Tocotrienols Reverse Cardiovascular, Metabolic and Liver Changes in High Carbohydrate, High Fat Diet-Fed Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Rats and Diets
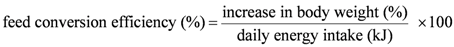
2.2. Echocardiography
2.3. Body Composition Measurements
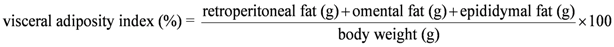
2.4. Assessment of Physiological Parameters
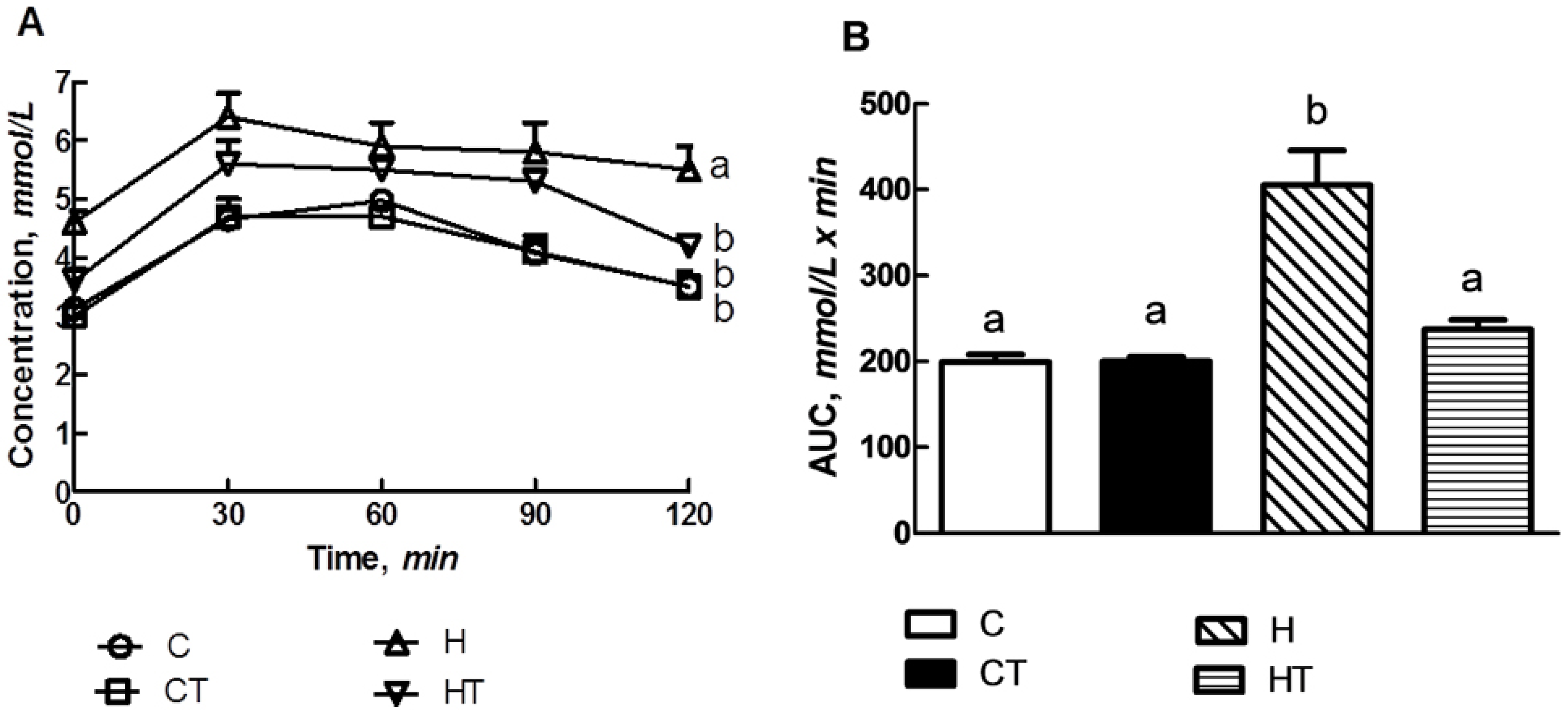
2.5. Oral Glucose Tolerance Test and Insulin Tolerance Test
2.6. Organ Bath Studies
2.7. Isolated Heart Preparation
2.8. Organ Weights
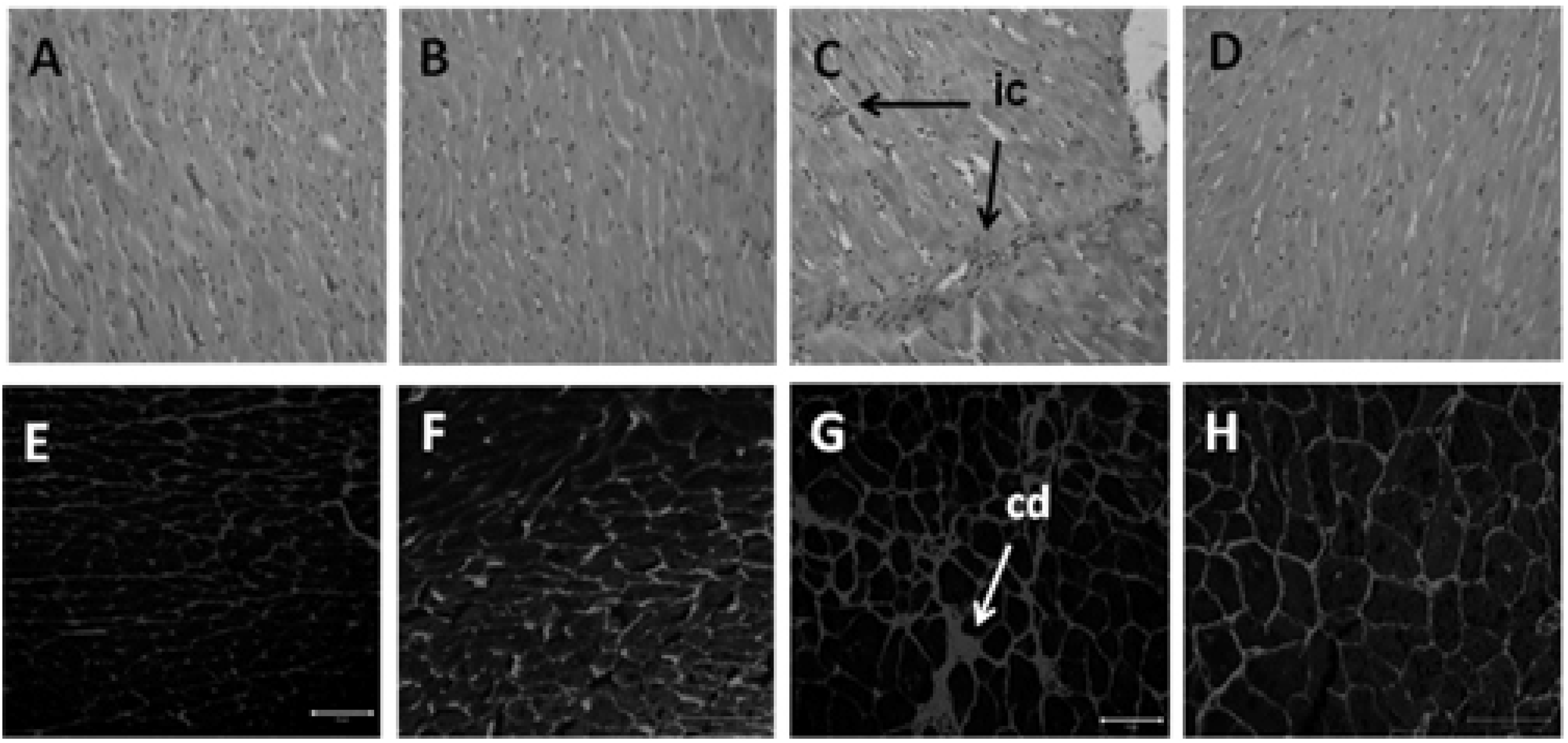
2.9. Histology of Heart and Liver
2.10. Plasma Analyses
2.11. Statistical Analysis
3. Results
3.1. Cardiovascular Structure and Function


| Variables | C | CT | H | HT | p | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||
| LVIDd, mm | 6.40 ± 0.21 a | 7.42 ± 0.22 b | 7.64 ± 0.26 b | 8.02 ± 0.10 b | 0.0001 | 0.0020 | 0.1280 |
| LVPWd, mm | 1.78 ± 0.03 | 1.86 ± 0.07 | 1.83 ± 0.03 | 1.85 ± 0.06 | 0.7014 | 0.2958 | 0.5658 |
| Relative wall thickness | 0.50 ± 0.02 a,b | 0.47 ± 0.04 a,b | 0.54 ± 0.02 a | 0.45 ± 0.01 b | 0.7876 | 0.0123 | 0.2189 |
| Fractional shortening, % | 48.0 ± 1.1 a | 51.5 ± 1.5 a | 42.5 ± 0.6 b | 48.6 ± 1.8 a | 0.0038 | 0.0011 | 0.3175 |
| Ejection fraction, % | 83.3 ± 0.8 b,c | 88.3 ± 1.1 a | 81.0 ± 0.6 c | 86.1 ± 1.5 a,b | 0.0414 | <0.0001 | 0.9736 |
| Heart rate, bpm | 268 ± 22 | 288 ± 25 | 264 ± 24 | 308 ± 18 | 0.7448 | 0.1659 | 0.6057 |
| Stroke volume, mL | 0.28 ± 0.01 b | 0.38 ± 0.03 a | 0.42 ± 0.04 a | 0.46 ± 0.01 a | 0.0005 | 0.0096 | 0.3497 |
| Cardiac output, mL/min | 74.2 ± 3.9 a,b | 98.1 ± 7.3 a,b | 108.9 ± 12.25 b | 143.1 ± 9.1 c | <0.0001 | 0.0045 | 0.091 |
| LV developed pressure, mmHg | 64.7 ± 8.8 a | 43.8 ± 4.5 b | 32.7 ± 2.3 b | 33.0 ± 2.6 b | 0.0003 | 0.0601 | 0.0540 |
| (+)dP/dt, mmHg/s | 1079 ± 104 a | 844 ± 89 a,b | 599 ± 39 b | 766 ± 97 a,b | 0.0031 | 0.6956 | 0.0266 |
| (−)dP/dt, mmHg/s | 614 ± 66 a | 507 ± 49 a,b | 359 ± 39 b | 512 ± 89 a,b | 0.0606 | 0.7235 | 0.0520 |
