Human Leukocyte Antigen (HLA) Class I Down-Regulation by Human Immunodeficiency Virus Type 1 Negative Factor (HIV-1 Nef): What Might We Learn From Natural Sequence Variants?
Abstract
:1. Introduction
1.1. HIV-1 Infection and Therapy
1.2. The Nef Protein
1.3. HIV-1 Immune Evasion Strategies
2. HLA Class I-Mediated Control of HIV-1
2.1. Role of HLA-I in Viral Infection
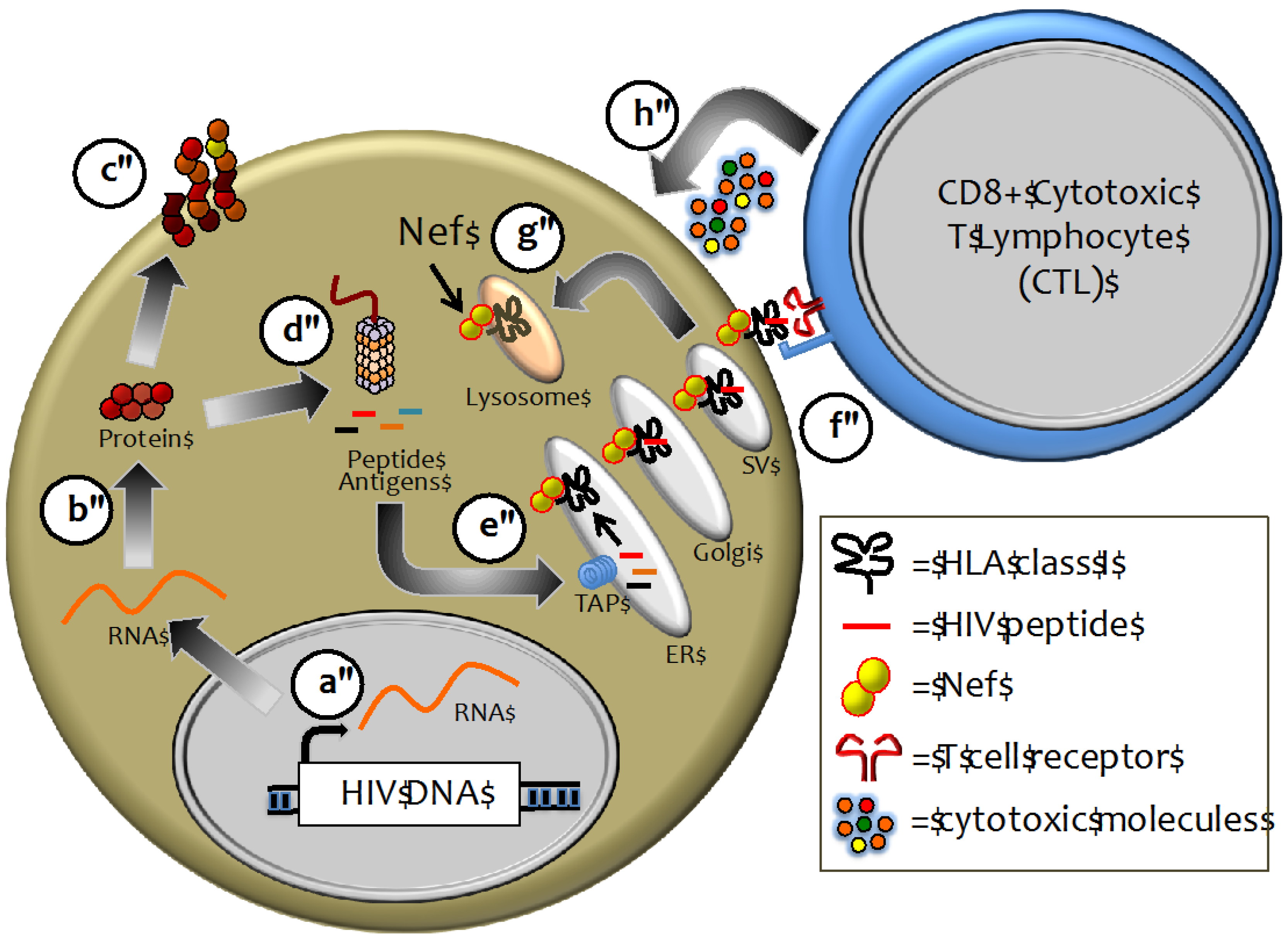
2.2. HLA Class I as a Major Determinant of HIV-1 Pathogenesis
3. Nef-Dependent Immune Evasion: In Vivo and in Vitro Observations
4. Nef Structure and HLA Class I Down-Regulation Function
4.1. General Features of Nef and its Domain Structure
4.2. Functional Motifs and Host Proteins Interactions
4.3. Proposed Mechanisms of HLA Class I Down-Regulation
5. Natural Variation in Nef Sequence and Implications for Immune Evasion
5.1. Sequence Variability within Described HLA-I Down-Regulation Motifs
| Nef Domain a | Role | AA | Frequency b | Entropy b | References |
|---|---|---|---|---|---|
| MG2xxxS6 | Myristoylation | G2 | 100 % | 0 | [116] |
| S6 | 99.2 % | 0.05 | [116] | ||
| W13 | Stability (?) | W13 | 100 % | 0 | [76] |
| R17xR19 | ß-COP | R17 | 97.5 % | 0.15 | [87] |
| E18 | 96.7% | 0.18 | [115] | ||
| R19 | 90.5 % | 0.35 | [87] | ||
| M20 | Stability (?) | M20 | 90.5 % | 0.37 | [93] |
| ? | Unknown | N52 | 98.8% | 0.08 | [115] |
| E62EEE65 | PACS-1/2 | E62 | 92.1 % | 0.31 | [95,115] |
| E63 | 75.2 % | 0.72 | [95] | ||
| E64 | 88.8 % | 0.48 | [95] | ||
| E65 | 91.7 % | 0.35 | [95] | ||
| P72xxPxR77 | SH3 binding, | P72 | 100 % | 0 | [76,81,117] |
| and (PxxP)3 | HLA-I “clamp” | Q73 | 99.6 % | 0.03 | [76,81,117] |
| V74 | 99.4 % | 0.05 | [76,81,115,117] | ||
| P75 | 100 % | 0 | [76,81,117] | ||
| L76 | 96.7 % | 0.16 | [76,81,117] | ||
| R77 | 100 % | 0 | [76,81,117] | ||
| P78 | 99.6 % | 0.03 | [76,81,117] | ||
| G83 | 56.2% | 0.73 | [90,115] | ||
| ? | Unknown | A84 | 99.2% | 0.05 | [115] |
| D123 | Oligomerization and Stability (?) | D123 | 100 % | 0 | [74,76,82,115] |
| ? | Unknown | Y135 | 75.2% | 0.64 | [115] |
| ? | Unknown | G140 | 100% | 0 | [76,115] |
| ? | Unknown | S169 | 89.7% | 0.47 | [76,115] |
| D175 | Trafficking | D175 | 99.6% | 0.03 | [76,88,115,118] |
| ? | Unknown | V180 | 99.2% | 0.05 | [76,115] |
| Y202 | Stability (?) | Y202 | 87.6 % | 0.39 | [76] |
| F203c | Stability (?) | F203 | 9.5 % | 0.31 | [76] |
| Y203 | 90.5 % | 0.31 | [76] | ||
| D123 | Oligomerization and Stability (?) | D123 | 100 % | 0 | [74,76,82,115] |
| ? | Unknown | Y135 | 75.2% | 0.64 | [115] |
| ? | Unknown | G140 | 100% | 0 | [76,115] |
| ? | Unknown | S169 | 89.7% | 0.47 | [76,115] |
| D175 | Trafficking | D175 | 99.6% | 0.03 | [76,88,115,118] |
5.2. Immune-Mediated Attenuation of Nef Function?
6. Conclusions
Acknowledgments
Conflict of Interest
Correction
A correction was published on 5 October 2012: https://www.mdpi.com/1999-4915/4/10/2014 (PDF, 364 KB)
References
- Dandekar, S. Pathogenesis of HIV in the gastrointestinal tract. Curr. HIV/AIDS Rep. 2007, 4, 10–15. [Google Scholar] [CrossRef]
- Guadalupe, M.; Reay, E.; Sankaran, S.; Prindiville, T.; Flamm, J.; McNeil, A.; Dandekar, S. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 2003, 77, 11708–11717. [Google Scholar] [CrossRef]
- Lim, S.G.; Condez, A.; Lee, C.A.; Johnson, M.A.; Elia, C.; Poulter, L.W. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin. Exp. Immunol. 1993, 92, 448–454. [Google Scholar]
- Lyles, R.H.; Munoz, A.; Yamashita, T.E.; Bazmi, H.; Detels, R.; Rinaldo, C.R.; Margolick, J.B.; Phair, J.P.; Mellors, J.W. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J. Infect. Dis. 2000, 181, 872–880. [Google Scholar] [CrossRef]
- Sierra-Aragon, S.; Walter, H. Targets for inhibition of HIV replication: Entry, enzyme action, release and maturation. Intervirology 2012, 55, 84–97. [Google Scholar] [CrossRef]
- Mayer, K.H.; Venkatesh, K.K. Antiretroviral therapy as HIV prevention: Status and prospects. Am. J. Public Health 2010, 100, 1867–1876. [Google Scholar] [CrossRef]
