Chemopreventive Potential of Flavonoids in Oral Squamous Cell Carcinoma in Human Studies
Abstract
:1. Introduction
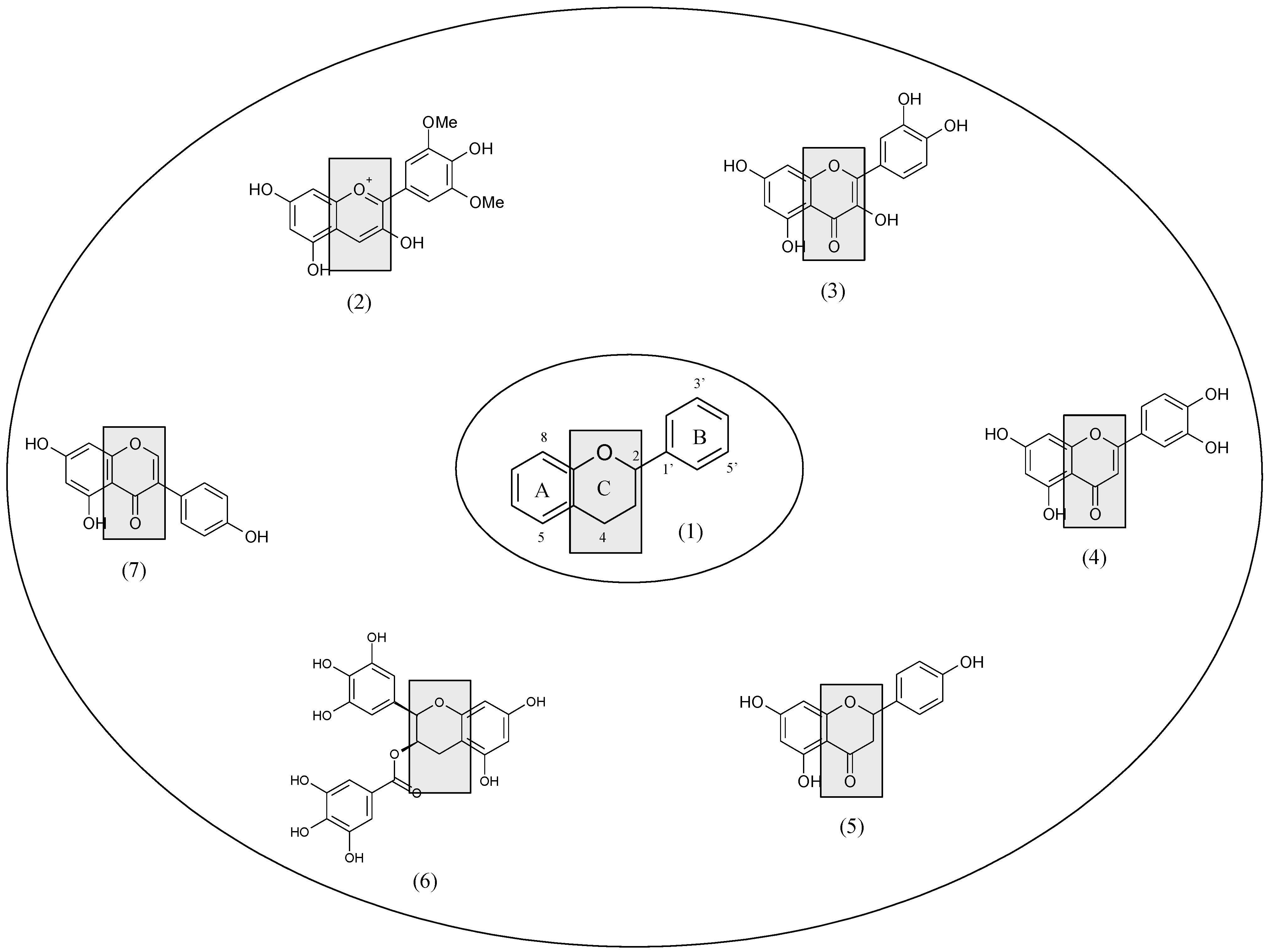
2. Oral Cancer Chemoprevention and Preclinical Evidence
3. Epidemiological Studies
4. Clinical Studies
5. Oral Bioavailability of Flavonoids
6. Future Perspectives: Nanoparticles and Oral Transmucosal Delivery to Improve Systemic and Local Bioavailability
7. Conclusions
Acknowledgments
Conflict of Interest
References
- Lucenteforte, E.; Garavello, W.; Bosetti, C.; La Vecchia, C. Dietary factors and oral and pharyngeal cancer risk. Oral Oncol. 2009, 45, 461–467. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Bioactivity of grape chemicals for human health. Nat. Prod. Commun. 2009, 4, 611–634. [Google Scholar]
- Iriti, M.; Faoro, F. Chemical diversity and defence metabolism: How plants cope with pathogens and ozone pollution. Int. J. Mol. Sci. 2009, 10, 3371–3399. [Google Scholar] [CrossRef]
- Iriti, M.; Faoro, F. Plant defense and human nutrition: Phenylpropanoids on the menu. Curr. Top. Nutr. Res. 2004, 2, 47–65. [Google Scholar]
- Iriti, M. Introduction to polyphenols, plant chemicals for human health. Mini-Rev. Med. Chem. 2011, 11, 1183–1185. [Google Scholar]
- Petersen, P.E. Oral cancer prevention and control? The approach of the World Health Organization. Oral Oncol. 2009, 45, 454–460. [Google Scholar]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef]
- Tanaka, T.; Tanaka, M.; Tanaka, T. Oral carcinogenesis and oral cancer chemoprevention: A review. Pathol. Res. Int. 2011, 2011. [Google Scholar] [CrossRef]
- Carnelio, S.; Rodrigues, G.S.; Shenoy, R.; Fernandes, D. A brief review of common oral premalignant lesions with emphasis on their management and cancer prevention. Indian J. Surg. 2011, 73, 256–261. [Google Scholar] [CrossRef]
- Lodi, G.; Franchini, R.; Bez, C.; Sardella, A.; Moneghini, L.; Pellegrini, C.; Bosari, S.; Manfredi, M.; Vescovi, P.; Carrassi, A. Detection of survivin mRNA in healthy oral mucosa, oral leucoplakia and oral cancer. Oral Dis. 2010, 16, 61–67. [Google Scholar] [CrossRef]
- Lodi, G.; Carrozzo, M.; Furness, S.; Thongprasom, K. Interventions for treating oral lichen planus: A systematic review. Br. J. Dermatol. 2012, 166, 938–947. [Google Scholar] [CrossRef]
- Syrjänen, S.; Lodi, G.; von Bültzingslöwen, I.; Aliko, A.; Arduino, P.; Campisi, G.; Challacombe, S.; Ficarra, G.; Flaitz, C.; Zhou, H.M.; et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: A systematic review. Oral Dis. 2011, 17 (Suppl. 1), 58–72. [Google Scholar]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- De Moura, C.F.; Noguti, J.; de Jesus, G.P.; Ribeiro, F.A.; Garcia, F.A.; Gollucke, A.P.; Aguiar, O., Jr.; Ribeiro, D.A. Polyphenols as a chemopreventive agent in oral carcinogenesis: Putative mechanisms of action using in-vitro and in-vivo test systems. Eur. J. Cancer Prev. 2012. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lodi, G.; Sardella, A.; Carrassi, A.; Iriti, M. Plant polyphenols and oral health: Old phytochemicals for new fields. Curr. Med. Chem. 2012, 19, 1706–1720. [Google Scholar] [CrossRef]