| Diastolic stiffness, κ | 22.8 ± 0.7 c | 23.5 ± 0.3 c | 28.8 ± 0.5 a | 26.4 ± 0.3 b | <0.0001 | 0.1003 | 0.0037 |
| Ascending aortic flow, m/s | 0.90 ± 0.02 | 0.91 ± 0.07 | 0.95 ± 0.04 | 1.05 ± 0.07 | 0.1064 | 0.3305 | 0.4432 |
| Descending aortic flow, m/s | 0.87 ± 0.04 | 0.92 ± 0.09 | 0.93 ± 0.03 | 0.88 ± 0.07 | 0.8777 | 0.9607 | 0.4405 |
| Ejection time, s | 84.5 ± 3.2 a | 89.6 ± 3.0 a | 98.1 ± 2.5 b | 88.3 ± 2.2 a | 0.0349 | 0.3974 | 0.0112 |
| Estimated LV mass, Litwin, g | 0.89 ± 0.03 | 0.89 ± 0.07 | 0.98 ± 0.05 | 1.01 ± 0.03 | 0.0310 | 0.7471 | 0.7316 |
| LV + septum wet weight, mg/mm | 19.5 ± 0.7 | 17.8 ± 0.8 | 20.7 ± 0.9 | 20.3 ± 1.1 | 0.0427 | 0.2536 | 0.5082 |
| Right ventricle wet weight, mg/mm | 4.8 ± 0.7 a,b | 3.5 ± 0.4 b | 5.4 ± 0.5 a | 4.0 ± 0.4 a,b | 0.2726 | 0.0099 | 0.9280 |
| Heart wet weight, mg/mm | 24.8 ± 1.2 | 21.3 ± 0.8 | 25.3 ± 1.9 | 24.3 ± 1.1 | 0.2402 | 0.0789 | 0.4212 |
| Systolic wall stress, mmHg | 81.9 ± 1.8 | 80.6 ± 5.0 | 101.1 ± 8.1 | 96.1 ± 6.6 | 0.0060 | 0.5944 | 0.7547 |
3.2. Dietary Intake, Body Parameters and Lipid Profile
| Variables | C | CT | H | HT | p | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||
| Food intake, g/day | 38.2 ± 1.0 a | 34.2 ± 0.8 b | 22.0 ± 0.9 c | 25.6 ± 0.5 d | <0.0001 | 0.7595 | 0.0001 |
| Water intake, mL/day | 32.4 ± 2.8 a | 26.2 ± 1.0 b | 23.3 ± 1.2 b | 26.1 ± 1.4 b | 0.0132 | 0.3377 | 0.0148 |
| Body weight gain, % | 11.0 ± 1.0 a,b | 8.6 ± 0.9 b | 15.0 ± 1.5 a | 14.4 ± 1.4 a | 0.0005 | 0.2425 | 0.4535 |
| Energy intake, kJ/day | 443.2 ± 5.6 a | 397.5 ± 11.6 b | 480.1 ± 13.5 c | 556.3 ± 14.7 d | <0.0001 | 0.2093 | <0.0001 |
| Feed conversion efficiency, % | 2.4 ± 0.2 | 2.2 ± 0.3 | 3.0 ± 0.3 | 2.8 ± 0.2 | 0.0364 | 0.4079 | 0.9615 |
| Bone mineral content, g | 12.8 ± 0.1 | 13.0 ± 1.3 | 15.0 ± 0.6 | 14.9 ± 0.3 | 0.0092 | 0.9109 | 0.8408 |
| Total fat mass, g | 90.6 ± 7.0 b | 83.2 ± 6.2 b | 193.7 ± 20.1 a | 181.2 ± 9.8 a | <0.0001 | 0.4197 | 0.8362 |
| Total lean mass, g | 335.9 ± 9.2 a | 288.8 ± 8.1 b | 257.7 ± 8.7 c | 270.1 ± 11.1 b | <0.0001 | 0.0738 | 0.0036 |