- Montaner, J.S.; Lima, V.D.; Barrios, R.; Yip, B.; Wood, E.; Kerr, T.; Shannon, K.; Harrigan, P.R.; Hogg, R.S.; Daly, P.; et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: A population-based study. Lancet 2010, 376, 532–539. [Google Scholar]
- Richter, S.N.; Frasson, I.; Palu, G. Strategies for inhibiting function of HIV-1 accessory proteins: A necessary route to AIDS therapy? Curr. Med. Chem. 2009, 16, 267–286. [Google Scholar] [CrossRef]
- Kestler, H.W., 3rd; Ringler, D.J.; Mori, K.; Panicali, D.L.; Sehgal, P.K.; Daniel, M.D.; Desrosiers, R.C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 1991, 65, 651–662. [Google Scholar] [CrossRef]
- Deacon, N.J.; Tsykin, A.; Solomon, A.; Smith, K.; Ludford-Menting, M.; Hooker, D.J.; McPhee, D.A.; Greenway, A.L.; Ellett, A.; Chatfield, C.; et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 1995, 270, 988–991. [Google Scholar]
- Kirchhoff, F.; Greenough, T.C.; Brettler, D.B.; Sullivan, J.L.; Desrosiers, R.C. Brief report: Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 1995, 332, 228–232. [Google Scholar] [CrossRef]
- Kirchhoff, F.; Easterbrook, P.J.; Douglas, N.; Troop, M.; Greenough, T.C.; Weber, J.; Carl, S.; Sullivan, J.L.; Daniels, R.S. Sequence variations in human immunodeficiency virus type 1 Nef are associated with different stages of disease. J. Virol. 1999, 73, 5497–5508. [Google Scholar]
- Pushker, R.; Jacque, J.M.; Shields, D.C. Meta-analysis to test the association of HIV-1 nef amino acid differences and deletions with disease progression. J. Virol. 2010, 84, 3644–3653. [Google Scholar] [CrossRef]
- Corro, G.; Rocco, C.A.; de Candia, C.; Catano, G.; Turk, G.; Mangano, A.; Aulicino, P.C.; Bologna, R.; Sen, L. Genetic and functional analysis of HIV type 1 nef gene derived from long-term nonprogressor children: Association of attenuated variants with slow progression to pediatric AIDS. AIDS Res. Hum. Retrovir. 2012. doi:10.1089/aid.2012.0020. [Google Scholar]
- Crotti, A.; Neri, F.; Corti, D.; Ghezzi, S.; Heltai, S.; Baur, A.; Poli, G.; Santagostino, E.; Vicenzi, E. Nef alleles from human immunodeficiency virus type 1-infected long-term-nonprogressor hemophiliacs with or without late disease progression are defective in enhancing virus replication and CD4 down-regulation. J. Virol. 2006, 80, 10663–10674. [Google Scholar] [CrossRef]
- Lewis, M.J.; Balamurugan, A.; Ohno, A.; Kilpatrick, S.; Ng, H.L.; Yang, O.O. Functional adaptation of Nef to the immune milieu of HIV-1 infection in vivo. J. Immunol. 2008, 180, 4075–4081. [Google Scholar]
- Zuo, J.; Suen, J.; Wong, A.; Lewis, M.; Ayub, A.; Belzer, M.; Church, J.; Yang, O.O.; Krogstad, P. Functional analysis of HIV type 1 Nef gene variants from adolescent and adult survivors of perinatal infection. AIDS Res. Hum. Retrovi. 2012, 28, 486–492. [Google Scholar]
- Klotman, M.E.; Kim, S.; Buchbinder, A.; de Rossi, A.; Baltimore, D.; Wong-Staal, F. Kinetics of expression of multiply spliced RNA in early human immunodeficiency virus type 1 infection of lymphocytes and monocytes. Proc. Natl. Acad. Sci. USA 1991, 88, 5011–5015. [Google Scholar]
- Landi, A.; Iannucci, V.; Nuffel, A.V.; Meuwissen, P.; Verhasselt, B. One protein to rule them all: Modulation of cell surface receptors and molecules by HIV Nef. Curr. HIV Res. 2011, 9, 496–504. [Google Scholar] [CrossRef]
- Miller, M.D.; Warmerdam, M.T.; Gaston, I.; Greene, W.C.; Feinberg, M.B. The human immunodeficiency virus-1 nef gene product: A positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 1994, 179, 101–113. [Google Scholar] [CrossRef]
- Munch, J.; Rajan, D.; Schindler, M.; Specht, A.; Rucker, E.; Novembre, F.J.; Nerrienet, E.; Muller-Trutwin, M.C.; Peeters, M.; Hahn, B.H.; et al. Nef-mediated enhancement of virion infectivity and stimulation of viral replication are fundamental properties of primate lentiviruses. J. Virol. 2007, 81, 13852–13864. [Google Scholar] [CrossRef]
- Aiken, C.; Konner, J.; Landau, N.R.; Lenburg, M.E.; Trono, D. Nef induces CD4 endocytosis: Requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 1994, 76, 853–864. [Google Scholar] [CrossRef]
