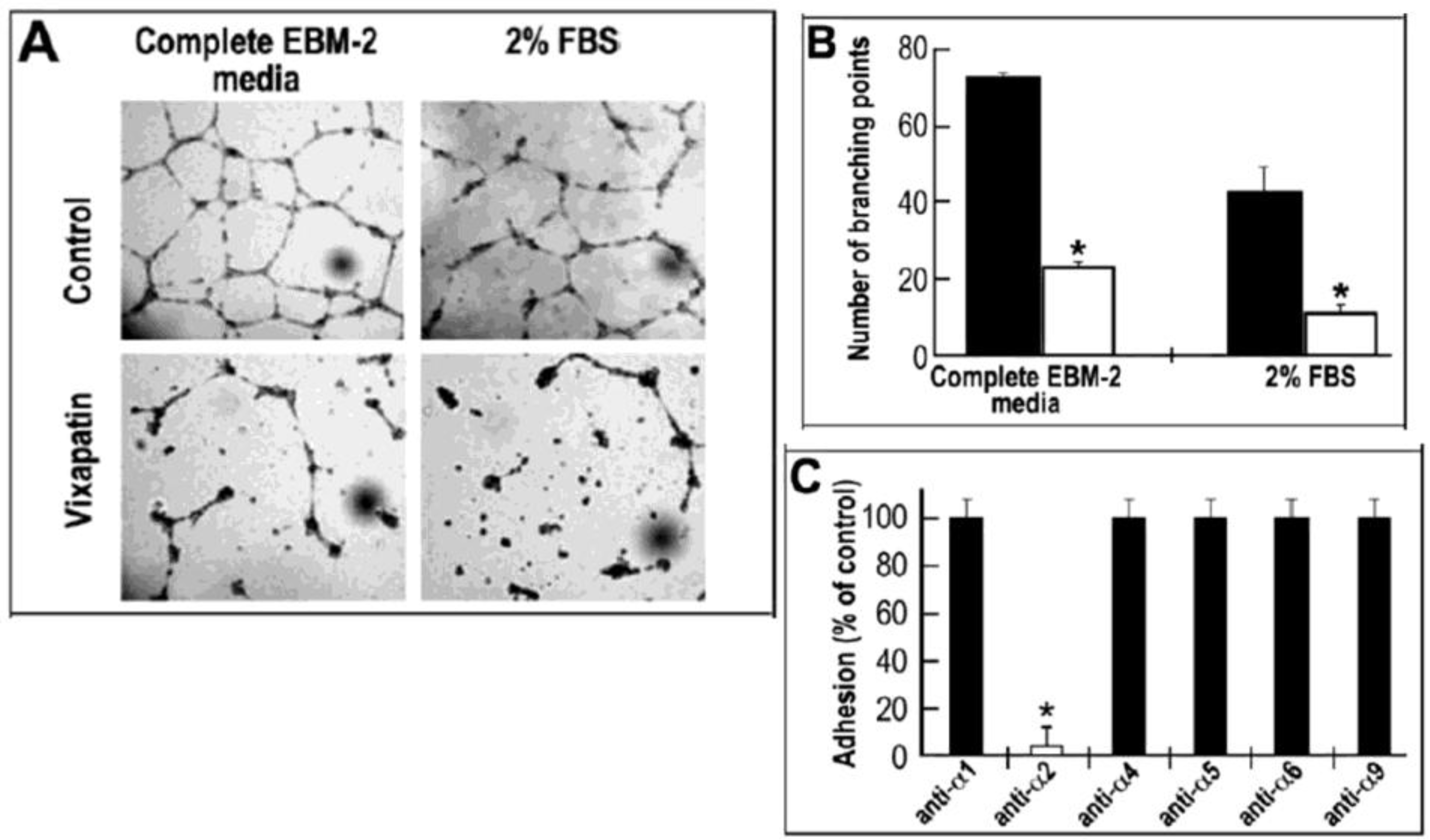
2.4. Effect of Vixapatin on Tube Formation in Matrigel Assay
2.5. Effect of Vixapatin on bFGF Induced Physiological Angiogenesis in the CAM Quail Embryonic Model

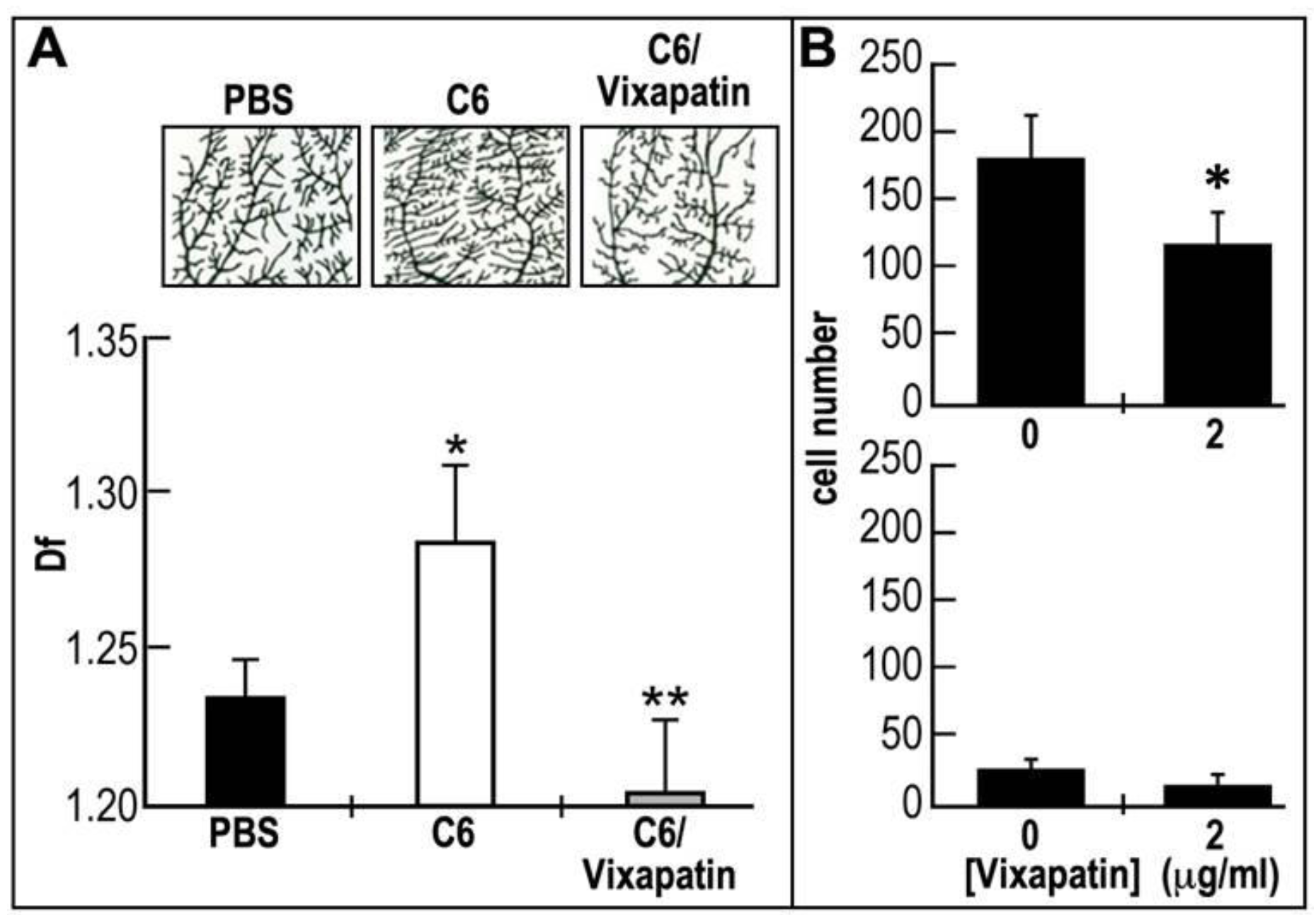
2.6. Effect of Vixapatin on C6-Induced Pathological Angiogenesis in the CAM Quail Embryonic Model
2.7. Discussion
3. Experimental Section
3.1. Materials
3.2. C-Type Lectin-Like Proteins
3.3. Cell Lines
3.4. Cell Adhesion Studies
3.5. Cell Proliferation Assay
3.6. Cell Migration Assay
3.7. Human Aortic Endothelial Cells Tube Formation in Matrigel Assay
3.8. Angiogenesis in Chorioallantoic Membrane (CAM) Quail Embryonic Model
3.9. Statistics
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Kini, R.M. Toxins in thrombosis and haemostasis: Potential beyond imagination. J. Thromb. Haemost. 2011, 9, 195–208. [Google Scholar] [CrossRef]
- Vanhoorelbeke, K.; Ulrichts, H.; Schoolmeester, A.; Deckmyn, H. Inhibition of platelet adhesion to collagen as a new target for antithrombotic drugs. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2003, 3, 125–140. [Google Scholar] [CrossRef]
- Chung, C.-H.; Wu, W.-B.; Huang, T.-F. Aggretin, a snake venom-derived endothelial integrin alpha2beta1 angiogenesis drug development agonist, induces angiogenesis via expression of vascular endothelial growth factor. Blood 2004, 103, 2105–2113. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S.; Dennes, A.; Schmidt-Hederich, A.; Bruckner, P.; Brunner, G. Rhodocetin antagonizes stromal tumor invasion in vitro and other alpha2beta1 angiogenesis drug developmen tintegrin-mediated cell functions. Matrix Biol. 2002, 21, 547–558. [Google Scholar] [CrossRef]
- Marcinkiewicz, C. Functional characteristic of snake venom disintegrins: Potential therapeutic implication. Curr. Pharm. Des. 2005, 11, 815–827. [Google Scholar] [CrossRef]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef]
- Drickamer, K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999, 9, 585–590. [Google Scholar] [CrossRef]
- Lu, Q.; Clementson, K.J.; Clementson, J.M. Snake venom c-type lectins interacting with platelet receptors. Tox. Rev. 2007, 26, 77–93. [Google Scholar] [CrossRef]
- Morita, T. Structures and functions of snake venom clps (C-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet-modulating activities. Toxicon 2005, 45, 1099–1114. [Google Scholar] [CrossRef]
