Adherence to the Mediterranean Diet and Inflammatory Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. General Questionnaire
2.3. Mediterranean Dietary Pattern
2.4. Anthropometric Measurements
2.5. Metabolic Syndrome Definition
2.6. Biochemical Measurements
2.7. Statistical Analysis
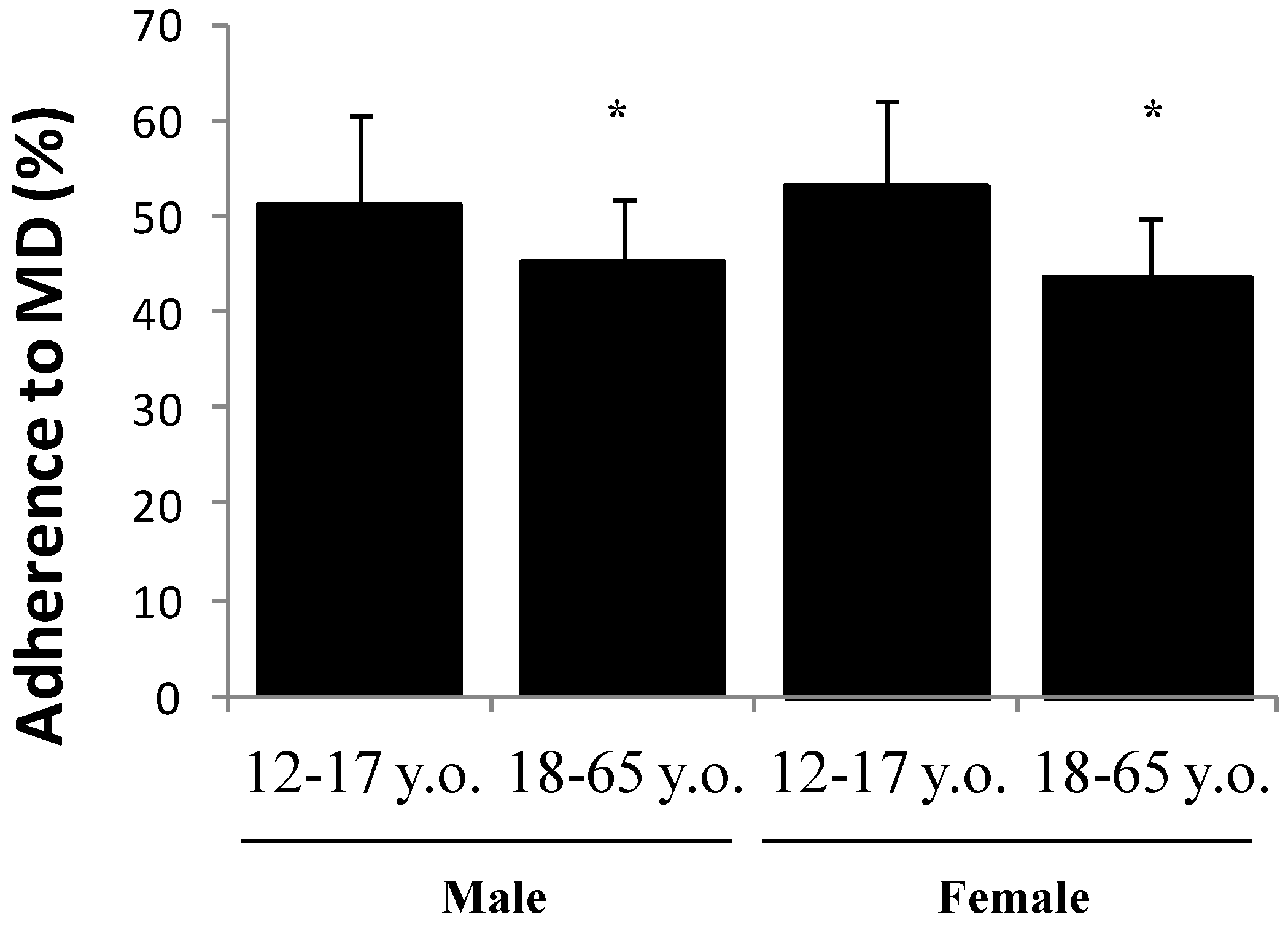
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Bach, A.; Roman, B. Recognition of the Mediterranean diet: Going step further. Public Health Nutr. 2006, 9, 101–102. [Google Scholar] [CrossRef]
- Tur, J.A. Nutritional survey of the Balearic Islands (ENIB, 1999–2000). Rev. Cienc. 2002, 27–30. [Google Scholar]
- Grosso, G.; Mistretta, A.; Frigiola, A.; Gruttadauria, S.; Biondi, A.; Basile, F.; Vitaglione, P.; D’Orazio, N.; Galvano, F. Mediterranean diet and cardiovascular risk factors: A systematic review. Crit. Rev. Food Sci. Nutr. 2014, 54, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Roman, B.; Estruch, R. Scientific evidence of interventions using the Mediterranean diet: A systematic review. Nutr. Rev. 2006, 64, S27–S47. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Bosetti, C.; Rossi, M.; Negri, E.; La Vecchia, C. Selected aspects of Mediterranean diet and cancer risk. Nutr. Cancer 2009, 61, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’armiento, M.; D’andrea, F.; Giugliano, D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Newby, P.K.; Muller, D.; Tucker, K.L. Associations of empirically derived eating patterns with plasma lipid biomarkers: A comparison of factor and cluster analysis methods. Am. J. Clin. Nutr. 2004, 80, 759–767. [Google Scholar] [PubMed]
- Van Dam, R.M.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann. Intern. Med. 2002, 136, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Lakka, H.M.; Laaksonen, D.E.; Lakka, T.A.; Niskanen, L.K.; Kumpusalo, E.; Tuomilehto, J.; Salonen, J.T. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002, 288, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Marsland, A.L.; McCaffery, J.M.; Muldoon, M.F.; Manuck, S.B. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism 2010, 59, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Clement, K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 2006, 113, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Wellen, K.E.; Hotamisligil, G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Investig. 2003, 112, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Brown, N.J.; Vaughan, D.E.; Harrison, D.G.; Mehta, J.L. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation 2004, 109, IV6–IV19. [Google Scholar] [CrossRef] [PubMed]
- Alikasifoglu, A.; Gonc, N.; Ozon, Z.A.; Sen, Y.; Kandemir, N. The relationship between serum adiponectin, tumor necrosis factor-alpha, leptin levels and insulin sensitivity in childhood and adolescent obesity: Adiponectin is a marker of metabolic syndrome. J. Clin. Res. Pediatr. Endocrinol. 2009, 1, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Stumvoll, M. Adiponectin—Its role in metabolism and beyond. Horm. Metab. Res. 2002, 34, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Miller, A.H.; Bremner, J.D.; Goldberg, J.; Jones, L.; Shallenberger, L.; Buckham, R.; Murrah, N.V.; Veledar, E.; Wilson, P.W.; et al. