Binge Drinking Effects on EEG in Young Adult Humans
Abstract
:1. Introduction
1.1. EEG and Alcoholism
1.2. EEG and Drinking Amount
1.3. Present Study
2. Results and Discussion
2.1. Demographics
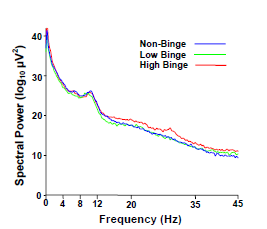
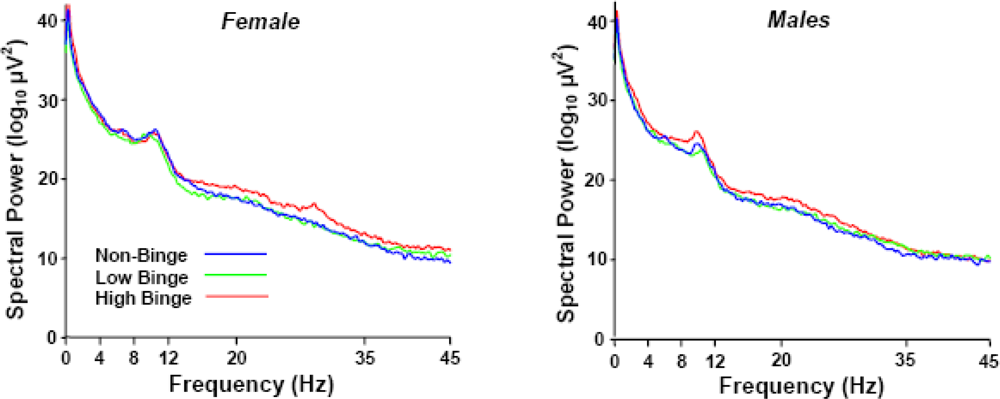
2.2. Spectral Power Findings
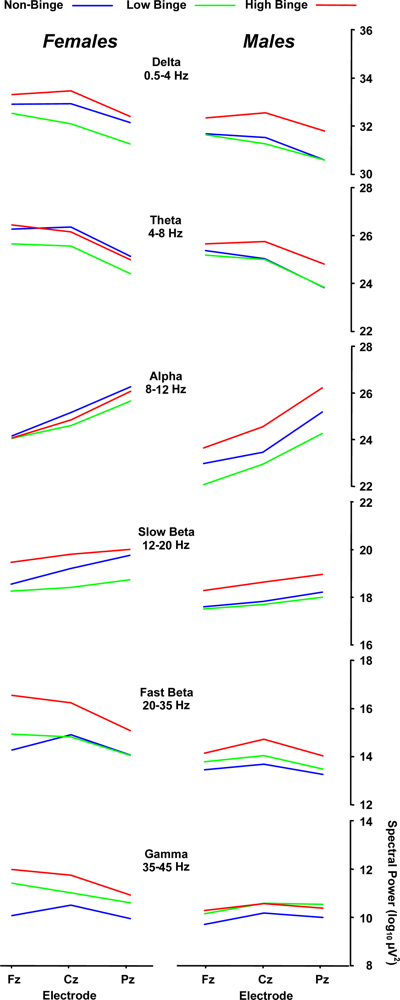
2.3. Delta (0–4 Hz) Band
2.4. Beta (12–35 Hz)
2.5. Sex Effects
3. Experimental Section
3.1. Participants
3.2. Recording Conditions
3.3. Analysis Procedures
4. Conclusions
4.1. Limitations and Future Directions
Acknowledgments
References
- Wechsler, H; Davenport, A; Dowdall, G; Moeykens, B; Castillo, S. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. JAMA 1994, 272, 1672–1677. [Google Scholar]
- Courtney, KE; Polich, J. Binge drinking in young adults: Data, deficits, and determinants. Psychol. Bull 2009, 135, 142–156. [Google Scholar]
- Nichols, JM; Martin, F. The effect of lorazepam on long-term verbal recall in heavy and light social drinkers. Alcohol 1997, 14, 455–461. [Google Scholar]
- Hartley, DE; Elsabagh, S; File, SE. Binge drinking and sex: effects on mood and cognitive function in healthy young volunteers. Pharmacol. Biochem. Behav 2004, 78, 611–619. [Google Scholar]
- Weissenborn, R; Duka, T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology 2003, 165, 306–312. [Google Scholar]
- Maurage, PM; Pesenti, M; Philippot, P; Joassin, F; Campanella, S. Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. J. Psychiatry. Neurosci 2009, 34, 111–118. [Google Scholar]
- Ehlers, CL; Phillips, E; Finnerman, G; Gilder, D; Lau, P; Criado, J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicol. Teratol 2007, 29, 153–163. [Google Scholar]
- Saletu, B. Advances in Pharmaco-EEG. In Pharmacodynamics and EEG; Krijzer, F, Herrmann, WM, Eds.; Zentrale Universitätsdruckerei: Freie Universität, Berlin, Germany, 1996; pp. 187–204. [Google Scholar]
- Saletu, B. Visualizing the living human brian. The techniques and promise of EEG and event-related potentials mapping. Bibl. Psychiatr 1997, 167, 54–62. [Google Scholar]
- Saletu-Zyhlarz, GM; Arnold, O; Anderer, P; Oberndorfer, S; Walter, H; Lesch, OM; Böning, J; Saletu, B. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol. Alcsm 2004, 39, 233–240. [Google Scholar]
- Rangaswamy, M; Porjesz, B; Chorlian, DB; Wang, K; Jones, KA; Bauer, LO; Rohrbaugh, J; O’Connor, SJ; Kuperman, S; Reich, T; Begleiter, H. Beta power in the EEG of alcoholics. Biol. Psychiatry 2002, 52, 831–842. [Google Scholar]
- Kiloh, LG; McComas, AJ; Osselton, JW; Upton, ARM. Clinical Electroencephalography; Butterworths: London, UK, Boston, MA, USA, 1981. [Google Scholar]
