Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Experimental Protocol
2.3. Agent Preparations
2.4. MTT Test
2.5. Caco-2 Permeability Assay
2.6. GTL-16 Permeability Assay
2.7. Apparent Permeability Coefficient (Papp)
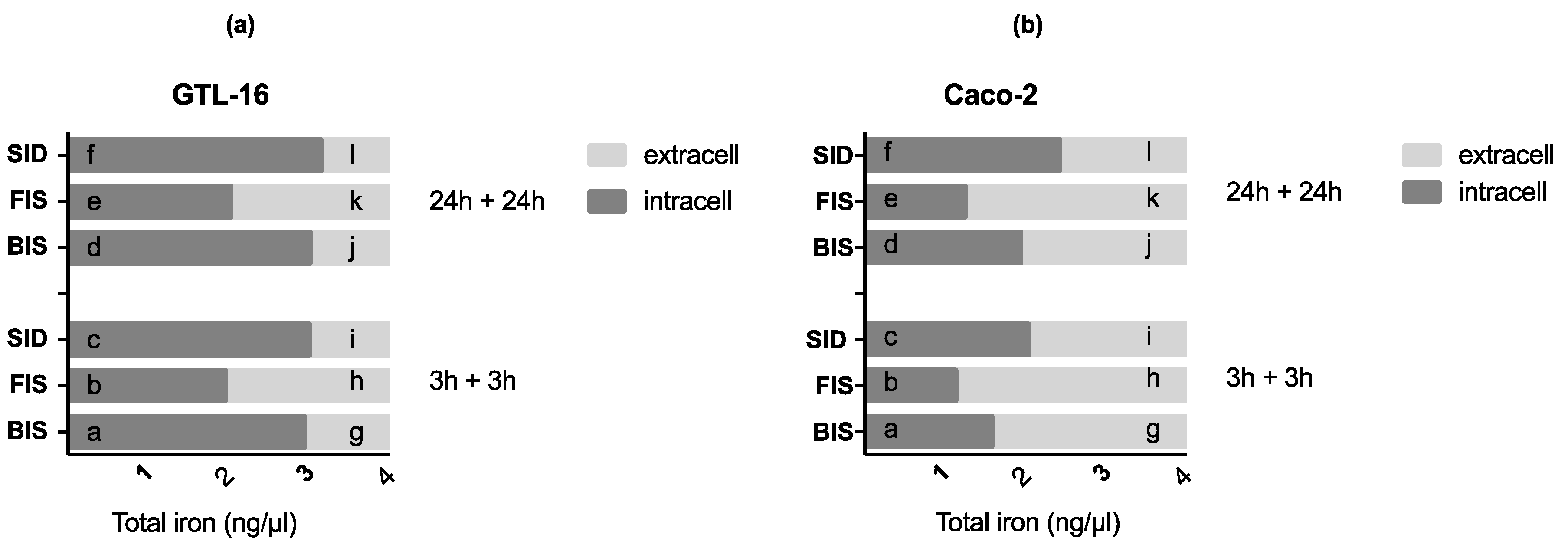
2.8. Iron Quantification Assay
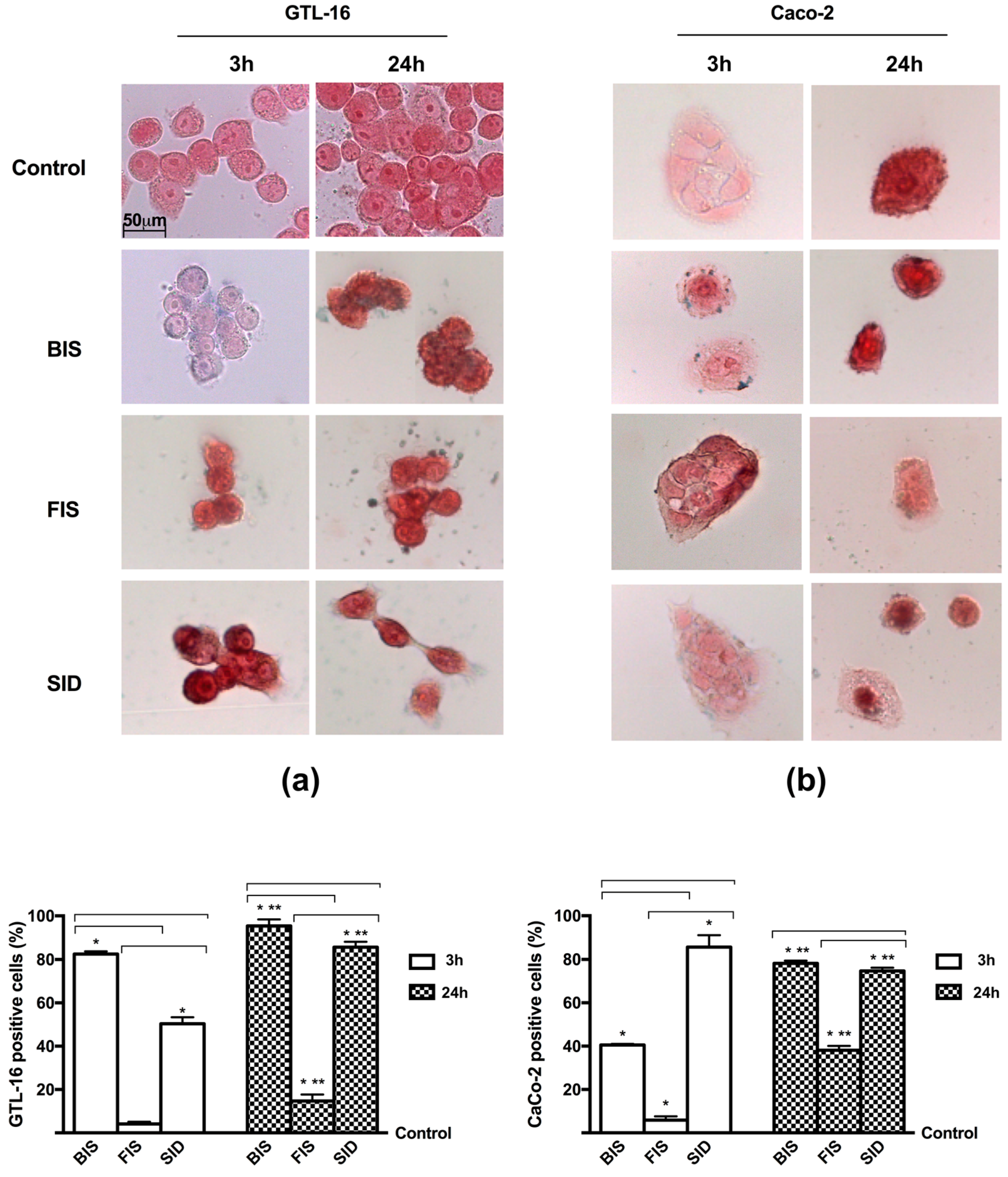
2.9. Iron Histochemistry
2.10. IL-8 Assay Kit
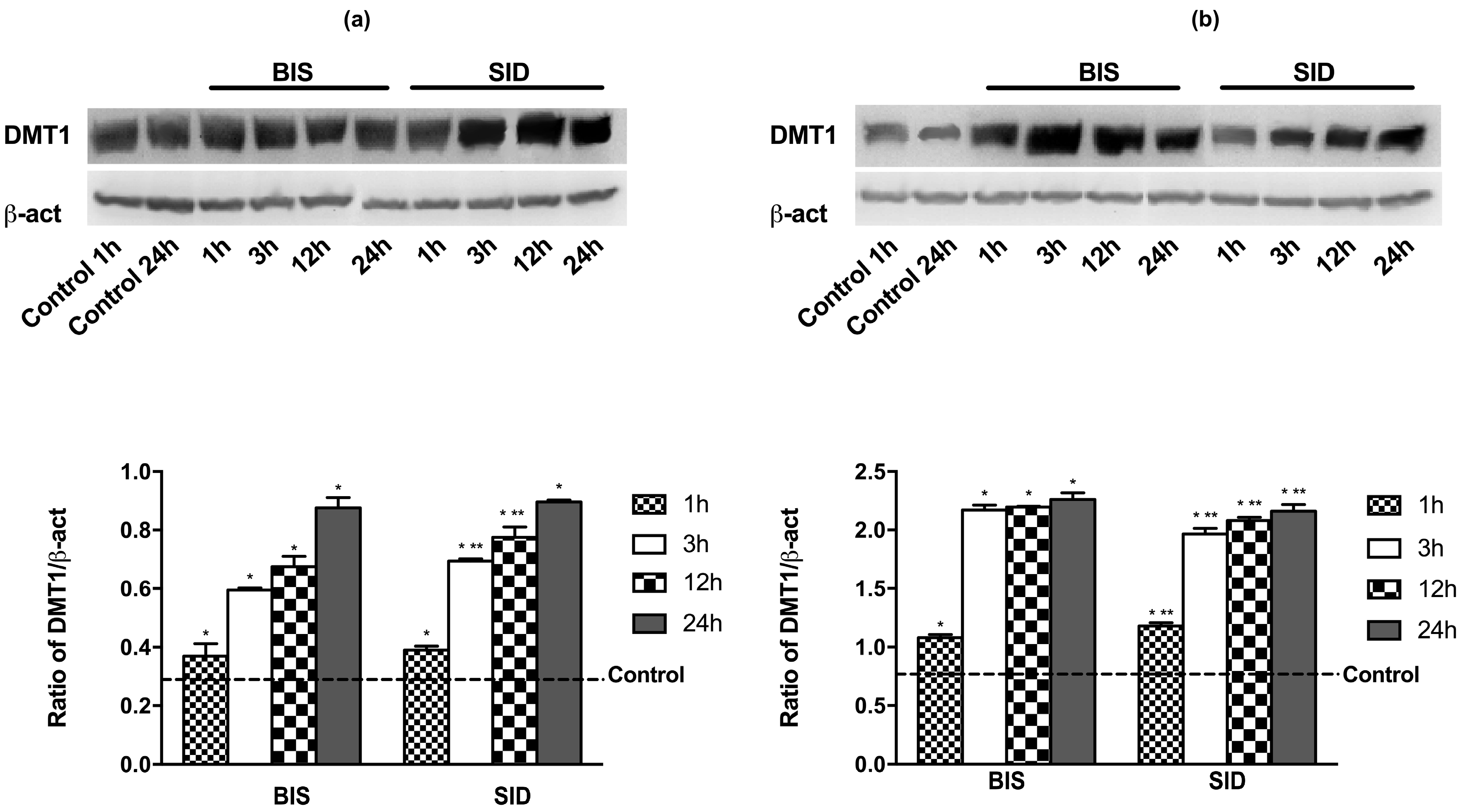
2.11. Western Blot of Cell Lysates
2.12. Statistical Analysis
3. Results
3.1. Time-Course Study on DMT1 Expression
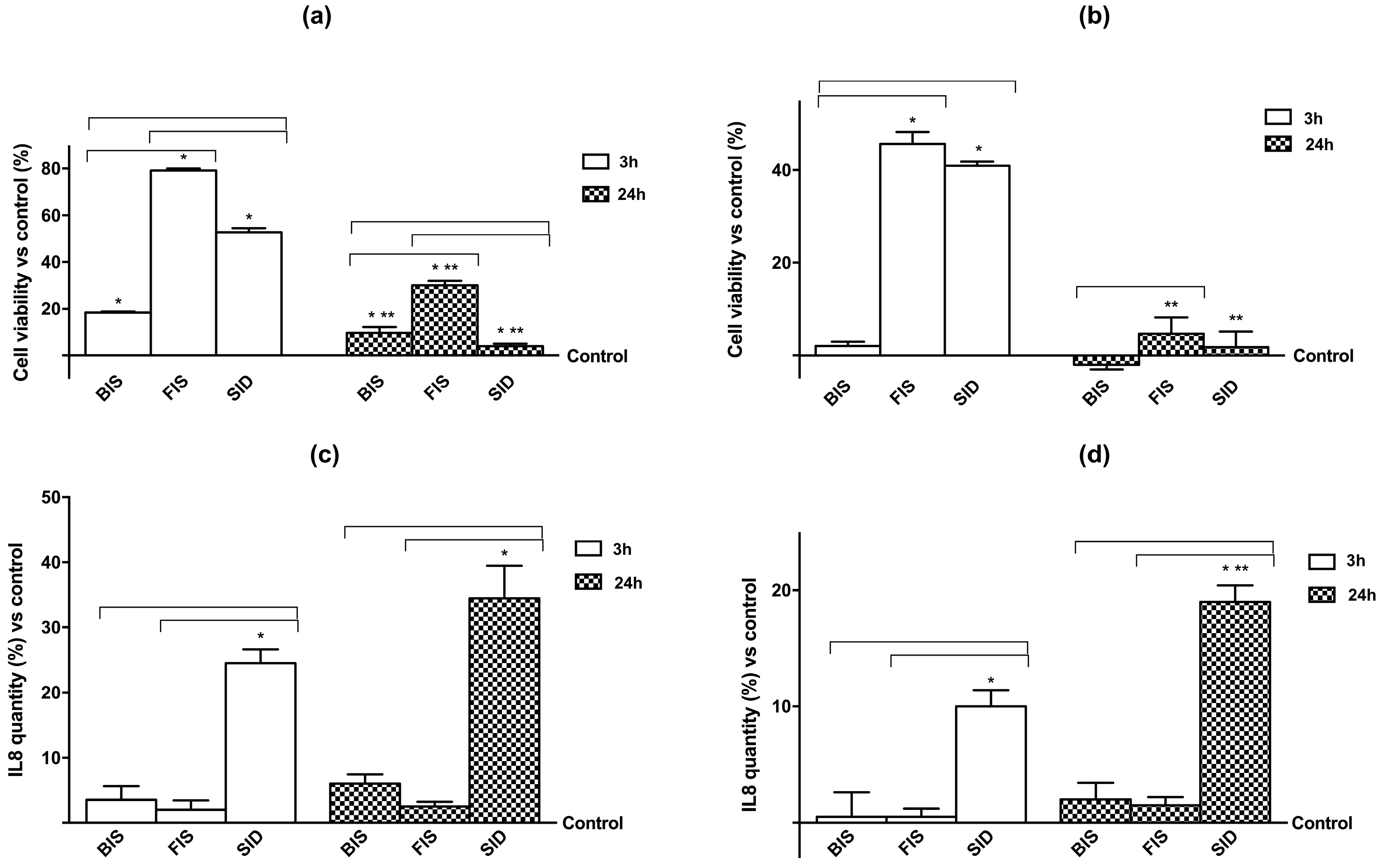
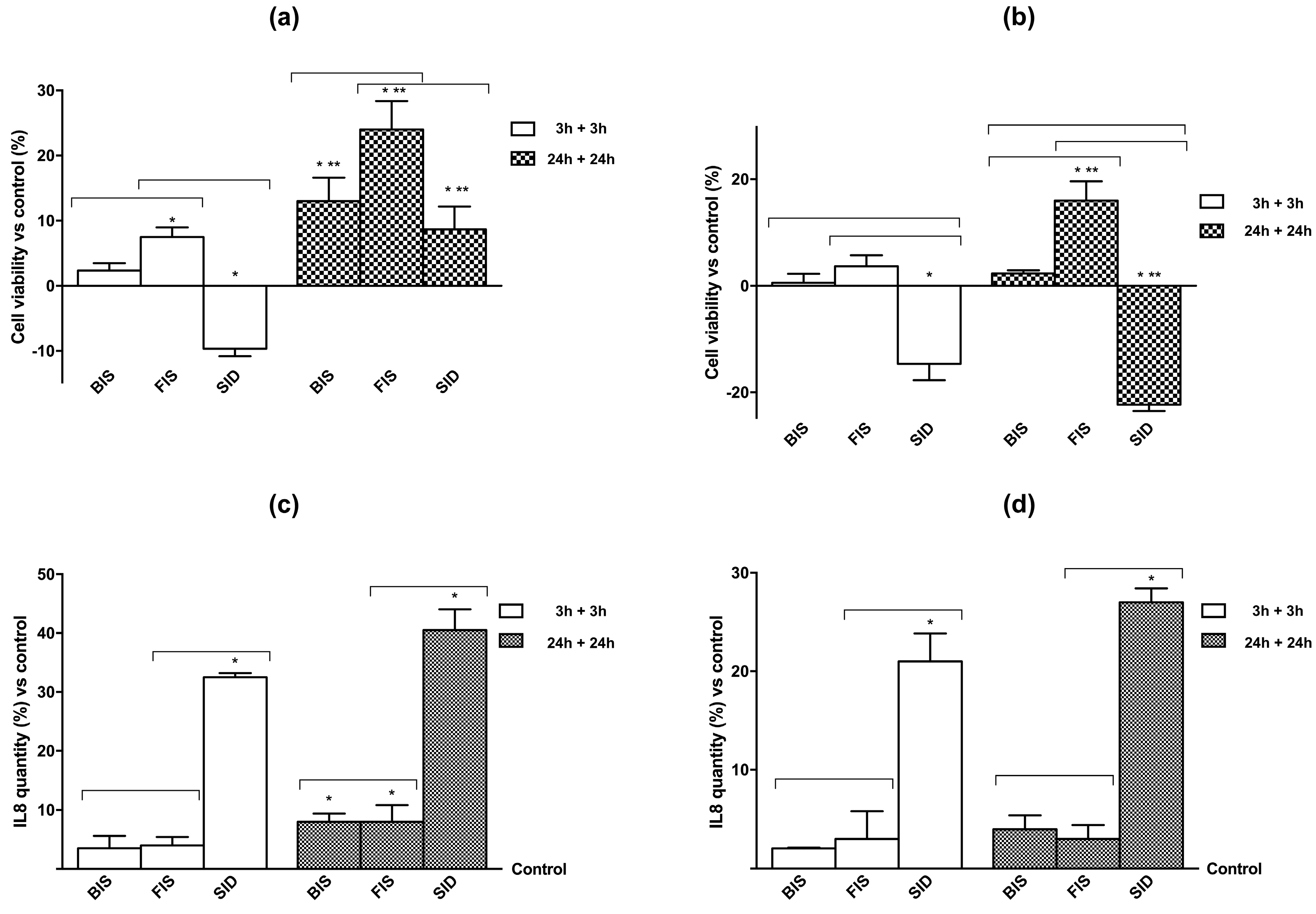
3.2. Cell Viability and Cell Regulation
3.3. Iron Deposition and Quantification
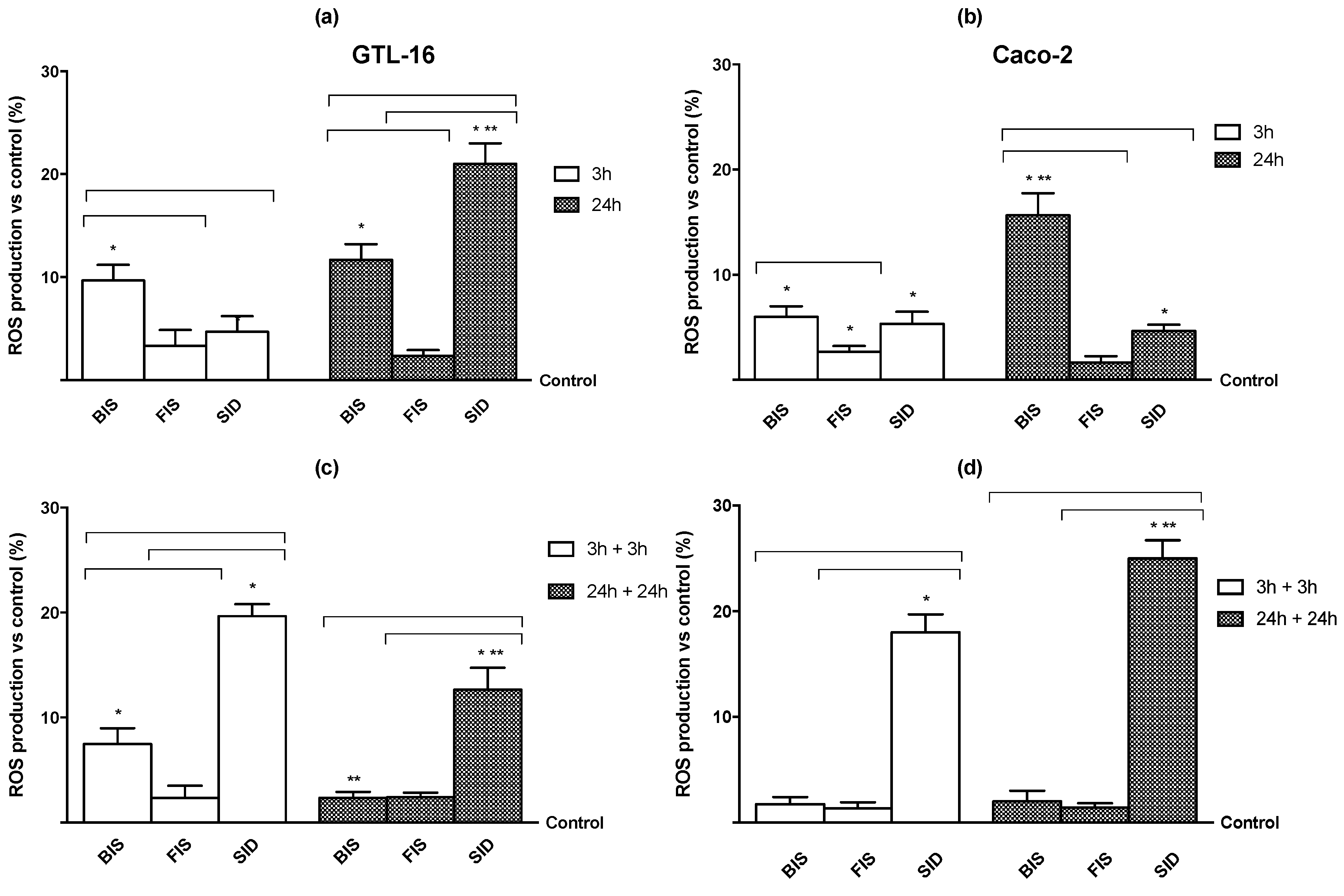
3.4. Iron Mechanisms
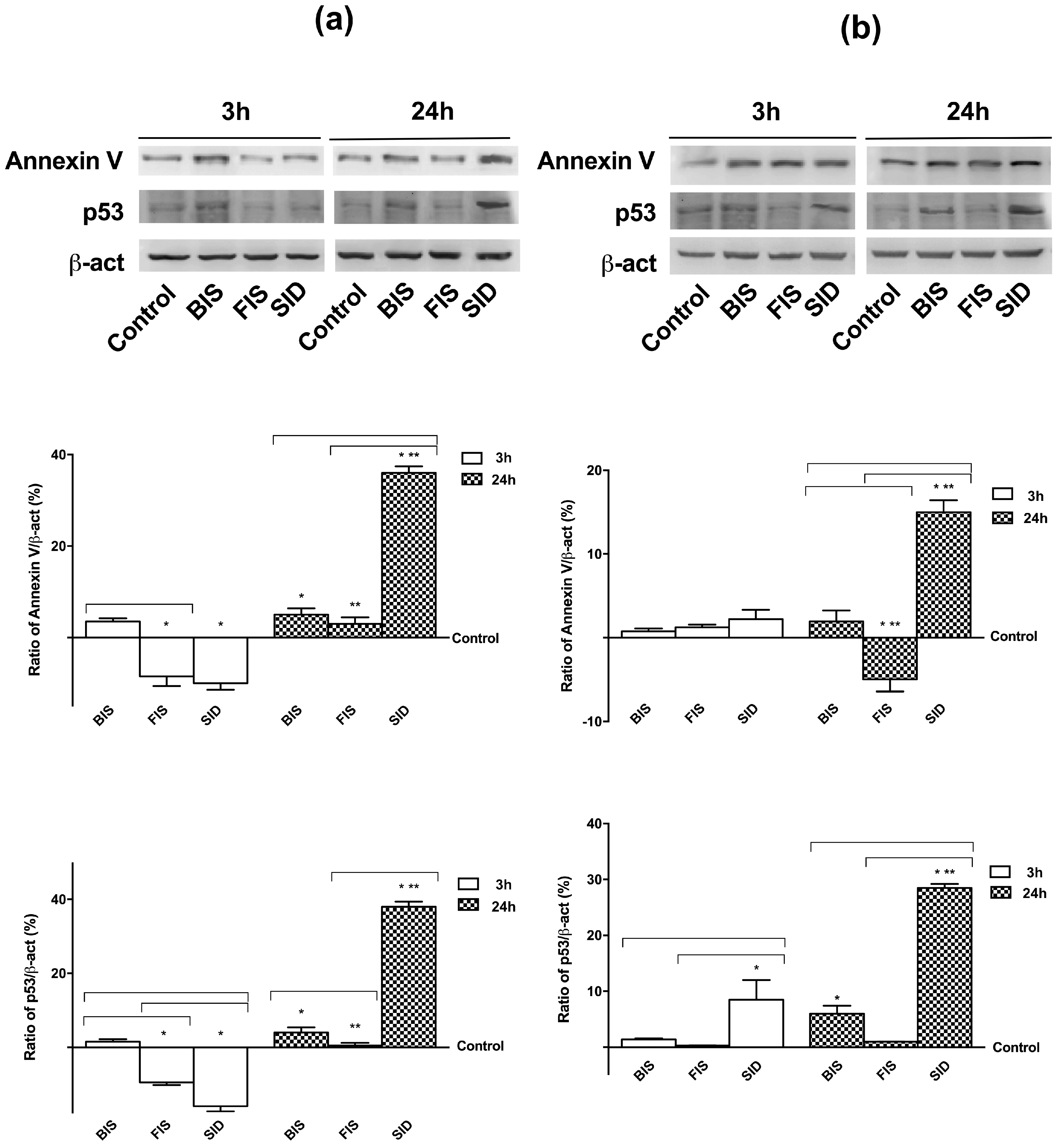
3.5. Irreversible Effects of Iron
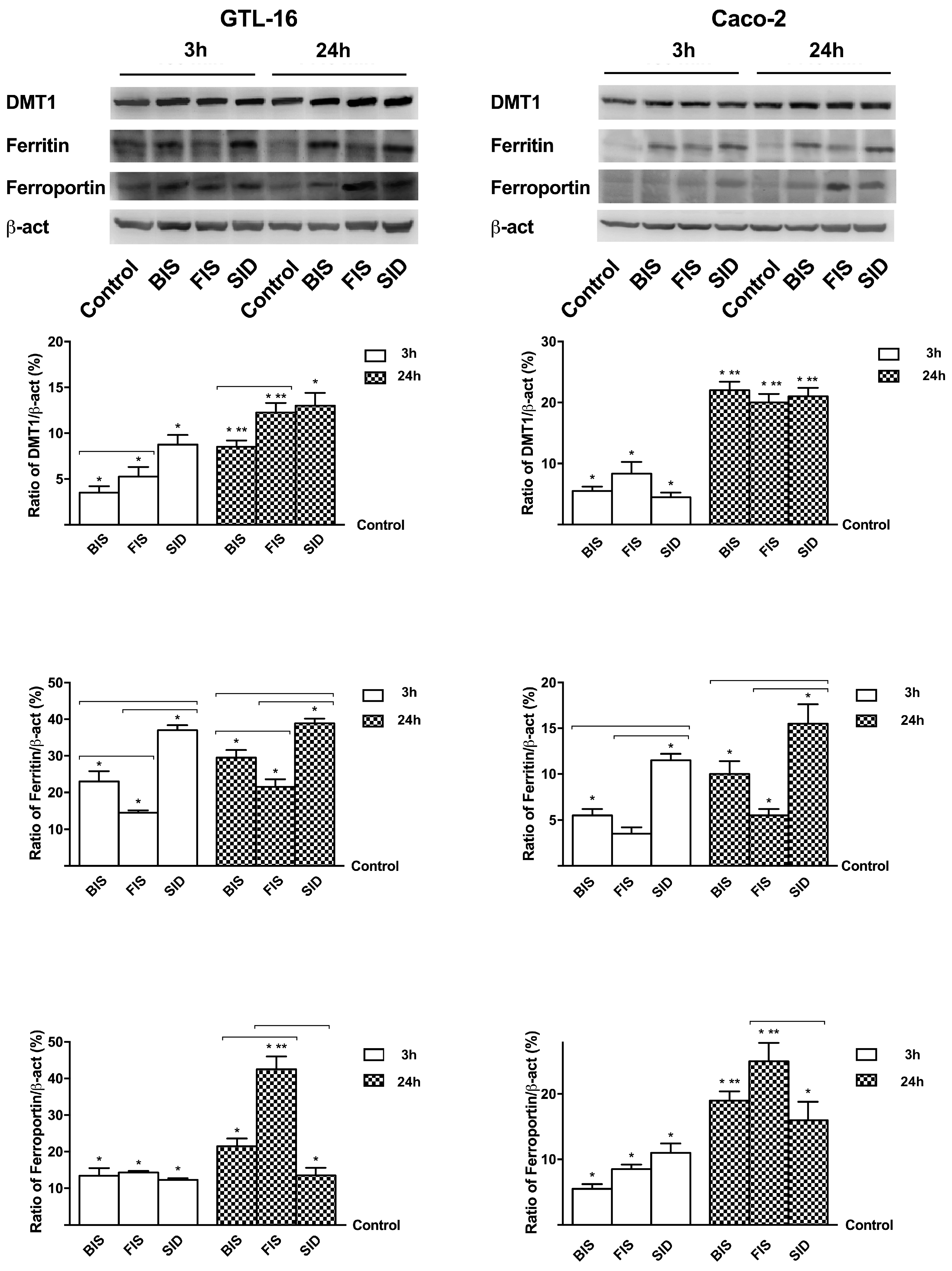
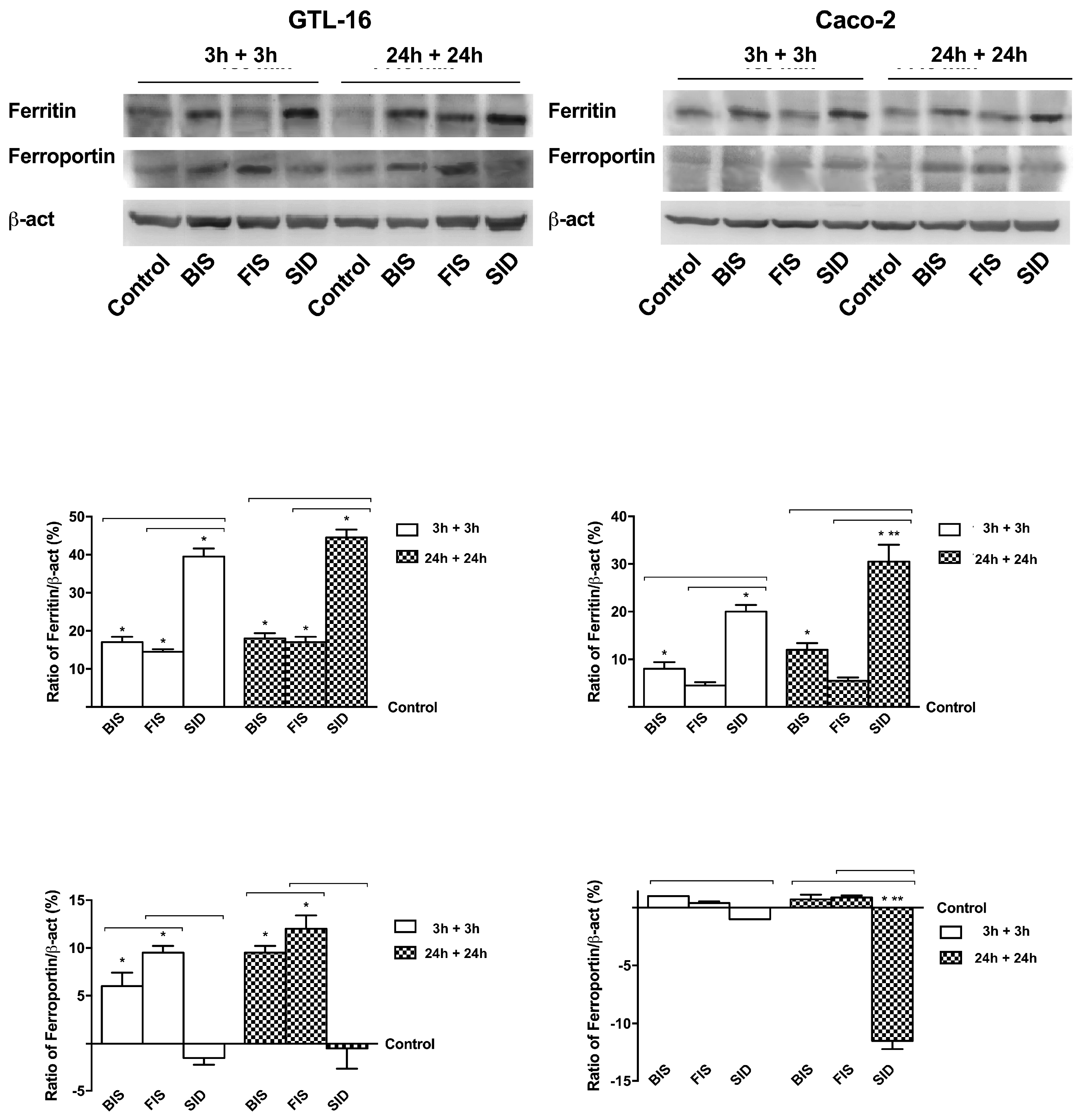
3.6. Iron Metabolism