| Abdominal circumference, cm | 19.8 ± 0.1 c | 19.7 ± 0.1c | 22.4 ± 0.3 a | 21.8 ± 0.2 b | <0.0001 | 0.0742 | 0.2337 |
| Visceral adiposity index, % | 3.6 ± 0.2 a | 4.3 ± 0.4 a | 6.8 ± 0.6 b | 6.6 ± 0.3 b | <0.0001 | 0.6177 | 0.2546 |
| Tissue wet weight, mg/mm tibial length | |||||||
| Retroperitoneal fat | 136.9 ± 11.6 a | 148.7 ± 11.0 a | 350.9 ± 29.3 b | 316.7 ± 21.9 b | <0.0001 | 0.5777 | 0.2583 |
| Epididymal fat | 108.7 ± 8.8 a | 119.5 ± 12.9 a | 204.3 ± 23.3 b | 206.8 ± 16.7 b | <0.0001 | 0.6883 | 0.8005 |
| Omental fat | 63.0 ± 6.7 c | 77.9 ± 9.2 c | 160.1 ±16.8 a | 127.8 ± 6.9 b | <0.0001 | 0.4207 | 0.0365 |
| Total abdominal fat | 302.5 ± 23.5 a | 346.0 ± 29.1 a | 690.6 ± 71.2 b | 651.2 ± 35.9 b | <0.0001 | 0.0018 | 0.0161 |
| Plasma lipid profile | |||||||
| Total cholesterol, mmol/L | 1.23 ± 0.07 a | 1.63 ± 0.07 b | 1.74 ± 0.09 b | 1.60 ± 0.09 b | 0.0062 | 0.1252 | 0.0026 |
| Triglyceride, mmol/L | 0.34 ± 0.04 a | 0.44 ± 0.05 a | 0.90 ± 0.17 b | 0.60 ± 0.09 b | 0.0082 | 0.6539 | 0.2530 |
| NEFA, mmol/L | 0.98 ± 0.14 a | 1.46 ± 0.11a | 2.28 ± 0.25 b | 1.74 ± 0.16 a | 0.0050 | 0.0394 | 0.0183 |
| Liver enzymes | |||||||
| ALT, U/L | 24.8 ± 2.8 a | 28.7 ± 2.2 a | 43.9 ± 6.5 b | 25.8 ± 2.0 a | 0.0473 | 0.0779 | 0.0087 |
| AST, U/L | 67.1 ± 6.8 a | 65.7 ± 3.9 a | 96.6 ± 3.8 b | 58.0 ± 2.3 a | 0.0233 | 0.0002 | 0.0004 |
| Liver wet weight, mg/mm | 234.9 ± 10.6 a | 240.4 ± 8.8 a | 330.2 ± 13.8 b | 324.6 ± 4.6 b | <0.0001 | 0.9966 | 0.5774 |
| Fasting plasma glucose, mmol/L | |||||||
| 0 week | 3.5 ± 0.1 | 3.6 ± 0.1 | 3.7 ± 0.1 | 3.5 ± 0.1 | 0.0016 | 0.3305 | 0.0585 |
| 8 weeks | 3.8 ± 0.2 a | 3.6 ± 0.2 a | 5.0 ± 0.1 b | 5.0 ± 01 b | <0.0001 | 0.5322 | 0.5322 |
| 16 weeks | 3.1 ± 0.2 c | 3.0 ± 0.1 c | 4.6 ± 0.2 a | 3.6 ± 0.2 b | <0.0001 | 0.0050 | 0.0187 |
3.3. Glucose Handling
3.4. Hepatic Structure and Function