- Johnson, T.L.; Lai, M.B.; Lai, J.C.; Bhushan, A. Inhibition of cell proliferation and MAP kinase and Akt pathways in oral squamous cell carcinoma by genistein and biochanin A. Evid. Based Complement. Altern. Med. 2010, 7, 351–358. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Li, L.A.; Lin, P.; Cheng, L.C.; Hung, C.H.; Chang, N.W.; Lin, C. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol. Appl. Pharmacol. 2012, 263, 360–367. [Google Scholar] [CrossRef]
- Chen, S.F.; Nien, S.; Wu, C.H.; Liu, C.L.; Chang, Y.C.; Lin, Y.S. Reappraisal of the anticancer efficacy of quercetin in oral cancer cells. J. Chin. Med. Assoc. 2013, 76, 146–152. [Google Scholar] [CrossRef]
- Yang, S.H.; Liao, P.H.; Pan, Y.F.; Chen, S.L.; Chou, S.S.; Chou, M.Y. The novel p53-dependent metastatic and apoptotic pathway induced by vitexin in human oral cancer OC2 cells. Phytother. Res. 2012. [Google Scholar] [CrossRef]
- Silvan, S.; Manoharan, S.; Baskaran, N.; Anusuya, C.; Karthikeyan, S.; Prabhakar, M.M. Chemopreventive potential of apigenin in 7,12-dimethylbenz(a)anthracene induced experimental oral carcinogenesis. Eur. J. Pharmacol. 2011, 670, 571–577. [Google Scholar] [CrossRef]
- Gómez-García, F.J.; López-Jornet, M.P.; Álvarez-Sánchez, N.; Castillo-Sánchez, J.; Benavente-García, O.; Vicente Ortega, V. Effect of the phenolic compounds apigenin and carnosic acid on oral carcinogenesis in hamster induced by DMBA. Oral Dis. 2013, 19, 279–286. [Google Scholar]
- Kim, J.W.; Amin, A.R.; Shin, D.M. Chemoprevention of head and neck cancer with green tea polyphenols. Cancer Prev. Res. 2010, 3, 900–909. [Google Scholar] [CrossRef]
- Lee, U.L.; Choi, S.W. The chemopreventive properties and therapeutic modulation of green tea polyphenols in oral squamous cell carcinoma. ISRN Oncol. 2011, 2011. [Google Scholar] [CrossRef]
- Narotzki, B.; Reznick, A.Z.; Aizenbud, D.; Levy, Y. Green tea: A promising natural product in oral health. Arch. Oral Biol. 2012, 57, 429–435. [Google Scholar]
- Winn, D.M.; Ziegler, R.G.; Pickle, L.W.; Gridley, G.; Blot, W.J.; Hoover, R.N. Diet in the etiology of oral and pharyngeal cancer among women from the southern United States. Cancer Res. 1984, 44, 1216–1222. [Google Scholar]
- Chuang, S.C.; Jenab, M.; Heck, J.E.; Bosetti, C.; Talamini, R.; Matsuo, K.; Castellsague, X.; Franceschi, S.; Herrero, R.; Winn, D.M.; et al. Diet and the risk of head and neck cancer: A pooled analysis in the INHANCE consortium. Cancer Causes Control 2011, 23, 69–88. [Google Scholar]
- De Stefani, E.; Ronco, A.L.; Mendilaharsu, M.; Deneo-Pellegrini, H. Diet and risk of cancer of the upper aerodigestive tract—II. Nutrients. Oral Oncol. 1999, 35, 22–26. [Google Scholar] [CrossRef]
- Rossi, M.; Garavello, W.; Talamini, R.; Negri, E.; Bosetti, C.; Dal Maso, L.; Lagiou, P.; Tavani, A.; Polesel, J.; Barzan, L.; et al. Flavonoids and the risk of oral and pharyngeal cancer: A case-control study from Italy. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1621–1625. [Google Scholar] [CrossRef]