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix A.1. Method: Iron Histochemistry
Appendix A.2. Method: Radical Oxygen Species (ROS) Analysis
Appendix B

References
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2013, 1, a011866. [Google Scholar] [CrossRef] [PubMed]
- Scheers, N.M.; Almgren, A.B.; Sandberg, A.S. Proposing a caco-2/hepG2 cell model for in vitro iron absorption studies. J. Nutr. Biochem. 2014, 25, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Miret, S.; Tascoglu, S.; Van der Burg, M.; Frenken, L.; Klaffke, W. In vitro bioavailability of iron from the heme analogue sodium iron chlorophyllin. J. Agric. Food Chem. 2010, 58, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.A. Intestinal iron absorption: Regulation by dietary & systemic factors. Int. J. Vitam. Nutr. Res. 2010, 80, 231–242. [Google Scholar] [CrossRef]
- López, M.A.; Martos, F.C. Iron availability: An updated review. Int. J. Food Sci. Nutr. 2004, 55, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, B.K.; Vulpe, C.D.; Anderson, G.J. Intestinal iron absorption. J. Trace Elem. Med. Biol. 2012, 26, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Fossati, L.; Dechaume, R.; Hardillier, E.; Chevillon, D.; Prevost, C.; Bolze, S.; Maubon, N. Use of simulated intestinal fluid for Caco-2 permeability assay of lipophilic drugs. Int. J. Pharm. 2008, 360, 148–155. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, R.L.; Hunt, D.R.; Dewi, R.E.; Heilshorn, S.C. Improvement of paracellular transport in the Caco-2 drug screening model using protein-engineered substrates. Biomaterials 2017, 129, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Christides, T.; Ganis, J.C.; Sharp, P.A. In vitro assessment of iron availability from commercial Young Child Formulae supplemented with prebiotics. Eur. J. Nut. 