4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Srinath, R.K.; Katan, M.B. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004, 7, 167–186. [Google Scholar]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Sirtori, E.; Arnoldi, A. Functional foods for dyslipidaemia and cardiovascular risk prevention. Nutr. Res. Rev. 2009, 22, 244–261. [Google Scholar] [CrossRef]
- Zhao, J. Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: A perspective on plant biotechnology application. Recent Pat. Biotechnol. 2007, 1, 75–97. [Google Scholar] [CrossRef]
- Weng-Yew, W.; Brown, L. Nutrapharmacology of tocotrienols for metabolic syndrome. Curr. Pharm. Des. 2011, 17, 2206–2214. [Google Scholar] [CrossRef]
- Gee, P. Unleashing the untold and misunderstood observations on vitamin E. Genes Nutr. 2010, 6, 1–12. [Google Scholar]
- Saremi, A.; Arora, R. Vitamin E and cardiovascular disease. Am. J. Ther. 2010, 17, e56–e65. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- Nesaretnam, K.; Wong, W.Y.; Wahid, M.B. Tocotrienols and cancer: Beyond antioxidant activity. Eur. J. Lipid Sci. Technol. 2007, 109, 445–452. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Parinandi, N.L.; Maurer, M.; Sen, C.K. Characterization of the potent neuroprotective properties of the natural vitamin E, α-tocotrienol. J. Neurochem. 2006, 98, 1474–1486. [Google Scholar] [CrossRef]
- Yam, M.L.; Abdul Hafid, S.; Cheng, H.M.; Nesaretnam, K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids 2009, 44, 787–797. [Google Scholar] [CrossRef]
- Wong, W.Y.; Selvaduray, K.R.; Cheng, H.M.; Nesaretnam, K. Suppression of tumor growth by palm tocotrienols via the attenuation of angiogenesis. Nutr. Cancer 2009, 61, 367–373. [Google Scholar] [CrossRef]
- Nakagawa, K.; Shibata, A.; Yamashita, S.; Tsuzuki, T.; Kariya, J.; Oikawa, S.; Miyazawa, T. In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J. Nutr. 2007, 137, 1938–1943. [Google Scholar]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.; Brown, L. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br. J. Nutr. 2010, 104, 1322–1332. [Google Scholar] [CrossRef]