- Garcia, J.V.; Miller, A.D. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 1991, 350, 508–511. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Iafrate, A.J.; Skowronski, J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 1998, 17, 2777–2789. [Google Scholar] [CrossRef]
- Schwartz, O.; Marechal, V.; le Gall, S.; Lemonnier, F.; Heard, J.M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 1996, 2, 338–342. [Google Scholar] [CrossRef]
- Iafrate, A.J.; Carl, S.; Bronson, S.; Stahl-Hennig, C.; Swigut, T.; Skowronski, J.; Kirchhoff, F. Disrupting surfaces of nef required for downregulation of CD4 and for enhancement of virion infectivity attenuates simian immunodeficiency virus replication in vivo. J Virol 2000, 74, 9836–9844. [Google Scholar]
- Stoddart, C.A.; Geleziunas, R.; Ferrell, S.; Linquist-Stepps, V.; Moreno, M.E.; Bare, C.; Xu, W.; Yonemoto, W.; Bresnahan, P.A.; McCune, J.M.; et al. Human immunodeficiency virus type 1 Nef-mediated downregulation of CD4 correlates with Nef enhancement of viral pathogenesis. J. Virol. 2003, 77, 2124–2133. [Google Scholar]
- Tanaka, M.; Ueno, T.; Nakahara, T.; Sasaki, K.; Ishimoto, A.; Sakai, H. Downregulation of CD4 is required for maintenance of viral infectivity of HIV-1. Virology 2003, 311, 316–325. [Google Scholar] [CrossRef]
- Ross, T.M.; Oran, A.E.; Cullen, B.R. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 1999, 9, 613–621. [Google Scholar] [CrossRef]
- Arganaraz, E.R.; Schindler, M.; Kirchhoff, F.; Cortes, M.J.; Lama, J. Enhanced CD4 down-modulation by late stage HIV-1 nef alleles is associated with increased Env incorporation and viral replication. J. Biol. Chem. 2003, 278, 33912–33919. [Google Scholar]
- Benson, R.E.; Sanfridson, A.; Ottinger, J.S.; Doyle, C.; Cullen, B.R. Downregulation of cell-surface CD4 expression by simian immunodeficiency virus Nef prevents viral super infection. J. Exp. Med. 1993, 177, 1561–1566. [Google Scholar] [CrossRef]
- Michel, N.; Allespach, I.; Venzke, S.; Fackler, O.T.; Keppler, O.T. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell-surface CCR5 and CD4. Curr. Biol. 2005, 15, 714–723. [Google Scholar] [CrossRef]
- Mwimanzi, P.; Hasan, Z.; Tokunaga, M.; Gatanaga, H.; Oka, S.; Ueno, T. Naturally arising HIV-1 Nef variants conferring escape from cytotoxic T lymphocytes influence viral entry co-receptor expression and susceptibility to superinfection. Biochem. Biophys. Res. Commun. 2010, 403, 422–427. [Google Scholar] [CrossRef]
- Carl, S.; Greenough, T.C.; Krumbiegel, M.; Greenberg, M.; Skowronski, J.; Sullivan, J.L.; Kirchhoff, F. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 2001, 75, 3657–3665. [Google Scholar] [CrossRef]
- Ueno, T.; Motozono, C.; Dohki, S.; Mwimanzi, P.; Rauch, S.; Fackler, O.T.; Oka, S.; Takiguchi, M. CTL-mediated selective pressure influences dynamic evolution and pathogenic functions of HIV-1 Nef. J. Immunol. 2008, 180, 1107–1116. [Google Scholar]
- Collins, K.L.; Chen, B.K.; Kalams, S.A.; Walker, B.D.; Baltimore, D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 1998, 391, 397–401. [Google Scholar]
- Leonard, J.A.; Filzen, T.; Carter, C.C.; Schaefer, M.; Collins, K.L. HIV-1 Nef disrupts intracellular trafficking of major histocompatibility complex class I, CD4, CD8, and CD28 by distinct pathways that share common elements. J. Virol. 2011, 85, 6867–6881. [Google Scholar] [CrossRef]
- Malim, M.H.; Emerman, M. HIV-1 accessory proteins–ensuring viral survival in a hostile environment. Cell Host Microbe 2008, 3, 388–398. [Google Scholar] [CrossRef]
- Carlson, J.M.; Brumme, Z.L. HIV evolution in response to HLA-restricted CTL selection pressures: A population-based perspective. Microbes Infect. 2008, 10, 455–461. [Google Scholar] [CrossRef]
- Goulder, P.J.; Watkins, D.I. HIV and SIV CTL escape: Implications for vaccine design. Nat. Rev. Immunol. 2004, 4, 630–640. [Google Scholar] [CrossRef]