2016, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.; Di Renzo, M.F.; Ferracini, R.; Chiadò-Piat, L.; Comoglio, P.M. A protein with associated tyrosine kinase activity in a human gastric carcinoma cell line. Mol. Cell Biol. 1988, 8, 3510–3517. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Bardelli, C.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Role of vitamin D3 combined to alginates in preventing acid and oxidative injury in cultured gastric epithelial cells. BMC Gastroenterol. 2016, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Christides, T.; Wray, D.; McBride, R.; Fairweather, R.; Sharp, P. Iron bioavailability from commercially available iron supplements. Eur. J. Nutr. 2015, 54, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D protects human endothelial cells from oxidative stress through the autophagic and survival pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; D’Onofrio, M.; Brandi, R.; Arisi, I.; Zucco, F.; Felsani, A. Cell growing density affects the structural and functional properties of Caco-2 differentiated monolayer. J. Cell Physiol. 2011, 226, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Obringer, C.; Manwaring, J.; Goebel, C.; Hewitt, N.J.; Rothe, H. Suitability of the in vitro Caco-2 assay to predict the oral absorption of aromatic amine hair dyes. Toxicol. In Vitro 2016, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; de Freitas, V.; Reis, C.; Mateus, N. A new approach on the gastric absorption of anthocyanins. Food Funct. 2012, 3, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, M.; Bouchard, F.; Gosselin, P.; Paquin, J.; Mateescu, M.A. The NCI-N87 cell line as a gastric epithelial barrier model for drug permeability assay. Biochem. Biophys. Res. Commun. 