Long-Term Single-Dose Efficacy of a Vesicular Stomatitis Virus-Based Andes Virus Vaccine in Syrian Hamsters
Abstract
:1. Introduction
2. Results and Discussion
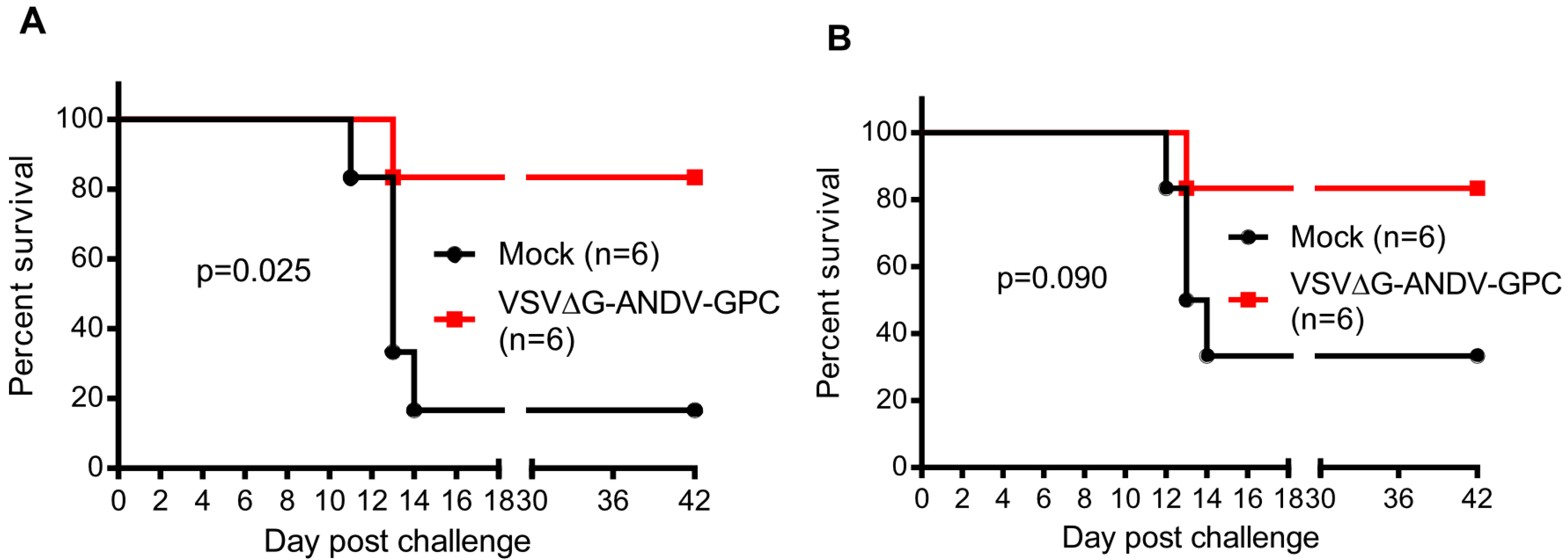
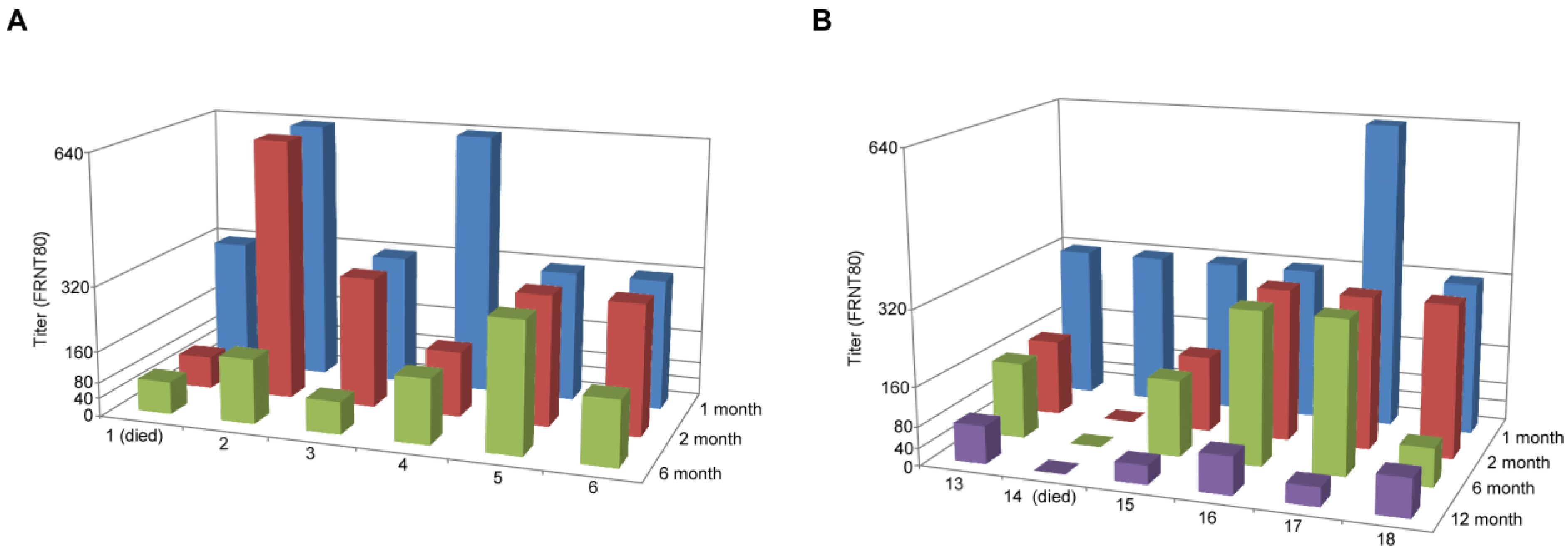
| Challenge 6 months post-vaccination | Challenge 12 months post-vaccination | ||||
|---|---|---|---|---|---|
| Animal | Vaccine | Titer | Animal | Vaccine | Titer |
| 1 | VSV∆G-ANDV-GPC | NA | 13 | VSV∆G-ANDV-GPC | ≥3200 |
| 2 | VSV∆G-ANDV-GPC | 800 | 14 | VSV∆G-ANDV-GPC | NA |
| 3 | VSV∆G-ANDV-GPC | 1600 | 15 | VSV∆G-ANDV-GPC | ≥3200 |
| 4 | VSV∆G-ANDV-GPC | 1600 | 16 | VSV∆G-ANDV-GPC | 1600 |
| 5 | VSV∆G-ANDV-GPC | ≥3200 | 17 | VSV∆G-ANDV-GPC | 800 |
| 6 | VSV∆G-ANDV-GPC | ≥3200 | 18 | VSV∆G-ANDV-GPC | ≥3200 |
| 7 | Mock | NA | 19 | Mock | ≥3200 |
| 8 | Mock | NA | 20 | Mock | ≥3200 |
| 9 | Mock | NA | 21 | Mock | NA |
| 10 | Mock | NA | 22 | Mock | NA |
| 11 | Mock | 1600 | 23 | Mock | NA |
| 12 | Mock | NA | 24 | Mock | NA |
3. Experimental Section
3.1. Hamster Vaccination and Challenge
3.2. Andes Virus Neutralization and ELISA
3.2. Statistics
3.3. Biosafety and Ethics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khan, A.S.; Ksiazek, T.G.; Peters, C.J. Hantavirus pulmonary syndrome. Lancet 1996, 347, 739–741. [Google Scholar] [CrossRef]
- Levis, S.; Rowe, J.E.; Morzunov, S.; Enria, D.A.; St Jeor, S. New hantaviruses causing hantavirus pulmonary syndrome in central Argentina. Lancet 1997, 349, 998–999. [Google Scholar] [CrossRef]
- Padula, P.J.; Edelstein, A.; Miguel, S.D.; López, N.M.; Rossi, C.M.; Rabinovich, R.D. Hantavirus pulmonary syndrome outbreak in Argentina: Molecular evidence for person-to-person transmission of Andes virus. Virology 1998, 241, 323–330. [Google Scholar] [CrossRef]