- Hynes, O.R. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Senger, D.R.; Claffey, K.P.; Benes, J.E.; Perruzzi, C.A.; Sergiou, A.P.; Detmar, M. Angiogenesis promoted by vascular endothelial growth factor: Regulation through α1β1and α2β1integrins. Proc. Natl. Acad. Sci. USA 1997, 94, 13612–13617. [Google Scholar]
- Lussier, C.; Basora, N.; Bouatrouss, Y.; Beaulieu, J.-F. Integrins as mediators of epithelial cell-matrix interactions in the human small intestinal mucosa. Microsc. Res. Tech. 2000, 51, 169–178. [Google Scholar] [CrossRef]
- Veit, G.; Zwolanek, D.; Eckes, B.; Niland, S.; Kapyla, J.; Zweers, M.C.; Ishada-Yamamoto, A.; Krieg, T.; Heino, J.; Eble, J.A.; et al. Collagen xxiii, novel ligand for integrin α2β1 in the epidermis. J. Biol. Chem. 2011, 286, 27804–27813. [Google Scholar]
- Dickeson, S.K.; Mathis, N.L.; Rahman, M.; Bergelson, J.M.; Santoro, S.A. Determinants of ligand binding specificity of the α1β1 and α2β1 integrins. J. Biol. Chem. 1999, 274, 32182–32191. [Google Scholar]
- Lecut, C.; Feijge, M.A.H.; Cosemans, J.M.E.M.; Jandrot-Perrus, M.; Heemskerk, J.W.M. Fibrillar type I collagens enhance platelet-dependent thrombin generation via glycoprotein vi with direct support of α2β1 but not αIIbβ3 integrin. Thromb. Haemost. 2005, 94, 107–114. [Google Scholar]
- Mergia, E.; Russwurm, M.; Zoidl, G.; Koesling, D. Major occurrence of the new α2β1 isoform of no-sensitive guanylyl cyclase in brain. Cell Signal. 2003, 15, 189–195. [Google Scholar] [CrossRef]
- Friedl, P.; Maaser, K.; Klein, C.E.; Niggemann, B.; Krohne, G.; Zanker, K.S. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of α2 and β1 integrins and CD44. Cancer Res. 1997, 57, 2061–2070. [Google Scholar]
- Maaser, K.; Wolf, K.; Klein, C.E.; Niggemann, B.; Zanker, K.S.; Brocker, E.-B.; Friedl, P. Functional hierarchy of simultaneously expressed adhesion receptors: Integrin α2β1 but not CD44 mediates MV3 melanoma cell migration and matrix reorganization within three-dimensional hyaluronan-containing collagen matrices. Mol. Biol. Cell 1999, 10, 3067–3079. [Google Scholar]
- Chan, B.; Matsuura, N.; Takada, Y.; Zetter, B.; Hemler, M. In vitro and in vivo consequences of vla-2 expression on rhabdomyosarcoma cells. Science 1991, 251, 1600–1602. [Google Scholar]