2011, 412, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Triguero, D.; Yang, J.; Cancilla, P.A. Comparison of in vitro and in vivo models of drug transcytosis through the blood–brain barrier. J. Pharmacol. Exp. Ther. 1990, 253, 884–891. [Google Scholar] [PubMed]
- Owen, J.E.; Bishop, G.M.; Robinson, S.R. Uptake and Toxicity of Hemin and Iron in Cultured Mouse Astrocytes. Neurochem. Res. 2016, 41, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Morsanuto, V.; Bardelli, C.; Molinari, C. Protective effects of 1α,25-Dihydroxyvitamin D3 on cultured neural cells exposed to catalytic iron. Physiol. Rep. 2016, 4, e12769. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Morsanuto, V.; Lattuada, D.; Colciaghi, B.; Cochis, A.; Bulfoni, A.; Colombo, P.; Bolis, G.; Molinari, C. Protective effects of vitamin D3 on fimbrial cells exposed to catalytic iron damage. J. Ovarian Res. 2016, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Hyder, S.M.; Persson, L.A.; Chowdhury, A.M.; Ekstrom, E.C. Do side-effects reduced compliance to iron supplementation? A study of daily- and weekly- dose regimens in pregnancy. J. Health Popul. Nutr. 2002, 20, 175–179. [Google Scholar] [PubMed]
- Yun, S.; Habicht, J.P.; Miller, D.D.; Glahn, R.P. An in vitro digestion Caco-2 cell culture system accurately predicts the effects of ascorbic acid and polyphenolic compounds on iron bioavailability in humans. J. Nutr. 2004, 134, 2717–2721. [Google Scholar] [PubMed]
- Garcia, M.N.; Flowers, C.; Cook, J.D. The Caco-2 cell culture system can be used as a model to study food iron availability. J. Nutr. 1996, 126, 251–258. [Google Scholar] [PubMed]
- Chana, K.; Fenwick, P.; Nicholson, A.; Barnes, P.; Donnelly, L. Identification of a distinct glucocorticosteroid-insensitive pulmonary macrophage phenotype in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2014, 133, 207–216. [Google Scholar] [CrossRef] [PubMed]


| Iron Formulation | Papp (10−6 cm/s) at 3 h | Papp (10−6 cm/s) at 24 h | ||
|---|---|---|---|---|
| GTL-16 | Caco-2 | GTL-16 | Caco-2 | |
| SID | 5.02 ± 1.1 ** | 15.07 ± 1.9 ** | 0.63 ± 0.035 | 1.57 ± 0.41 |
| FIS | 12.56 ± 1.43 *,** | 16.08 ± 2 ** | 1.38 ± 0.4 | 1.88 ± 0.43 |
| BIS | 10.35 ± 1.82 *,** | 13.56 ± 1.8 ** | 0.94 ± 0.6 | 1.44 ± 0.35 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uberti, F.; Morsanuto, V.; Ghirlanda, S.; Molinari, C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients 2017, 9, 1008. https://doi.org/10.3390/nu9091008
Uberti F, Morsanuto V, Ghirlanda S, Molinari C. Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines. Nutrients. 2017; 9(9):1008. https://doi.org/10.3390/nu9091008
Chicago/Turabian StyleUberti, Francesca, Vera Morsanuto, Sabrina Ghirlanda, and Claudio Molinari. 2017. "Iron Absorption from Three Commercially Available Supplements in Gastrointestinal Cell Lines" Nutrients 9, no. 9: 1008. https://doi.org/10.3390/nu9091008