- Martinez, V.P.; Bellomo, C.; San Juan, J.; Pinna, D.; Forlenza, R.; Elder, M.; Padula, P.J. Person-to-person transmission of Andes virus. Emerg. Infect. Dis. 2005, 11, 1848–1853. [Google Scholar] [CrossRef]
- Lázaro, M.E.; Cantoni, G.E.; Calanni, L.M.; Resa, A.J.; Herrero, E.R.; Iacono, M.A.; Enria, D.A.; González Cappa, S.M. Clusters of hantavirus infection, southern Argentina. Emerg. Infect. Dis. 2007, 13, 104–110. [Google Scholar] [CrossRef]
- Hooper, J.W.; Larsen, T.; Custer, D.M.; Schmaljohn, C.S. A lethal disease model for hantavirus pulmonary syndrome. Virology 2001, 289, 6–14. [Google Scholar] [CrossRef]
- Safronetz, D.; Zivcec, M.; Lacasse, R.; Feldmann, F.; Rosenke, R.; Long, D.; Haddock, E.; Brining, D.; Gardner, D.; Feldmann, H.; et al. Pathogenesis and host response in Syrian hamsters following intranasal infection with Andes virus. PLoS Pathog. 2011, 7, e1002426. [Google Scholar] [CrossRef]
- Brown, K.S.; Safronetz, D.; Marzi, A.; Ebihara, H.; Feldmann, H. Vesicular stomatitis virus-based vaccine protects hamsters against lethal challenge with Andes virus. J. Virol. 2011, 85, 12781–12791. [Google Scholar] [CrossRef]
- Jones, S.M.; Feldmann, H.; Ströher, U.; Geisbert, J.B.; Fernando, L.; Grolla, A.; Klenk, H.-D.; Sullivan, N.J.; Volchkov, V.E.; Fritz, E.A.; et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 2005, 11, 786–790. [Google Scholar] [CrossRef]
- Prescott, J.; Safronetz, D.; Haddock, E.; Robertson, S.; Scott, D.; Feldmann, H. The adaptive immune response does not influence hantavirus disease or persistence in the Syrian hamster. Immunology 2013, 140, 168–178. [Google Scholar]
- Safronetz, D.; Ebihara, H.; Feldmann, H.; Hooper, J.W. The Syrian hamster model of hantavirus pulmonary syndrome. Antivir. Res. 2012, 95, 282–292. [Google Scholar] [CrossRef]