- Chen, D.Y.; Balamurugan, A.; Ng, H.L.; Cumberland, W.G.; Yang, O.O. Epitope targeting and viral inoculum are determinants of Nef-mediated immune evasion of HIV-1 from cytotoxic T lymphocytes. Blood 2012, 120, 100–111. [Google Scholar] [CrossRef]
- Le Gall, S.; Erdtmann, L.; Benichou, S.; Berlioz-Torrent, C.; Liu, L.; Benarous, R.; Heard, J.M.; Schwartz, O. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 1998, 8, 483–495. [Google Scholar] [CrossRef]
- Williams, M.; Roeth, J.F.; Kasper, M.R.; Fleis, R.I.; Przybycin, C.G.; Collins, K.L. Direct binding of human immunodeficiency virus type 1 Nef to the major histocompatibility complex class I (MHC-I) cytoplasmic tail disrupts MHC-I trafficking. J. Virol. 2002, 76, 12173–12184. [Google Scholar]
- Cohen, G.B.; Gandhi, R.T.; Davis, D.M.; Mandelboim, O.; Chen, B.K.; Strominger, J.L.; Baltimore, D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 1999, 10, 661–671. [Google Scholar] [CrossRef]
- Rajapaksa, U.S.; Li, D.; Peng, Y.C.; McMichael, A.J.; Dong, T.; Xu, X.N. HLA-B may be more protective against HIV-1 than HLA-A because it resists negative regulatory factor (Nef) mediated down-regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 13353–13358. [Google Scholar] [CrossRef]
- Carrington, M.; O’Brien, S.J. The influence of HLA genotype on AIDS. Annu. Rev. Med. 2003, 54, 535–551. [Google Scholar] [CrossRef]
- Jenkins, M.R.; Griffiths, G.M. The synapse and cytolytic machinery of cytotoxic T cells. Curr. Opin. Immunol. 2010, 22, 308–313. [Google Scholar] [CrossRef]
- Horst, D.; Verweij, M.C.; Davison, A.J.; Ressing, M.E.; Wiertz, E.J. Viral evasion of T cell immunity: Ancient mechanisms offering new applications. Curr. Opin. Immunol. 2011, 23, 96–103. [Google Scholar]
- Kirchhoff, F.; Schindler, M.; Specht, A.; Arhel, N.; Munch, J. Role of Nef in primate lentiviral immunopathogenesis. Cell Mol. Life Sci. 2008, 65, 2621–2636. [Google Scholar] [CrossRef]
- Lin, A.; Xu, H.; Yan, W. Modulation of HLA expression in human cytomegalovirus immune evasion. Cell Mol. Immunol. 2007, 4, 91–98. [Google Scholar]
- Altfeld, M.; Kalife, E.T.; Qi, Y.; Streeck, H.; Lichterfeld, M.; Johnston, M.N.; Burgett, N.; Swartz, M.E.; Yang, A.; Alter, G.; et al. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8(+) T cell Response against HIV-1. PLoS Med. 2006, 3, e403. [Google Scholar] [CrossRef]
- Kiepiela, P.; Leslie, A.J.; Honeyborne, I.; Ramduth, D.; Thobakgale, C.; Chetty, S.; Rathnavalu, P.; Moore, C.; Pfafferott, K.J.; Hilton, L.; et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 2004, 432, 769–775. [Google Scholar]
- Borrow, P.; Lewicki, H.; Wei, X.; Horwitz, M.S.; Peffer, N.; Meyers, H.; Nelson, J.A.; Gairin, J.E.; Hahn, B.H.; Oldstone, M.B.; et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 1997, 3, 205–211. [Google Scholar]
- Koup, R.A.; Safrit, J.T.; Cao, Y.; Andrews, C.A.; McLeod, G.; Borkowsky, W.; Farthing, C.; Ho, D.D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 1994, 68, 4650–4655. [Google Scholar]
- Turnbull, E.L.; Lopes, A.R.; Jones, N.A.; Cornforth, D.; Newton, P.; Aldam, D.; Pellegrino, P.; Turner, J.; Williams, I.; Wilson, C.M.; et al. HIV-1 epitope-specific CD8+ T cell responses strongly associated with delayed disease progression cross-recognize epitope variants efficiently. J. Immunol. 2006, 176, 6130–6146. [Google Scholar]
- Schmitz, J.E.; Kuroda, M.J.; Santra, S.; Sasseville, V.G.; Simon, M.A.; Lifton, M.A.; Racz, P.; Tenner-Racz, K.; Dalesandro, M.; Scallon, B.J.; et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999, 283, 857–860. [Google Scholar]
- Deeks, S.G.; Walker, B.D. Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 2007, 27, 406–416. [Google Scholar] [CrossRef]
- Fellay, J.; Ge, D.; Shianna, K.V.; Colombo, S.; Ledergerber, B.; Cirulli, E.T.; Urban, T.J.; Zhang, K.; Gumbs, C.E.; Smith, J.P.; et al. Common genetic variation and the control of HIV-1 in humans. PLoS Genet 2009, 5, e1000791. [Google Scholar] [CrossRef] [Green Version]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A whole-genome association study of major determinants for host control of HIV-1. Science 2007, 317, 944–947. [Google Scholar]