- Ide, R.; Fujino, Y.; Hoshiyama, Y.; Mizoue, T.; Kubo, T.; Pham, T.M.; Shirane, K.; Tokui, N.; Sakata, K.; Tamakoshi, A.; et al. A prospective study of green tea consumption and oral cancer incidence in Japan. Ann. Epidemiol. 2007, 17, 821–826. [Google Scholar] [CrossRef]
- Hildebrand, J.S.; Patel, A.V.; McCullough, M.L.; Gaudet, M.M.; Chen, A.Y.; Hayes, R.B.; Gapstur, S.M. Coffee, tea, and fatal oral/pharyngeal cancer in a large prospective US cohor. Am. J. Epidemiol. 2013, 177, 50–58. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef]
- Radoï, L.; Paget-Bailly, S.; Menvielle, G.; Cyr, D.; Schmaus, A.; Carton, M.; Guida, F.; Cénée, S.; Sanchez, M.; Guizard, A.V.; et al. Tea and coffee consumption and risk of oral cavity cancer: Results of a large population-based case-control study, the ICARE study. Cancer Epidemiol. 2013, 37, 284–289. [Google Scholar] [CrossRef]
- Boehm, K.; Borrelli, F.; Ernst, E.; Habacher, G.; Hung, S.K.; Milazzo, S.; Horneber, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Li, N.; Sun, Z.; Han, C.; Chen, J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc. Soc. Exp. Biol. Med. 2003, 220, 218–224. [Google Scholar]
- Schwartz, J.L.; Baker, V.; Larios, E.; Chung, F.L. Molecular and cellular effects of green tea on oral cells of smokers: A pilot study. Mol. Nutr. Food Res. 2005, 49, 43–51. [Google Scholar] [CrossRef]
- Tsao, A.S.; Liu, D.; Martin, J.; Tang, X.M.; Lee, J.J.; El-Naggar, A.K.; Wistuba, I.; Culotta, K.S.; Mao, L.; Gillenwater, A.; et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev. Res. 2009, 2, 931–941. [Google Scholar] [CrossRef]
- Yoon, A.J.; Shen, J.; Santella, R.M.; Philipone, E.M.; Wu, H.C.; Eisig, S.B.; Blitzer, A.; Close, L.G.; Zegarelli, D.J. Topical application of green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) for prevention of recurrent oral neoplastic lesions. J. Orofac. Sci. 2012, 4, 43–50. [Google Scholar]
- Chow, H.H.S.; Hakim, I.A. Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol. Res. 2011, 64, 105–112. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar]
- Mallery, S.R.; Budendorf, D.E.; Larsen, M.P.; Pei, P.; Tong, M.; Holpuch, A.S.; Larsen, P.E.; Stoner, G.D.; Fields, H.W.; Chan, K.K.; et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011, 4, 1209–1221. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Mukhtar, H. Nanochemoprevention by bioactive food components: A perspective. Pharm. Res. 2010, 27, 1054–1060. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Adhami, V.M.; Christopher, J. Impact of nanotechnology in cancer: Emphasis on nanochemoprevention. Int. J. Nanomed. 2012, 7, 591–605. [Google Scholar]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing nanochemoprevention as a novel approach for cancer control: Proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef]