- Nakamura, H.; Furukawa, F.; Nishikawa, A.; Miyauchi, M.; Son, H.Y.; Imazawa, T.; Hirose, M. Oral toxicity of a tocotrienol preparation in rats. Food Chem. Toxicol. 2001, 39, 799–805. [Google Scholar] [CrossRef]
- Brown, L.; Fenning, A.; Chan, V.; Loch, D.; Wilson, K.; Anderson, B.; Burstow, D. Echocardiographic assessment of cardiac structure and function in rats. Heart Lung Circ. 2002, 11, 167–173. [Google Scholar] [CrossRef]
- Ward, L.C.; Battersby, K.J. Assessment of body composition of rats by bioimpedance spectroscopy validation against dual-energy X-ray absorptiometry. Scand. J. Lab. Anim. Sci. 2009, 36, 253–261. [Google Scholar]
- Jeyakumar, S.M.; Vajreswari, A.; Giridharan, N.V. Chronic dietary vitamin A supplementation regulates obesity in an obese mutant WNIN/Ob rat model. Obesity 2006, 14, 52–59. [Google Scholar] [CrossRef]
- Panchal, S.; Poudyal, H.; Arumugam, T.; Brown, L. Rutin attenuates metabolic changes, non-alcoholic steatohepatitis, and cardiovascular remodeling in high carbohydrate-, high fat-fed rats. J. Nutr. 2011, 141, 1062–1069. [Google Scholar] [CrossRef]
- Panchal, S.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 153–162. [Google Scholar]
- Khanna, S.; Patel, V.; Rink, C.; Roy, S.; Sen, C.K. Delivery of orally supplemented α-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic. Biol. Med. 2005, 39, 1310–1319. [Google Scholar] [CrossRef]
- Patel, V.; Khanna, S.; Roy, S.; Ezziddin, O.; Sen, C.K. Natural vitamin E α-tocotrienol: Retention in vital organs in response to long-term oral supplementation and withdrawal. Free Radic. Res. 2006, 40, 763–771. [Google Scholar] [CrossRef]
- Grundy, S.M. Drug therapy of the metabolic syndrome: Minimizing the emerging crisis in polypharmacy. Nat. Rev. Drug Discov. 2006, 5, 295–309. [Google Scholar] [CrossRef]
- Generally recognised as safe determination for the use of palm tocotrienol rich fractions (TRF) as ingredients in food. Available online: http://www.accessdata.fda.gov/scripts/fcn/gras_notices/grn_307.pdf (accessed on 3 October 2012).