- Niedermeyer, E; Lopes da Silva, F. Electroencephalography Basic Principles, Clinical Applications and Related Fields; Urban and Schwarzenberg: Baltimore, MD, USA, 1982. [Google Scholar]
- Bauer, LO. Electroencephalographic and autonomic predictors of relapse in alcohol-dependent patients. Alcsm. Clin. Exp. Res 1994, 18, 755–760. [Google Scholar]
- Davis, PA; Gibbs, FA; Davis, H; Jetter, WW; Trowbridge, IS. The effects of alcohol upon the electroencephalogram (brian waves). Q. J. Stud. Alcohol 1941, 1, 626–637. [Google Scholar]
- Engel, GL; Rosenbaum, M. Studies of the elctroencephalogram in acute alcoholic intoxication. Proc. Cent. Soc. Clin. Res 1944, 17, 62–63. [Google Scholar]
- Bauer, LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology 2001, 25, 332–340. [Google Scholar]
- Enoch, MA. Genetic and environmental influences on the development of alcoholism: Resilience vs. risk. Ann. N. Y. Acad. Sci 2006, 1094, 193–201. [Google Scholar]
- Stacey, D; Clarke, TK; Schumann, G. The genetics of alcoholism. Curr. Psychiatry Rep 2009, 5, 364–369. [Google Scholar]
- Heath, AC; Madden, PA; Bucholz, KK; Dinwiddie, SH; Slutske, WS; Bierut, LJ; Rohrbaugh, W; Statham, DJ; Dunne, MP; Whitfield, B; Martin, NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol. Med 1999, 29, 1069–1081. [Google Scholar]
- Schuckit, MA; Edenberg, HJ; Kalmijn, J; Flury, L; Smith, TL; Reich, T; Bierut, L; Goate, A; Foroud, T. A genome-wide search for genes that relate to a low level of response to alcohol. Alcsm. Clin. Exp. Res 2001, 25, 323–329. [Google Scholar]
- Newlin, DB; Thomson, JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol. Bull 1990, 108, 383–402. [Google Scholar]
- Enoch, MA; Shen, PH; Ducci, F; Yuan, Q; Liu, J; White, KV; Albaugh, B; Hodgkinson, CA; Goldman, D. Common genetic origins for EEG, alcoholism and anxiety: The role of CRH-BP. PLoS ONE 2008, 3, e3620. [Google Scholar]
- Vogel, F. The genetic basis of the normal human electroencephalogram. Humangenetik 1970, 10, 91–114. [Google Scholar]
- van Beijsterveldt, CE; Molenaar, PC; De Geus, EJ; Boomsma, DI. Heritability of human brain functioning as assessed by electroencephalography. Am. J. Hum. Genet 1996, 58, 562–573. [Google Scholar]
- Gabrielli, WF; Nednick, SA; Volavka, J; Pollock, VE; Schulsinger, F; Itil, TM. Electroencephalograms in children of alcoholic fathers. Psychophysiology 1982, 19, 404–407. [Google Scholar]
- Pollock, VE; Voivaka, J; Goodwin, DW; Mednick, SA; Gabrielli, WF; Knop, J; Schulsinger, F. The EEG after alcohol administration in men at risk for alcoholism. Arch. Gen. Psychiatry 1983, 40, 847–861. [Google Scholar]