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men: A twin study. Circulation 2008, 117, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Bibiloni, M.d.M.; Martinez, E.; Llull, R.; Juarez, M.D.; Pons, A.; Tur, J.A. Prevalence and risk factors for obesity in Balearic Islands adolescents. Br. J. Nutr. 2010, 103, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.L.; Bibiloni, M.M.; Salas, R.; Pons, A.; Tur, J.A. Prevalence and Related Risk Factors of Overweight and Obesity among the Adult Population in the Balearic Islands, a Mediterranean Region. Obes. Facts 2015, 8, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Alonso, J.; Domingo, A.; Regidor, E. La Medición de la Clase Social en Ciencias de la Salud; SG-Sociedad Española de Epidemiología: Barcelona, Spain, 1995. [Google Scholar]
- Martin-moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and validation of a food frequency questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Mataix, J.; Mañas, M.; Llopis, J.; Martínez de Victoria, E.; Juan, J.; Borregón, A. Tablas de Composición de Alimentos Españoles (Composition Tables of Spanish Foods), 3rd ed.; INTA-Universidad de Granada: Granada, Spain, 1998. [Google Scholar]
- Moreiras, O.; Carvajal, A.; Cabrera, L.; Cuadrado, C. Tablas de Composición de Alimentos (Food Composition Tables), 7th ed.; Pirámide: Madrid, Spain, 2003. [Google Scholar]
- Favier, J.C.; Ireland-Ripert, J.; Toque, C.; Feinberg, M. Répertoire Géneral des Aliments: Table de Composition (Foods General Repertory: Composition Tables), 2nd ed.; Tec & Doc Lavoisier: Paris, France, 1995. [Google Scholar]
- Ripoll, L. La Cocina de las Islas Baleares (The Balearic Islands Cookery); EAE Editorial Academia Espanola: Palma de Mallorca, Spain, 1992. [Google Scholar]
- Tur, J.A.; Romaguera, D.; Pons, A. Adherence to the Mediterranean dietary pattern among the population of the Balearic Islands. Br. J. Nutr. 2004, 92, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar] [PubMed]
- Livingstone, M.B.; Black, A.E. Markers of the validity of reported energy intake. J. Nutr. 2003, 133 (Suppl. 3), 895S–920S. [Google Scholar] [PubMed]
- Sanchez-Villegas, A.; Martinez, J.A.; De Irala, J.; Martinez-Gonzalez, M.A. Determinants of the adherence to an “a priori” defined Mediterranean dietary pattern. Eur. J. Nutr. 2002, 41, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute; American Academy of Family Physicians. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar]
- De Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef] [PubMed]
- Serra, L.; Aranceta, J.; Pérez, C.; Moreno, B.; Tojo, R.; Delgado, A.; AEP-SENC-SEEDO, Collaborative Group. Curvas de Referencia Para la Tipificación Ponderal; IM&C, SA: Madrid, Spain, 2002; pp. 9–69. [Google Scholar]
- National Heart, Lung, and Blood Institute. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: A working group report from the National High Blood Pressure Education Program. Pediatrics 1996, 98, 649–658. [Google Scholar]