- Limou, S.; le Clerc, S.; Coulonges, C.; Carpentier, W.; Dina, C.; Delaneau, O.; Labib, T.; Taing, L.; Sladek, R.; Deveau, C.; et al. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J. Infect. Dis. 2009, 199, 419–426. [Google Scholar] [CrossRef]
- McLaren, P.J.; Ripke, S.; Pelak, K.; Weintrob, A.C.; Patsopoulos, N.A.; Jia, X.; Erlich, R.L.; Lennon, N.J.; Kadie, C.M.; Heckerman, D.; et al. Fine-mapping classical HLA variation associated with durable host control of HIV-1 infection in African Americans. Hum. Mol. Genet. 2012, 21, 4334–4347. [Google Scholar] [CrossRef]
- Pereyra, F.; Jia, X.; McLaren, P.J.; Telenti, A.; de Bakker, P.I.; Walker, B.D.; Ripke, S.; Brumme, C.J.; Pulit, S.L.; Carrington, M.; et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010, 330, 1551–1557. [Google Scholar]
- Tomiyama, H.; Akari, H.; Adachi, A.; Takiguchi, M. Different effects of Nef-mediated HLA class I down-regulation on human immunodeficiency virus type 1-specific CD8(+) T-cell cytolytic activity and cytokine production. J. Virol. 2002, 76, 7535–7543. [Google Scholar] [CrossRef]
- Munch, J.; Stolte, N.; Fuchs, D.; Stahl-Hennig, C.; Kirchhoff, F. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 2001, 75, 10532–10536. [Google Scholar] [CrossRef]
- Swigut, T.; Alexander, L.; Morgan, J.; Lifson, J.; Mansfield, K.G.; Lang, S.; Johnson, R.P.; Skowronski, J.; Desrosiers, R. Impact of Nef-mediated downregulation of major histocompatibility complex class I on immune response to simian immunodeficiency virus. J. Virol. 2004, 78, 13335–13344. [Google Scholar]
- Friedrich, T.C.; Piaskowski, S.M.; Leon, E.J.; Furlott, J.R.; Maness, N.J.; Weisgrau, K.L.; Mac Nair, C.E.; Weiler, A.M.; Loffredo, J.T.; Reynolds, M.R.; et al. High viremia is associated with high levels of in vivo major histocompatibility complex class I Downregulation in rhesus macaques infected with simian immunodeficiency virus SIVmac239. J. Virol. 2010, 84, 5443–5447. [Google Scholar] [CrossRef]
- Noviello, C.M.; Pond, S.L.; Lewis, M.J.; Richman, D.D.; Pillai, S.K.; Yang, O.O.; Little, S.J.; Smith, D.M.; Guatelli, J.C. Maintenance of Nef-mediated modulation of major histocompatibility complex class I and CD4 after sexual transmission of human immunodeficiency virus type 1. J. Virol. 2007, 81, 4776–4786. [Google Scholar] [CrossRef]
- Shankar, P.; Xu, Z.; Lieberman, J. Viral-specific cytotoxic T lymphocytes lyse human immunodeficiency virus-infected primary T lymphocytes by the granule exocytosis pathway. Blood 1999, 94, 3084–3093. [Google Scholar]
- Yang, O.O.; Kalams, S.A.; Rosenzweig, M.; Trocha, A.; Jones, N.; Koziel, M.; Walker, B.D.; Johnson, R.P. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 1996, 70, 5799–5806. [Google Scholar]
- Yang, O.O.; Nguyen, P.T.; Kalams, S.A.; Dorfman, T.; Gottlinger, H.G.; Stewart, S.; Chen, I.S.; Threlkeld, S.; Walker, B.D. Nef-mediated resistance of human immunodeficiency virus type 1 to antiviral cytotoxic T lymphocytes. J. Virol. 2002, 76, 1626–1631. [Google Scholar]
- Ali, A.; Ng, H.L.; Dagarag, M.D.; Yang, O.O. Evasion of cytotoxic T lymphocytes is a functional constraint maintaining HIV-1 Nef expression. Eur. J. Immunol. 2005, 35, 3221–3228. [Google Scholar] [CrossRef]
- Lewinsohn, D.A.; Lines, R.; Lewinsohn, D.M.; Riddell, S.R.; Greenberg, P.D.; Emerman, M.; Bartz, S.R. HIV-1 Vpr does not inhibit CTL-mediated apoptosis of HIV-1 infected cells. Virology 2002, 294, 13–21. [Google Scholar] [CrossRef]
- Wong, J.K.; Strain, M.C.; Porrata, R.; Reay, E.; Sankaran-Walters, S.; Ignacio, C.C.; Russell, T.; Pillai, S.K.; Looney, D.J.; Dandekar, S. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 2010, 6, e1000748. [Google Scholar] [CrossRef]
- Foster, J.L.; Denial, S.J.; Temple, B.R.; Garcia, J.V. Mechanisms of HIV-1 Nef function and intracellular signaling. J. Neuroimmune Pharmacol. 2011, 6, 230–246. [Google Scholar] [CrossRef]