- Newaz, M.A.; Nawal, N.N.A. Effect of γ-tocotrienol on blood pressure, lipid peroxidation and total antioxidant status in spontaneously hypertensive rats (SHR). Clin. Exp. Hypertens. 1999, 21, 1297–1313. [Google Scholar] [CrossRef]
- Koba, K.; Abe, K.; Ikeda, I.; Sugano, M. Effects of α-tocopherol and tocotrienols on blood pressure and linoleic acid metabolism in the spontaneously hypertensive rat (SHR). Biosci. Biotechnol. Biochem. 1992, 56, 1420–1423. [Google Scholar] [CrossRef]
- Jiang, F.; Liao, Z.; Hu, L.H.; Du, Y.Q.; Man, X.H.; Gu, J.J.; Gao, J.; Gong, Y.F.; Li, Z.S. Comparison of antioxidative and antifibrotic effects of α-tocopherol with those of tocotrienol-rich fraction in a rat model of chronic pancreatitis. Pancreas 2011, 40, 1091–1096. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Wan Nazaimoon, W.M.; Khalid, B.A.K. Tocotrienols-rich diet decreases advanced glycosylation endproducts in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays. J. Pathol. 2002, 24, 77–82. [Google Scholar]
- Fang, F.; Kang, Z.; Wong, C. Vitamin E tocotrienols improve insulin sensitivity through activating peroxisome proliferator-activated receptors. Mol. Nutr. Food Res. 2010, 54, 345–352. [Google Scholar] [CrossRef]
- Patel, J.; Matnor, N.A.; Iyer, A.; Brown, L. A regenerative antioxidant protocol of vitamin E and α-lipoic acid ameliorates cardiovascular and metabolic changes in fructose-fed rats. Evid. Based Complement. Alternat. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Coppack, S.W.; Evans, R.D.; Fisher, R.M.; Frayn, K.N.; Gibbons, G.F.; Humphreys, S.M.; Kirk, M.L.; Potts, J.L.; Hockaday, T.D.R. Adipose tissue metabolism in obesity: Lipase action in vivo before and after a mixed meal. Metabolism 1992, 41, 264–272. [Google Scholar] [CrossRef]
- Ferrannini, E.; Camastra, S.; Coppack, S.W.; Fliser, D.; Golay, A.; Mitrakou, A. Insulin action and non-esterified fatty acids. Proc. Nutr. Soc. 1997, 56, 753–761. [Google Scholar] [CrossRef]
- Iyer, A.; Fairlie, D.P.; Prins, J.B.; Hammock, B.D.; Brown, L. Inflammatory lipid mediators in adipocyte function and obesity. Nat. Rev. Endocrinol. 2010, 6, 71–82. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Tocotrienol attenuates oxidative–nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 2009, 57, 456–462. [Google Scholar] [CrossRef]
- Matsunaga, T.; Shoji, A.; Gu, N.; Joo, E.; Li, S.; Adachi, T.; Yamazaki, H.; Yasuda, K.; Kondoh, T.; Tsuda, K. γ-Tocotrienol attenuates TNF-α-induced changes in secretion and gene expression of MCP-1, IL-6 and adiponectin in 3T3-L1 adipocytes. Mol. Med. Report 2012, 5, 905–909. [Google Scholar]
- Hattori, R.; Otani, H.; Uchiyama, T.; Imamura, H.; Cui, J.; Maulik, N.; Cordis, G.A.; Zhu, L.; Das, D.K. Src tyrosine kinase is the trigger but not the mediator of ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1066–H1074. [Google Scholar]