- Cartier, A.; Côté, M.; Lemieux, I.; Pérusse, L.; Tremblay, A.; Bouchard, C.; Després, J.P. Sex differences in inflammatory markers: What is the contribution of visceral adiposity? Am. J. Clin. Nutr. 2009, 89, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.; Sanghavi, M.; Ayers, C.R.; McGuire, D.K.; Omland, T.; Atzler, D.; Gore, M.O.; Neeland, I.; Berry, J.D.; Khera, A.; et al. Sex-Based Differences in Cardiometabolic Biomarkers. Circulation 2017, 135, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Bibiloni, M.M.; Gonzalez, M.; Julibert, A.; Llompart, I.; Pons, A.; Tur, J.A. Ten-Year Trends (1999–2010) of Adherence to the Mediterranean Diet among the Balearic Islands’ Adult Population. Nutrients 2017, 9, 749. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Llull, R.; Bibiloni, M.M.; Pons, A.; Tur, J.A. Adherence to the Mediterranean dietary pattern among Balearic Islands adolescents. Br. J. Nutr. 2010, 103, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- JafariNasabian, P.; Inglis, J.E.; Reilly, W.; Kelly, O.J.; Ilich, J.Z. Aging human body: Changes in bone, muscle and body fat with consequent changes in nutrient intake. J. Endocrinol. 2017, 234, R37–R51. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, E.; Collazo-Clavell, M.L.; Faubion, S.S. Weight Gain in Women at Midlife: A Concise Review of the Pathophysiology and Strategies for Management. Mayo Clin. Proc. 2017, 92, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh. Hig. Rada Toksikol. 2014, 65, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Kastorini, C.M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and metabolic syndrome: An updated systematic review. Rev. Endocr. Metab. Disord. 2013, 14, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S.; Vassallo, J.; Calleja, N.; Pace, N.; Mamo, J. The effect of age, gender, TG/HDL-C ratio and behavioral lifestyles on the metabolic syndrome in the high risk Mediterranean Island population of Malta. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S321–S327. [Google Scholar] [CrossRef] [PubMed]
- Viscogliosi, G.; Cipriani, E.; Liguori, M.L.; Marigliano, B.; Saliola, M.; Ettorre, E.; Andreozzi, P. Mediterranean dietary pattern adherence: Associations with prediabetes, metabolic syndrome, and related microinflammation. Metab. Syndr. Relat. Disord. 2013, 11, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Izadi, V.; Azadbakht, L. Specific dietary patterns and concentrations of adiponectin. J. Res. Med. Sci. 2015, 20, 178–184. [Google Scholar] [PubMed]
- Kubota, N.; Terauchi, Y.; Yamauchi, T.; Kubota, T.; Moroi, M.; Matsui, J.; Eto, K.; Yamashita, T.; Kamon, J.; Satoh, H.; et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002, 277, 25863–25866. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Kihara, S.; Arita, Y.; Okamoto, Y.; Maeda, K.; Kuriyama, H.; Hotta, K.; Nishida, M.; Takahashi, M.; Muraguchi, M.; et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000, 102, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Bellastella, G.; Petrizzo, M.; Scappaticcio, L.; Giugliano, D.; Esposito, K. Mediterranean diet cools down the inflammatory milieu in type 2 diabetes: The MEDITA randomized controlled trial. Endocrine 2016, 54, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Bedard, A.; Tchernof, A.; Lamarche, B.; Corneau, L.; Dodin, S.; Lemieux, S. Effects of the traditional Mediterranean diet on adiponectin and leptin concentrations in men and premenopausal women: Do sex differences exist? Eur. J. Clin. Nutr. 2014, 68, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.F.; Sinha, M.K.; Kolaczynski, J.W.; Zhang, P.L.; Considine, R.V. Leptin: The tale of an obesity gene. Diabetes 1996, 45, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.; Zulet, M.A.; Abete, I.; Martinez, J.A. Discriminated benefits