- Foster, J.L.; Garcia, J.V. HIV-1 Nef: At the crossroads. Retrovirology 2008, 5. [Google Scholar] [CrossRef]
- Jia, X.; Singh, R.; Homann, S.; Yang, H.; Guatelli, J.; Xiong, Y. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat. Struct. Mol. Biol. 2012, 19, 701–706. [Google Scholar] [CrossRef]
- Giese, S.I.; Woerz, I.; Homann, S.; Tibroni, N.; Geyer, M.; Fackler, O.T. Specific and distinct determinants mediate membrane binding and lipid raft incorporation of HIV-1(SF2) Nef. Virology 2006, 355, 175–191. [Google Scholar] [CrossRef]
- Das, S.R.; Jameel, S. Biology of the HIV Nef protein. Indian J. Med. Res. 2005, 121, 315–332. [Google Scholar]
- Franken, P.; Arold, S.; Padilla, A.; Bodeus, M.; Hoh, F.; Strub, M.P.; Boyer, M.; Jullien, M.; Benarous, R.; Dumas, C. HIV-1 Nef protein: Purification, crystallizations, and preliminary X-ray diffraction studies. Protein Sci. 1997, 6, 2681–2683. [Google Scholar]
- Grzesiek, S.; Bax, A.; Clore, G.M.; Gronenborn, A.M.; Hu, J.S.; Kaufman, J.; Palmer, I.; Stahl, S.J.; Wingfield, P.T. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat. Struct. Biol. 1996, 3, 340–345. [Google Scholar] [CrossRef]
- Fackler, O.T.; Luo, W.; Geyer, M.; Alberts, A.S.; Peterlin, B.M. Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 1999, 3, 729–739. [Google Scholar] [CrossRef]
- Liu, L.X.; Heveker, N.; Fackler, O.T.; Arold, S.; Le Gall, S.; Janvier, K.; Peterlin, B.M.; Dumas, C.; Schwartz, O.; Benichou, S.; et al. Mutation of a conserved residue (D123) required for oligomerization of human immunodeficiency virus type 1 Nef protein abolishes interaction with human thioesterase and results in impairment of Nef biological functions. J. Virol. 2000, 74, 5310–5319. [Google Scholar]
- Aldrovandi, G.M.; Gao, L.; Bristol, G.; Zack, J.A. Regions of human immunodeficiency virus type 1 nef required for function in vivo. J. Virol. 1998, 72, 7032–7039. [Google Scholar]
- Chaudhuri, R.; Mattera, R.; Lindwasser, O.W.; Robinson, M.S.; Bonifacino, J.S. A basic patch on alpha-adaptin is required for binding of human immunodeficiency virus type 1 Nef and cooperative assembly of a CD4-Nef-AP-2 complex. J. Virol. 2009, 83, 2518–2530. [Google Scholar] [CrossRef]
- Lindwasser, O.W.; Smith, W.J.; Chaudhuri, R.; Yang, P.; Hurley, J.H.; Bonifacino, J.S. A diacidic motif in human immunodeficiency virus type 1 Nef is a novel determinant of binding to AP-2. J. Virol. 2008, 82, 1166–1174. [Google Scholar]
- Piguet, V.; Gu, F.; Foti, M.; Demaurex, N.; Gruenberg, J.; Carpentier, J.L.; Trono, D. Nef-induced CD4 degradation: A diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell 1999, 97, 63–73. [Google Scholar] [CrossRef]
- Schaefer, M.R.; Wonderlich, E.R.; Roeth, J.F.; Leonard, J.A.; Collins, K.L. HIV-1 Nef targets MHC-I and CD4 for degradation via a final common beta-COP-dependent pathway in T cells. PLoS Pathog. 2008, 4, e1000131. [Google Scholar] [CrossRef]
- Geyer, M.; Yu, H.; Mandic, R.; Linnemann, T.; Zheng, Y.H.; Fackler, O.T.; Peterlin, B.M. Subunit H of the V-ATPase binds to the medium chain of adaptor protein complex 2 and connects Nef to the endocytic machinery. J. Biol. Chem. 2002, 277, 28521–28529. [Google Scholar]
- Mandic, R.; Fackler, O.T.; Geyer, M.; Linnemann, T.; Zheng, Y.H.; Peterlin, B.M. Negative factor from SIV binds to the catalytic subunit of the V-ATPase to internalize CD4 and to increase viral infectivity. Mol. Biol. Cell 2001, 12, 463–473. [Google Scholar]
- Mangasarian, A.; Piguet, V.; Wang, J.K.; Chen, Y.L.; Trono, D. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 1999, 73, 1964–1973. [Google Scholar]
- Greenberg, M.; de Tulleo, L.; Rapoport, I.; Skowronski, J.; Kirchhausen, T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 1998, 8, 1239–1242. [Google Scholar] [CrossRef]
- Williams, M.; Roeth, J.F.; Kasper, M.R.; Filzen, T.M.; Collins, K.L. Human immunodeficiency virus type 1 Nef domains required for disruption of major histocompatibility complex class I trafficking are also necessary for coprecipitation of Nef with HLA-A2. J. Virol. 2005, 79, 632–636. [Google Scholar] [CrossRef]