- Fishman, D.A.; Kearns, A.; Chilukuri, K.; Bafetti, L.M.; O’Toole, E.A.; Georgacopoulos, J.; Ravosa, M.J.; Stack, M.S. Metastatic dissemination of human ovarian epithelial carcinoma is promoted by α2β1-integrin-mediated interaction with type I collagen. Invasion Metastasis 1998, 18, 15–26. [Google Scholar] [CrossRef]
- Lochter, A.; Navre, M.; Werb, Z.; Bissell, M.J. α1 and α2 integrins mediate invasive activity of mouse mammary carcinoma cells through regulation of stromelysin-1 expression. Mol. Biol. Cell 1999, 10, 271–282. [Google Scholar]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef]
- Marcinkiewicz, C.; Lobb, R.R.; Marcinkiewicz, M.M.; Daniel, J.L.; Smith, J.B.; Dangelmaier, C.; Weinreb, P.H.; Beacham, D.A.; Niewiarowski, S. Isolation and characterization of EMS16, a C-lectin type protein from Echis multisquamatus venom, a potent and selective inhibitor of the α2β1 integrin. Biochemistry 2000, 39, 9859–9867. [Google Scholar]
- Eble, J.A.; Beermann, B.; Hinz, H.-J.; Schmidt-Hederich, A. α2β1 integrin is not recognized by rhodocytin but is the specific, high affinity target of rhodocetin, an RGD-independent disintegrin and potent inhibitor of cell adhesion to collagen. J. Biol. Chem. 2001, 276, 12274–12284. [Google Scholar]
- Staniszewska, I.; Walsh, E.M.; Rothman, V.L.; Gaathon, A.; Tuszynski, G.P.; Calvete, J.J.; Lazarovici, P.; Marcinkiewicz, C. Effect of VP12 and viperistatin on inhibition of collagen receptors: Dependent melanoma metastasis. Cancer Biol. Ther. 2009, 8, 1507–1516. [Google Scholar] [CrossRef]
- Momic, T.; Arlinghaus, F.T.; Arien-Zakay, H.; Katzhendler, J.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Pharmacological aspects of Vipera xantina palestinae venom. Toxins 2011, 3, 1420–1432. [Google Scholar] [CrossRef]
- Eble, J.A.; Tuckwell, D.S. The alpha2beta1 integrin inhibitor rhodocetin binds to the A-domain of the integrin alpha2 subunit proximal to the collagen-binding site. Biochem. J. 2003, 376, 77–85. [Google Scholar] [CrossRef]
- Arlinghaus, F.T.; Momic, T.; Abu Ammar, N.; Shai, E.; Spectre, G.; Varon, D.; Marcinkiewicz, C.; Lazarovici, P.; Eble, J.A. Detection of α2β1 integrin inhibitor(s) with anti-platelet properties in the venom of Vipera palaestinae. Toxicon 2012. submitted for publication. [Google Scholar]