of a Mediterranean dietary pattern within a hypocaloric diet program on plasma RBP4 concentrations and other inflammatory markers in obese subjects. Endocrine 2009, 36, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Royer, M.M.; Couture, P.; Cianflone, K.; Rezvani, R.; Desroches, S.; Lamarche, B. Effect of the Mediterranean diet on plasma adipokine concentrations in men with metabolic syndrome. Metabolism 2013, 62, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Chiefari, E.; Montalcini, T.; Accattato, F.; Costanzo, F.S.; Pujia, A.; Foti, D.; Brunetti, A.; Gulletta, E. Early effects of a hypocaloric, Mediterranean diet on laboratory parameters in obese individuals. Mediat. Inflamm. 2014, 2014, 750860. [Google Scholar] [CrossRef] [PubMed]
- Trifiletti, A.; Scamardi, R.; Gaudio, A.; Lasco, A.; Frisina, N. Hemostatic effects of diets containing olive or soy oil in hypertensive patients. J. Thromb. Haemost. 2005, 3, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, D.; Leighton, F.; Martinez, C.; Marshall, G.; Cuevas, A.; Castillo, O.; Panes, O.; Munoz, B.; Perez, D.D.; Mizon, C.; et al. Complementary effects of Mediterranean diet and moderate red wine intake on haemostatic cardiovascular risk factors. Eur. J. Clin. Nutr. 2001, 55, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.N.; Brown, L.M.; Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Estrogens, inflammation and obesity: An overview. Front. Biol. 2012, 7, 40–47. [Google Scholar] [CrossRef]
- Taylor, L.E.; Sullivan, J.C. Sex differences in obesity-induced hypertension and vascular dysfunction: A protective role for estrogen in adipose tissue inflammation? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R714–R720. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Pontillo, A.; Di Palo, C.; Giugliano, G.; Masella, M.; Marfella, R.; Giugliano, D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA 2003, 289, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpí-Sardà, M.; Chiva-Blanch, G.; Ros, E.; Martínez-González, M.A.; Covas, M.I.; Lamuela-Raventos, R.M.; Salas-Salvadó, J.; Fiol, M.; et al. The effects of the Mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Zhang, J.; Steck, S.E.; Fung, T.T.; Hazlett, L.J.; Han, K.; Ko, S.H.; Merchant, A.T. Obesity Mediates the Association between Mediterranean Diet Consumption and Insulin Resistance and Inflammation in US Adults. J. Nutr. 2017, 147, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Garcia-Arellano, A.; Estruch, R.; Marquez-Sandoval, F.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Viñoles, E.; Arós, F.; Herrera, C.; et al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur. J. Clin. Nutr. 2008, 62, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [PubMed]
- Rumawas, M.E.; Meigs, J.B.; Dwyer, J.T.; McKeown, N.M.; Jacques, P.F. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2009, 90, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Arpon, A.; Riezu-Boj, J.I.; Milagro, F.I.; Marti, A.; Razquin, C.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Casas, R.; Fitó, M.; et al. Adherence to Mediterranean diet is associated with methylation changes in inflammation-related genes in peripheral blood cells. J. Physiol. Biochem. 2016, 73, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Marques-Rocha, J.L.; Milagro, F.I.; Mansego, M.L.; Zulet, M.A.; Bressan, J.; Martinez, J.A. Expression of inflammation-related miRNAs in white blood cells from subjects with metabolic syndrome after 8 weeks of following a Mediterranean diet-based weight loss program. Nutrition 2016, 32, 48–55. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | ||