- Sulfikkarali, N.; Krishnakumar, N.; Manoharan, S.; Nirmal, R.M. Chemopreventive efficacy of naringenin-loaded nanoparticles in 7,12-dimethylbenz(a)anthracene induced experimental oral carcinogenesis. Pathol. Oncol. Res. 2013, 19, 287–296. [Google Scholar]
- Satheesh Madhav, N.V.; Semwal, R.; Semwal, D.K.; Semwal, R.B. Recent trends in oral transmucosal drug delivery systems: An emphasis on the soft palatal route. Exp. Opin. Drug Deliv. 2012, 9, 629–647. [Google Scholar] [CrossRef]
- Senel, S.; Rathbone, M.J.; Cansiz, M.; Pather, I. Recent developments in buccal and sublingual delivery systems. Exp. Opin. Drug Deliv. 2012, 9, 615–628. [Google Scholar] [CrossRef]
- Holpuch, A.S.; Desai, K.H.; Schwendeman, S.P.; Mallery, S.R. Optimizing therapeutic efficacy of chemopreventive agents: A critical review of delivery strategies in oral cancer chemoprevention clinical trials. J. Carcinog. 2011, 10, 23. [Google Scholar] [CrossRef]
- Wu, X.; Desai, K.G.H.; Mallery, S.R.; Holpuch, A.S.; Phelps, M.P.; Schwendeman, S.P. Mucoadhesive fenretinide patches for site-specific chemoprevention of oral cancer: Enhancement of oral mucosal permeation of fenretinide by coincorporation of propylene glycol and menthol. Mol. Pharm. 2012, 9, 937–945. [Google Scholar]
- Ling, Y.; Ren, C.; Mallery, S.R.; Ugalde, C.M.; Pei, P.; Saradhi, U.V.; Stoner, G.D.; Chan, K.K.; Liu, Z. A rapid and sensitive LC-MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel. J. Chromatogr. B 2009, 877, 4027–4034. [Google Scholar] [CrossRef]
- Ugalde, C.M.; Liu, Z.; Ren, C.; Chan, K.K.; Rodrigo, K.A.; Ling, Y.; Larsen, P.E.; Chacon, G.E.; Stoner, G.D.; Mumper, R.J.; et al. Distribution of anthocyanins delivered from a bioadhesive black raspberry gel following topical intraoral application in normal healthy volunteers. Pharm. Res. 2009, 26, 977–986. [Google Scholar] [CrossRef]
- Shumway, B.S.; Kresty, L.A.; Larsen, P.E.; Zwick, J.C.; Lu, B.; Fields, H.W.; Mumper, R.J.; Stoner, G.D.; Mallery, S.R. Effects of a topically applied bioadhesive berry gel on loss of heterozygosity indices in premalignant oral lesions. Clin. Cancer Res. 2008, 14, 2421–2430. [Google Scholar] [CrossRef]
- Mallery, S.R.; Zwick, J.C.; Pei, P.; Tong, M.; Larsen, P.E.; Shumway, B.S.; Lu, B.; Fields, H.W.; Mumper, R.J.; Stoner, G.D. Topical application of a bioadhesive black raspberry gel modulates gene expression and reduces cyclooxygenase 2 protein in human premalignant oral lesions. Cancer Res. 2008, 68, 4945–4957. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Iriti, M.; Varoni, E.M. Chemopreventive Potential of Flavonoids in Oral Squamous Cell Carcinoma in Human Studies. Nutrients 2013, 5, 2564-2576. https://doi.org/10.3390/nu5072564
Iriti M, Varoni EM. Chemopreventive Potential of Flavonoids in Oral Squamous Cell Carcinoma in Human Studies. Nutrients. 2013; 5(7):2564-2576. https://doi.org/10.3390/nu5072564
Chicago/Turabian StyleIriti, Marcello, and Elena Maria Varoni. 2013. "Chemopreventive Potential of Flavonoids in Oral Squamous Cell Carcinoma in Human Studies" Nutrients 5, no. 7: 2564-2576. https://doi.org/10.3390/nu5072564