- Kaikai, S.; Yuchen, S.; Lili, J.; Zhengtao, W. Critical role of c-Jun N-terminal kinase in regulating bFGF-induced angiogenesis in vitro. J. Biochem. 2011, 150, 189–197. [Google Scholar] [CrossRef]
- Zutter, M.M.; Santoro, S.A.; Staatz, W.D.; Tsung, Y.L. Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc. Natl. Acad. Sci. USA 1995, 92, 7411–7415. [Google Scholar] [CrossRef]
- Tuckwell, D.S.; Smith, L.; Korda, M.; Askari, J.A.; Santoso, S.; Barnes, M.J.; Farndale, R.W.; Humphries, M.J. Monoclonal antibodies identify residues 199-216 of the integrin alpha2 vWFA domain as a functionally important region within alpha2beta1. Biochem. J. 2000, 350, 485–493. [Google Scholar]
- Kern, A.; Marcantonio, E.E. Role of the I-domain in collagen binding specificity and activation of the integrins α1β1 and α2β1. J. Cell Physiol. 1998, 176, 634–641. [Google Scholar] [CrossRef]
- Ruggiero, F.; Comte, J.; Cabanas, C.; Garrone, R. Structural requirements for alpha 1 beta 1 and alpha 2 beta 1 integrin mediated cell adhesion to collagen V. J. Cell Sci. 1996, 109, 1865–1874. [Google Scholar]
- Lichtner, R.B.; Howlett, A.R.; Lerch, M.; Xuan, J.-A.; Brink, J.; Langton-Webster, B.; Schneider, M.R. Negative cooperativity between alpha3beta1 and alpha2beta1 integrins in human mammary carcinoma MDA MB 231 cells. Exp. Cell Res. 1998, 240, 368–376. [Google Scholar] [CrossRef]
- Obeso, J.; Weber, J.; Auerbach, R. A hemangioendothelioma-derived cell line: Its use as a model for the study of endothelial cell biology. Lab Invest. 1990, 63, 259–269. [Google Scholar]
- Zhang, Z.; Ramirez, N.E.; Yankeelov, T.E.; Li, Z.; Ford, L.E.; Qi, Y.; Pozzi, A.; Zutter, M.M. Alpha2beta1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood 2008, 111, 1980–1988. [Google Scholar] [CrossRef]
- Parsons-Wingerter, P.; Lwai, B.; Yang, M.C.; Elliott, K.E.; Milaninia, A.; Redlitz, A.; Clark, J.I.; Sage, E.H. A novel assay of angiogenesis in the quail chorioallantoic membrane: Stimulation by bfgf and inhibition by angiostatin according to fractal dimension and grid intersection. Microvasc. Res. 1998, 55, 201–214. [Google Scholar] [CrossRef]