|---|---|---|
| Male (n = 219) | 12–17 (n = 146) | 18–65 (n = 73) |
| Weight (kg), mean ± SD | 62.7 ± 12.6 | 82.2 ± 14.7 * |
| Height (cm), mean ± SD | 170.5 ± 9.4 | 174.9 ± 6.4 * |
| BMI (kg/m2), mean ± SD | 21.4 ± 3.1 | 26.9 ± 5.1 * |
| TSF (mm) ± SD | 10.1 ± 4.0 | 16.3 ± 7.1 * |
| WC (cm) ± SD | 72.2 ± 7.8 | 89.6 ± 13.3 * |
| HC (cm) ± SD | 91.5 ± 9.0 | 103.6 ± 8.2 * |
| WHR ± SD | 0.79 ± 0.05 | 0.86 ± 0.06 * |
| Total body fat (%) | 12.9 ± 6.0 | 20.6 ± 7.1 * |
| MetS (%) | 8.6 | 16.3 * |
| Female (n = 379) | (n = 218) | (n = 161) |
| Weight (kg), mean ± SD | 57.3 ± 9.6 | 64.3 ± 13.1 * |
| Height (cm), mean ± SD | 161.4 ± 6.2 | 162.8 ± 11.2 * |
| BMI (kg/m2), mean ± SD | 21.9 ± 3.3 | 26.7 ± 5.0 * |
| TSF (mm) ± SD | 14.3 ± 4.4 | 23.7 ± 7.3 * |
| WC (cm) ± SD | 67.5 ± 6.5 | 76.9 ± 11.1 * |
| HC (cm) ± SD | 94.7 ± 8.0 | 102.5 ± 9.4 * |
| WHR ± SD | 0.71 ± 0.06 | 0.75 ± 0.05 * |
| Total body fat (%) | 15.2 ± 5.8 | 28.9 ± 6.2 * |
| MetS (%) | 3.4 | 7.2 * |
| Adherence to MDP (%) | n | Adiponectin (µg/mL) | Leptin (ng/mL) | TNF-α (pg/mL) | PAI-1 (ng/mL) | hs-CRP (mg/mL) |
|---|---|---|---|---|---|---|
| 12–17 years old | ||||||
| Above median value (≥50%) | 68 | 13.8 ± 5.8 | 10.4 ± 8.6 | 5.4 ± 4.7 | 577 ± 212 | 0.16 ± 0.26 |
| Under median value (<50%) | 78 | 14.4 ± 5.8 | 7.6 ± 9.1 | 6.0 ± 5.1 | 563 ± 229 | 0.19 ± 0.38 |
| 18–65 years old | ||||||
| Above median value (≥50%) | 40 | 13.1 ± 6.7 | 9.4 ± 7.3 | 7.9 ± 2.4 | 201 ± 29 | 0.17 ± 0.18 |
| Under median value (<50%) | 33 | 9.5 ± 2.4 * | 16.0 ± 9.5 * | 12.3 ± 3.0 * | 262 ± 32 * | 0.41 ± 0.42 * |
| Adherence to MDP (%) | n | Adiponectin (µg/mL) | Leptin (ng/mL) | TNF-α (pg/mL) | PAI-1 (ng/mL) | hs-CRP (mg/mL) |
|---|---|---|---|---|---|---|
| 12–17 years old | ||||||
| Above median value (≥50%) | 90 | 16.4 ± 6.5 | 24.7 ± 11.0 | 5.4 ± 4.5 | 476 ± 236 | 0.07 ± 0.08 |
| Under median value (<50%) | 128 | 15.0 ± 6.0 | 36.3 ± 13.9 * | 5.3 ± 4.8 | 538 ± 266 | 0.16 ± 0.13 * |
| 18–65 years old | ||||||
| Above median value (≥50%) | 78 | 10.3 ± 1.5 | 44.3 ± 25.1 | 7.3 ± 3.1 | 204 ± 32 | 0.18 ± 0.23 |
| Under median value (<50%) | 83 | 11.1 ± 1.1 | 43.0 ± 24.0 | 7.7 ± 2.7 | 298 ± 43 * | 0.28 ± 0.32 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sureda, A.; Bibiloni, M.D.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. https://doi.org/10.3390/nu10010062
Sureda A, Bibiloni MDM, Julibert A, Bouzas C, Argelich E, Llompart I, Pons A, Tur JA. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients. 2018; 10(1):62. https://doi.org/10.3390/nu10010062
Chicago/Turabian StyleSureda, Antoni, Maria Del Mar Bibiloni, Alicia Julibert, Cristina Bouzas, Emma Argelich, Isabel Llompart, Antoni Pons, and Josep A. Tur. 2018. "Adherence to the Mediterranean Diet and Inflammatory Markers" Nutrients 10, no. 1: 62. https://doi.org/10.3390/nu10010062
APA StyleSureda, A., Bibiloni, M. D. M., Julibert, A., Bouzas, C., Argelich, E., Llompart, I., Pons, A., & Tur, J. A. (2018). Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients, 10(1), 62. https://doi.org/10.3390/nu10010062