- Hooper, J.W.; Josleyn, M.; Ballantyne, J.; Brocato, R. A novel Sin Nombre virus DNA vaccine and its inclusion in a candidate pan-hantavirus vaccine against hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS). Vaccine 2013, 31, 4314–4321. [Google Scholar] [CrossRef]
- Kapadia, S.U.; Rose, J.K.; Lamirande, E.; Vogel, L.; Subbarao, K.; Roberts, A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 2005, 340, 174–182. [Google Scholar] [CrossRef]
- Schwartz, J.A.; Buonocore, L.; Suguitan, A.L.; Silaghi, A.; Kobasa, D.; Kobinger, G.; Feldmann, H.; Subbarao, K.; Rose, J.K. Potent vesicular stomatitis virus-based avian influenza vaccines provide long-term sterilizing immunity against heterologous challenge. J. Virol. 2010, 84, 4611–4618. [Google Scholar] [CrossRef]
- Botten, J.; Mirowsky, K.; Kusewitt, D.; Bharadwaj, M.; Yee, J.; Ricci, R.; Feddersen, R.M.; Hjelle, B. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus). Proc. Natl. Acad. Sci. USA 2000, 97, 10578–10583. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prescott, J.; DeBuysscher, B.L.; Brown, K.S.; Feldmann, H. Long-Term Single-Dose Efficacy of a Vesicular Stomatitis Virus-Based Andes Virus Vaccine in Syrian Hamsters. Viruses 2014, 6, 516-523. https://doi.org/10.3390/v6020516
Prescott J, DeBuysscher BL, Brown KS, Feldmann H. Long-Term Single-Dose Efficacy of a Vesicular Stomatitis Virus-Based Andes Virus Vaccine in Syrian Hamsters. Viruses. 2014; 6(2):516-523. https://doi.org/10.3390/v6020516
Chicago/Turabian StylePrescott, Joseph, Blair L. DeBuysscher, Kyle S. Brown, and Heinz Feldmann. 2014. "Long-Term Single-Dose Efficacy of a Vesicular Stomatitis Virus-Based Andes Virus Vaccine in Syrian Hamsters" Viruses 6, no. 2: 516-523. https://doi.org/10.3390/v6020516