- Toth, M.J.; Ward, K.; van der Velden, J.; Miller, M.S.; VanBuren, P.; LeWinter, M.M.; Ades, P.A. Chronic heart failure reduces Akt phosphorylation in human skeletal muscle: Relationship to muscle size and function. J. Appl. Physiol. 2011, 110, 892–900. [Google Scholar] [CrossRef]
- Shiojima, I.; Walsh, K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006, 20, 3347–3365. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Slivka, A.; Craft, T.K.S.; Chaki, S.; Rink, C.; Notestine, M.A.; DeVries, A.C.; Parinandi, N.L.; Sen, C.K. Neuroprotective properties of the natural vitamin E α-tocotrienol. Stroke 2005, 36, e144–e152. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.K.; Waanders, J.; Ward, L.; Brown, L. Lipid redistribution by alpha-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J. Nutr. Biochem. 2012, 23, 153–162. [Google Scholar] [CrossRef]
- Burdeos, G.; Nakagawa, K.; Kimura, F.; Miyazawa, T. Tocotrienol attenuates triglyceride accumulation in HepG2 cells and F344 rats. Lipids 2012, 47, 471–481. [Google Scholar] [CrossRef]
- Uto-Kondo, H.; Ohmori, R.; Kiyose, C.; Kishimoto, Y.; Saito, H.; Igarashi, O.; Kondo, K. Tocotrienol suppresses adipocyte differentiation and Akt phosphorylation in 3T3-L1 preadipocytes. J. Nutr. 2009, 139, 51–57. [Google Scholar]
- Qureshi, A.A.; Pearce, B.C.; Nor, R.M.; Gapor, A.; Peterson, D.M.; Elson, C.E. Dietary α-tocopherol attenuates the impact of γ-tocotrienol on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J. Nutr. 1996, 126, 389–394. [Google Scholar]
- Ikeda, I.; Imasato, Y.; Sasaki, E.; Sugano, M. Lymphatic transport of α-, γ- and δ-tocotrienols and α-tocopherol in rats. Int. J. Vitam. Nutr. Res. 1996, 66, 217–221. [Google Scholar]
- Yap, S.P.; Yuen, K.H.; Lim, A.B. Influence of route of administration on the absorption and disposition of α-, γ- and δ-tocotrienols in rats. J. Pharm. Pharmacol. 2003, 55, 53–58. [Google Scholar]
- Tan, B.; Watson, R.; Preedy, V. Alpha-Tocopherol: A detriment to tocotrienol benefits. In Tocotrienols: Vitamin E beyond tocopherols, 2nd; Trias, A.M., Tan, B., Eds.; AOCS/CRC Press: Champaign, IL, USA, 2012. [Google Scholar]
- Uchida, T.; Abe, C.; Nomura, S.; Ichikawa, T.; Ikeda, S. Tissue distribution of α- and γ-tocotrienol and γ-tocopherol in rats and interference with their accumulation by α-tocopherol. Lipids 2012, 47, 129–139. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wong, W.-Y.; Poudyal, H.; Ward, L.C.; Brown, L. Tocotrienols Reverse Cardiovascular, Metabolic and Liver Changes in High Carbohydrate, High Fat Diet-Fed Rats. Nutrients 2012, 4, 1527-1541. https://doi.org/10.3390/nu4101527
Wong W-Y, Poudyal H, Ward LC, Brown L. Tocotrienols Reverse Cardiovascular, Metabolic and Liver Changes in High Carbohydrate, High Fat Diet-Fed Rats. Nutrients. 2012; 4(10):1527-1541. https://doi.org/10.3390/nu4101527
Chicago/Turabian StyleWong, Weng-Yew, Hemant Poudyal, Leigh C. Ward, and Lindsay Brown. 2012. "Tocotrienols Reverse Cardiovascular, Metabolic and Liver Changes in High Carbohydrate, High Fat Diet-Fed Rats" Nutrients 4, no. 10: 1527-1541. https://doi.org/10.3390/nu4101527