- Giannopoulou, E.; Katsoris, P.; Hatziapostolou, M.; Kardamakis, D.; Kotsaki, E.; Polytarchou, C.; Parthymou, A.; Papaioannou, S.; Papadimitriou, E. X-rays modulate extracellular matrix in vivo. Int. J. Cancer 2001, 94, 690–698. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Z.; Lin, J.; Weng, S.; Huang, Q.; Zhang, P.; Fu, J. Antitumor effect of endostatin overexpressed in C6 glioma cells is associated with the down-regulation of VEGF. Int. J. Oncol. 2011, 38, 465–471. [Google Scholar]
- Brill, A.; Dashevsky, O.; Rivo, J.; Gozal, Y.; Varon, D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [Google Scholar] [CrossRef]
- Niewiarowska, J.; Brézillon, S.; Sacewicz-Hofman, I.; Bednarek, R.; Maquart, F.-X.; Malinowski, M.; Wiktorska, M.; Wegrowski, Y.; Cierniewski, C.S. Lumican inhibits angiogenesis by interfering with α2β1 receptor activity and downregulating mmp-14 expression. Thromb. Res. 2011, 128, 452–457. [Google Scholar] [CrossRef]
- Bix, G.; Fu, J.; Gonzalez, E.M.; Macro, L.; Barker, A.; Campbell, S.; Zutter, M.M.; Santoro, S.A.; Kim, J.K.; Hook, M.; et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin. J. Cell Biol. 2004, 166, 97–109. [Google Scholar] [CrossRef]
- Woodall, B.P.; Nystrom, A.; Iozzo, R.A.; Eble, J.A.; Niland, S.; Krieg, T.; Eckes, B.; Pozzi, A.; Iozzo, R.V. Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J. Biol. Chem. 2008, 283, 2335–2343. [Google Scholar]
- Chung, C.-H.; Wu, W.-B.; Huang, T.-F. Aggretin, a snake venom-derived endothelial integrin α2β1 agonist, induces angiogenesis via expression of vascular endothelial growth factor. Blood 2004, 103, 2105–2113. [Google Scholar] [CrossRef]
- Sabherwal, Y.; Rothman, V.L.; Dimitrov, S.; L’Heureux, D.Z.; Marcinkiewicz, C.; Sharma, M.; Tuszynski, G.P. Integrin α2β1 mediates the anti-angiogenic and anti-tumor activities of angiocidin, a novel tumor-associated protein. Exp. Cell Res. 2006, 312, 2443–2453. [Google Scholar] [CrossRef]
- Kim, H.-K.; Oh, D.-S.; Lee, S.-B.; Ha, J.-M.; Joe, Y.A. Antimigratory effect of TK1-2 is mediated in part by interfering with integrin α2β1. Mol. Cancer Ther. 2008, 7, 2133–2141. [Google Scholar] [CrossRef]
- Antonio, S.J.D.; Zoeller, J.J.; Habursky, K.; Turner, K.; Pimtong, W.; Burrows, M.; Choi, S.; Basra, S.; Bennett, J.S.; DeGrado, W.F.; et al. A key role for the integrin α2β1 in experimental and developmental angiogenesis. Am. J. Pathol. 2009, 175, 1338–1347. [Google Scholar] [CrossRef]