- Ehlers, CL; Schuckit, MA. EEG fast frequency activity in the sons of alcoholics. Biol. Psychiatry 1990, 27, 631–641. [Google Scholar]
- Kokavec, A; Crowe, SF. A comparison of cognitive performance in binge versus regular chronic alcohol misusers. Alcohol. Alcsm 1999, 34, 601–608. [Google Scholar]
- Slawecki, CJ; Bentancourt, M; Li, TK; Ehlers, CL. Neurophysiological findings and drinking levels in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcsm. Clin. Exp. Res 2000, 24, 1492–1499. [Google Scholar]
- Ehlers, CL; Wall, TL; Schuckit, MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalogr. Clin. Neurophysiol 1989, 73, 179–187. [Google Scholar]
- de Bruin, EA; Bijl, S; Stam, CJ; Böcker, KB; Kenemans, JL; Verbaten, MN. Abnormal EEG synchronisation in heavily drinking students. Clin. Neurophysiol 2004, 115, 2048–2055. [Google Scholar]
- Buzsáki, G. The hippocampo-neocortical dialogue. Cereb. Cortex 1996, 6, 81–92. [Google Scholar]
- Hommer, DW; Momenan, R; Kaiser, E; Rawlings, RR. Evidence for a gender-related effect of alcoholism on brain volumes. Am. J. Psychiatry 2001, 158, 198–204. [Google Scholar]
- Agartz, I; Momenan, R; Rawlings, RR; Kerich, MJ; Hommer, DW. Hippocampal volume in patients with alcohol dependence. Arch. Gen. Psychiatry 1999, 56, 356–363. [Google Scholar]
- Ehlers, CL; Phillips, E; Gizer, IR; Gilder, DA; Wilhelmsen, KC. EEG spectral phenotypes: Heritability and association with marijuana and alcohol dependence in an American Indian community study. Drug. Alcohol Depend 2010, 106, 101–110. [Google Scholar]
- Scharbrough, F; Chatrian, GE; Lesser, R; Luders, H; Nuwer, M; Picton, T. Guidelines for Standard Electrode Position Nomenclature; American EEG Society: Bloomfield, CT, USA, 1990. [Google Scholar]
- Pollock, VE; Schneider, L; Lyness, S. Reliability of topographic quantitative EEG amplitudes in healthy, late middle-aged and elderly adults. Electroencephalogr. Clin. Neurophysiol 1992, 79, 20–26. [Google Scholar]
- Townshend, JM; Duka, T. Patterns of alcohol drinking in a population of young social drinkers: A comparison of questionnaire and diary measures. Alcohol. Alcsm 2002, 37, 187–192. [Google Scholar]
- Babor, TF; Steinberg, K; Anton, R; Del Boca, FK. Talk is cheap: Measuring drinking outcomes in clinical trials. J. Stud. Alcohol 2000, 61, 55–63. [Google Scholar]
- Del Boca, FK; Noll, JA. Truth or consequences: the validity of self-report data in health services research on addictions. Addiction 2000, 95, 347–360. [Google Scholar]
- Walker, S; Cosden, M. Reliability of college student self-reported drinking behavior. J. Subst. Abuse Treat 2007, 33, 405–409. [Google Scholar]