- Akari, H.; Arold, S.; Fukumori, T.; Okazaki, T.; Strebel, K.; Adachi, A. Nef-induced major histocompatibility complex class I down-regulation is functionally dissociated from its virion incorporation, enhancement of viral infectivity, and CD4 down-regulation. J. Virol. 2000, 74, 2907–2912. [Google Scholar] [CrossRef]
- Dikeakos, J.D.; Thomas, L.; Kwon, G.; Elferich, J.; Shinde, U.; Thomas, G. An interdomain binding site on HIV-1 Nef interacts with PACS-1 and PACS-2 on endosomes to down-regulate MHC-I. Mol. Biol. Cell 2012, 23, 2184–2197. [Google Scholar] [CrossRef]
- Piguet, V.; Wan, L.; Borel, C.; Mangasarian, A.; Demaurex, N.; Thomas, G.; Trono, D. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2000, 2, 163–167. [Google Scholar] [CrossRef]
- Bresnahan, P.A.; Yonemoto, W.; Ferrell, S.; Williams-Herman, D.; Geleziunas, R.; Greene, W.C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr. Biol. 1998, 8, 1235–1238. [Google Scholar] [CrossRef]
- Noviello, C.M.; Benichou, S.; Guatelli, J.C. Cooperative binding of the class I major histocompatibility complex cytoplasmic domain and human immunodeficiency virus type 1 Nef to the endosomal AP-1 complex via its mu subunit. J. Virol. 2008, 82, 1249–1258. [Google Scholar] [CrossRef]
- Singh, R.K.; Lau, D.; Noviello, C.M.; Ghosh, P.; Guatelli, J.C. An MHC-I cytoplasmic domain/HIV-1 Nef fusion protein binds directly to the mu subunit of the AP-1 endosomal coat complex. PLoS One 2009, 4, e8364. [Google Scholar]
- Wonderlich, E.R.; Williams, M.; Collins, K.L. The tyrosine binding pocket in the adaptor protein 1 (AP-1) mu1 subunit is necessary for Nef to recruit AP-1 to the major histocompatibility complex class I cytoplasmic tail. J. Biol. Chem. 2008, 283, 3011–3022. [Google Scholar]
- Roeth, J.F.; Williams, M.; Kasper, M.R.; Filzen, T.M.; Collins, K.L. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J. Cell Biol. 2004, 167, 903–913. [Google Scholar] [CrossRef]
- Hung, C.H.; Thomas, L.; Ruby, C.E.; Atkins, K.M.; Morris, N.P.; Knight, Z.A.; Scholz, I.; Barklis, E.; Weinberg, A.D.; Shokat, K.M.; Thomas, G. HIV-1 Nef assembles a Src family kinase-ZAP-70/Syk-PI3K cascade to downregulate cell-surface MHC-I. Cell Host Microbe 2007, 1, 121–133. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Cullen, P.J. Signalling via ADP-ribosylation factor 6 lies downstream of phosphatidylinositide 3-kinase. Biochem. J. 2000, 345 Pt 3, 719–724. [Google Scholar] [CrossRef]
- Blagoveshchenskaya, A.D.; Thomas, L.; Feliciangeli, S.F.; Hung, C.H.; Thomas, G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 2002, 111, 853–866. [Google Scholar] [CrossRef]
- Larsen, J.E.; Massol, R.H.; Nieland, T.J.; Kirchhausen, T. HIV Nef-mediated major histocompatibility complex class I down-modulation is independent of Arf6 activity. Mol. Biol. Cell 2004, 15, 323–331. [Google Scholar]
- Yi, L.; Rosales, T.; Rose, J.J.; Chowdhury, B.; Knutson, J.R.; Venkatesan, S. HIV-1 Nef binds a subpopulation of MHC-I throughout its trafficking itinerary and down-regulates MHC-I by perturbing both anterograde and retrograde trafficking. J. Biol. Chem. 2010, 285, 30884–30905. [Google Scholar]
- Lindwasser, O.W.; Chaudhuri, R.; Bonifacino, J.S. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr. Mol. Med. 2007, 7, 171–184. [Google Scholar] [CrossRef]
- Peterlin, B.M.; Trono, D. Hide, shield and strike back: How HIV-infected cells avoid immune eradication. Nat. Rev. Immunol. 2003, 3, 97–107. [Google Scholar] [CrossRef]
- Arhel, N.J.; Kirchhoff, F. Implications of Nef: Host cell interactions in viral persistence and progression to AIDS. Curr. Top. Microbiol. Immunol. 2009, 339, 147–175. [Google Scholar] [CrossRef]
- Shugars, D.C.; Smith, M.S.; Glueck, D.H.; Nantermet, P.V.; Seillier-Moiseiwitsch, F.; Swanstrom, R. Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J. Virol. 1993, 67, 4639–4650. [Google Scholar]
- HIV Sequence Database, Los Alamos National Laboratory. Available online: www.hiv.lanl.gov (accessed on August 1, 2012).