- Dolla, J.-P.; Rezvan, A.; Allen, F.D.; Lazarovici, P.; Lelkes, P.I. Nerve growth factor-induced migration of endothelial cells. J. Pharmacol. Exp. Ther. 2005, 315, 1220–1227. [Google Scholar] [CrossRef]
- Bazan-Socha, S.; Kisiel, D.G.; Young, B.; Theakston, R.D.G.; Calvete, J.J.; Sheppard, D.; Marcinkiewicz, C. Structural requirements of MLD-containing disintegrins for functional interaction with alpha4beta1 and alpha9beta1 integrins. Biochemistry 2004, 43, 1639–1647. [Google Scholar]
- Staniszewska, I.; Zaveri, S.; Valle, L.D.; Oliva, I.; Rothman, V.L.; Croul, S.E.; Roberts, D.D.; Mosher, D.F.; Tuszynski, G.P.; Marcinkiewicz, C. Interaction of α9β1 integrin with thrombospondin-1 promotes angiogenesis. Circ. Res. 2007, 100, 1308–1316. [Google Scholar] [CrossRef]
- Reich, R.; Royce, L.; Martin, G.R. Eicosapentaenoic acid reduces the invasive and metastatic activities of malignant tumor cells. Biochem. Biophys. Res. Commun. 1989, 160, 559–564. [Google Scholar] [CrossRef]
- Brown, M.C.; Staniszewska, I.; del Valle, L.; Tuszynski, G.P.; Marcinkiewicz, C. Angiostatic activity of obtustatin as α1β1 integrin inhibitor in experimental melanoma growth. Int. J. Cancer 2008, 123, 2195–2203. [Google Scholar] [CrossRef]
- Lazarovici, P.; Gazit, A.; Staniszewska, I.; Marcinkiewicz, C.; Lelkes, P.I. Nerve growth factor (NGF) promotes angiogenesis in the quail chorioallantoic membrane. Endothelium 2006, 13, 51–59. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Momic, T.; Cohen, G.; Reich, R.; Arlinghaus, F.T.; Eble, J.A.; Marcinkiewicz, C.; Lazarovici, P. Vixapatin (VP12), a C-Type Lectin-Protein from Vipera xantina palestinae Venom: Characterization as a Novel Anti-angiogenic Compound. Toxins 2012, 4, 862-877. https://doi.org/10.3390/toxins4100862
Momic T, Cohen G, Reich R, Arlinghaus FT, Eble JA, Marcinkiewicz C, Lazarovici P. Vixapatin (VP12), a C-Type Lectin-Protein from Vipera xantina palestinae Venom: Characterization as a Novel Anti-angiogenic Compound. Toxins. 2012; 4(10):862-877. https://doi.org/10.3390/toxins4100862
Chicago/Turabian StyleMomic, Tatjana, Gadi Cohen, Reuven Reich, Franziska T. Arlinghaus, Johannes A. Eble, Cezary Marcinkiewicz, and Philip Lazarovici. 2012. "Vixapatin (VP12), a C-Type Lectin-Protein from Vipera xantina palestinae Venom: Characterization as a Novel Anti-angiogenic Compound" Toxins 4, no. 10: 862-877. https://doi.org/10.3390/toxins4100862