| Non-Binge (>1 to 5/4 drinks within 2 hrs) | Low-Binge (5/4 to 7/6 drinks within 2 hrs) | High-Binge (≥10 drinks within 2 hrs) | ||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| Sample Size (n) | 16 | 16 | 16 | 16 | 16 | 16 |
| Age (years) | 20.38 [1.26] | 21.81 [1.68] | 20.38 [1.15] | 20.50 [0.97] | 19.94 [1.12] | 20.81 [1.97] |
| Education (college) | 2.93 [1.07] | 3.44 [0.81] | 3.14 [1.03] | 3.29 [0.83] | 2.77 [0.93] | 3 [1.36] |
| Height (ft) | 5.38 [0.23] | 5.75 [0.32] | 5.45 [0.44] | 5.76 [0.37] | 5.33 [0.39] | 5.59 [0.40] |
| Weight (pounds) | 127.88 [30.75] | 163.44 [26.76] | 148.93 [26.11] | 159.47 [27.99] | 143 [24.15] | 166.75 [22.37] |
| Years Drinking | 1.91 [0.97] | 3.69 [2.55] | 2.88 [1.50] | 2.69 [1.59] | 3.13 [1.54] | 3.06 [1.24] |
| Drink Days/Month | 3 [2.47] | 4 [4.00] | 4.81 [2.46] | 8 [7.58] | 7.69 [3.55] | 10 [4.89] |
| Drinks/Occasion | 3 [0.98] | 3 [1.54] | 5 [3.12] | 5 [2.28] | 6.75 [2.67] | 6 [2.85] |
| Amount: Drinks/2 hrs, Past 6 Months | 3 [1.07] | 3 [1.35] | 5.57 [0.85] | 6 [1.26] | 10 [3.61] | 11 [3.76] |
| Binge Frequency: Past 6 months | 0.11 [0.33] | 0 [0] | 9.43 [14.71] | 7 [6.42] | 14 [14.37] | 23 [23.72] |
| Factor (df) | Binge Group (2,90) | Sex (1,90) | Electrode (2,180) | BG × S (2,90) | BG × E (4,180) | S × E (2,180) | BG × S × E (4,180) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | η2 | F | η2 | F | η2 | F | η2 | F | η2 | F | η2 | F | η2 | |
| Delta (0–4 Hz) | 5.1** | 0.10 | 13.0*** | 0.13 | 22.7*** | 0.20 | --- | --- | --- | --- | --- | --- | --- | --- |
| Theta (4–8 Hz) | --- | --- | 3.9* | 0.04 | 135.8*** | 0.60 | --- | --- | --- | --- | --- | --- | --- | --- |
| Alpha (8–12 Hz) | --- | --- | --- | --- | 136.3*** | 0.60 | --- | --- | --- | --- | --- | --- | --- | --- |
| Slow–Beta (12–20 Hz) | --- | --- | 6.8* | 0.07 | 7.5*** | 0.08 | --- | --- | --- | --- | --- | --- | --- | --- |
| Fast–Beta (20–35 Hz) | 3.9* | 0.08 | 11.2** | 0.11 | 20.5*** | 0.19 | --- | --- | --- | --- | 4.2* | 0.05 | --- | --- |
| Gamma (35–45 Hz) | --- | --- | --- | --- | 3.5* | 0.04 | --- | --- | --- | --- | 5.5** | 0.06 | --- | --- |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Courtney, K.E.; Polich, J. Binge Drinking Effects on EEG in Young Adult Humans. Int. J. Environ. Res. Public Health 2010, 7, 2325-2336. https://doi.org/10.3390/ijerph7052325
Courtney KE, Polich J. Binge Drinking Effects on EEG in Young Adult Humans. International Journal of Environmental Research and Public Health. 2010; 7(5):2325-2336. https://doi.org/10.3390/ijerph7052325
Chicago/Turabian StyleCourtney, Kelly E., and John Polich. 2010. "Binge Drinking Effects on EEG in Young Adult Humans" International Journal of Environmental Research and Public Health 7, no. 5: 2325-2336. https://doi.org/10.3390/ijerph7052325