- Baugh, L.L.; Garcia, J.V.; Foster, J.L. Functional characterization of the human immunodeficiency virus type 1 Nef acidic domain. J. Virol. 2008, 82, 9657–9667. [Google Scholar] [CrossRef]
- Kuo, L.S.; Baugh, L.L.; Denial, S.J.; Watkins, R.L.; Liu, M.; Garcia, J.V.; Foster, J.L. Overlapping effector interfaces define the multiple functions of the HIV-1 Nef polyproline helix. Retrovirology 2012, 9. doi:10.1186/1742-4690-9-47. [Google Scholar]
- Lee, C.H.; Saksela, K.; Mirza, U.A.; Chait, B.T.; Kuriyan, J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell 1996, 85, 931–942. [Google Scholar] [CrossRef]
- Manninen, A.; Hiipakka, M.; Vihinen, M.; Lu, W.; Mayer, B.J.; Saksela, K. SH3-Domain binding function of HIV-1 Nef is required for association with a PAK-related kinase. Virology 1998, 250, 273–282. [Google Scholar] [CrossRef]
- Lewis, M.J.; Lee, P.; Ng, H.L.; Yang, O.O. Immune selection in vitro reveals human immunodeficiency virus type 1 nef sequence motifs important for its immune evasion function in vivo. J Virol 2012, 86, 7126–7135. [Google Scholar] [CrossRef]
- Geyer, M.; Munte, C.E.; Schorr, J.; Kellner, R.; Kalbitzer, H.R. Structure of the anchor-domain of myristoylated and non-myristoylated HIV-1 Nef protein. J. Mol. Biol. 1999, 289, 123–138. [Google Scholar] [CrossRef]
- Saksela, K.; Cheng, G.; Baltimore, D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995, 14, 484–491. [Google Scholar]
- Lu, X.; Yu, H.; Liu, S.H.; Brodsky, F.M.; Peterlin, B.M. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 1998, 8, 647–656. [Google Scholar] [CrossRef]
- Lichterfeld, M.; Yu, X.G.; Cohen, D.; Addo, M.M.; Malenfant, J.; Perkins, B.; Pae, E.; Johnston, M.N.; Strick, D.; Allen, T.M.; et al. HIV-1 Nef is preferentially recognized by CD8 T cells in primary HIV-1 infection despite a relatively high degree of genetic diversity. AIDS 2004, 18, 1383–1392. [Google Scholar] [CrossRef]
- Brumme, Z.L.; John, M.; Carlson, J.M.; Brumme, C.J.; Chan, D.; Brockman, M.A.; Swenson, L.C.; Tao, I.; Szeto, S.; Rosato, P.; et al. HLA-associated immune escape pathways in HIV-1 subtype B Gag, Pol and Nef proteins. PLoS One 2009, 4, e6687. [Google Scholar]
- Brumme, Z.L.; Brumme, C.J.; Carlson, J.; Streeck, H.; John, M.; Eichbaum, Q.; Block, B.L.; Baker, B.; Kadie, C.; Markowitz, M.; et al. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. J.Virol. 2008, 82, 9216–9227. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, Y.; Xu, K.Y.; Yan, H.; James, I.; Peng, Y.; Blais, M.E.; Gaudieri, S.; Chen, X.; Lun, W.; et al. Extensive HLA-driven viral diversity following a narrow-source HIV-1 outbreak in rural China. Blood 2011, 118, 98–106. [Google Scholar] [CrossRef]
- Brumme, Z.L.; Brumme, C.J.; Heckerman, D.; Korber, B.T.; Daniels, M.; Carlson, J.; Kadie, C.; Bhattacharya, T.; Chui, C.; Szinger, J.; Mo, T.; Hogg, R.S.; Montaner, J.S.; Frahm, N.; Brander, C.; Walker, B.D.; Harrigan, P.R. Evidence of differential HLA class I-mediated viral evolution in functional and accessory/regulatory genes of HIV-1. PLoS Pathog 2007, 3, e94. [Google Scholar] [CrossRef]
- Carlson, J.M.; Listgarten, J.; Pfeifer, N.; Tan, V.; Kadie, C.; Walker, B.D.; Ndung’u, T.; Shapiro, R.; Frater, J.; Brumme, Z.L.; Goulder, P.J.; Heckerman, D. Widespread impact of HLA restriction on immune control and escape pathways of HIV-1. J. Virol. 2012, 86, 5230–5243. [Google Scholar] [CrossRef]
- Ali, A.; Pillai, S.; Ng, H.; Lubong, R.; Richman, D.D.; Jamieson, B.D.; Ding, Y.; McElrath, M.J.; Guatelli, J.C.; Yang, O.O. Broadly increased sensitivity to cytotoxic T lymphocytes resulting from Nef epitope escape mutations. J. Immunol. 2003, 171, 3999–4005. [Google Scholar]
- Mwimanzi, P.; Hasan, Z.; Hassan, R.; Suzu, S.; Takiguchi, M.; Ueno, T. Effects of naturally-arising HIV Nef mutations on cytotoxic T lymphocyte recognition and Nef's functionality in primary macrophages. Retrovirology 2011, 8, 50. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mwimanzi, P.; Markle, T.J.; Ueno, T.; Brockman, M.A. Human Leukocyte Antigen (HLA) Class I Down-Regulation by Human Immunodeficiency Virus Type 1 Negative Factor (HIV-1 Nef): What Might We Learn From Natural Sequence Variants? Viruses 2012, 4, 1711-1730. https://doi.org/10.3390/v4091711
Mwimanzi P, Markle TJ, Ueno T, Brockman MA. Human Leukocyte Antigen (HLA) Class I Down-Regulation by Human Immunodeficiency Virus Type 1 Negative Factor (HIV-1 Nef): What Might We Learn From Natural Sequence Variants? Viruses. 2012; 4(9):1711-1730. https://doi.org/10.3390/v4091711
Chicago/Turabian StyleMwimanzi, Philip, Tristan J. Markle, Takamasa Ueno, and Mark A. Brockman. 2012. "Human Leukocyte Antigen (HLA) Class I Down-Regulation by Human Immunodeficiency Virus Type 1 Negative Factor (HIV-1 Nef): What Might We Learn From Natural Sequence Variants?" Viruses 4, no. 9: 1711-1730. https://doi.org/10.3390/v4091711
APA StyleMwimanzi, P., Markle, T. J., Ueno, T., & Brockman, M. A. (2012). Human Leukocyte Antigen (HLA) Class I Down-Regulation by Human Immunodeficiency Virus Type 1 Negative Factor (HIV-1 Nef): What Might We Learn From Natural Sequence Variants? Viruses, 4(9), 1711-1730. https://doi.org/10.3390/v4091711





