In vitro and in vivo Effects of Free and Chalcones-Loaded Nanoemulsions: Insights and Challenges in Targeted Cancer Chemotherapies
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
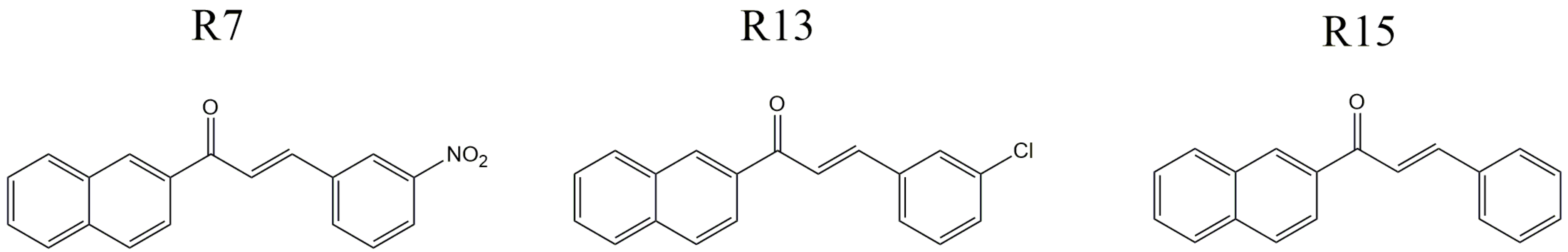
2.2. Nanoparticles Characterization
2.2.1. Nanoemulsion Preparation
2.2.2. Characterization Nanoemulsion Physicochemical Properties
2.2.3. High-Performance Liquid Chromatographic Analysis
2.2.4. Determination of Encapsulation Efficiency
2.2.5. Differential Scanning Calorimetry
2.3. In vitro Studies
2.3.1. Cell Culture
2.3.2. Viability Assay
2.4. In vivo Studies
2.4.1. Drug Treatment and Animals
2.5. Evaluation of Oxidative Stress in Liver
2.5.1. Preparation of Homogenates
2.5.2. ROS Determination
2.5.3. Enzyme Assays
2.5.4. Lipid Peroxidation Measurements
2.5.5. Non-Protein Thiol Determination
2.6. Biochemical Parameters
2.7. Hematological Evaluation
2.8. Histopathological Analysis
2.9. Statistical Analysis
3. Results
3.1. Nanoparticles Characterization
| Mean Diameter (nm) | PDI | ZP (mV) | EE (%) | |
|---|---|---|---|---|
| NE/R7 | 124 ± 6 | 0.24 ± 0.01 | −24 ± 5.1 | 86 |
| NE/R13 | 107 ± 2 | 0.19 ± 0.01 | −17 ± 2.8 | 93 |
| NE/R15 | 110 ± 3 | 0.17 ± 0.01 | −19 ± 4.2 | 93 |
| NE | 117 ± 4 | 0.19 ± 0.02 | −22 ± 1.4 | - |
3.1.1. Differential Scanning Calorimetry Investigation
3.2. In vitro Cell Toxicity
| AUC—24 h Incubation | |||
|---|---|---|---|
| Leukemic Cell | Non-Tumoral Cell | ||
| L1210 | VERO | SI | |
| Control (PBS) | 10,000 | 10,000 | - |
| NE | 8778 ± 52 | 9351 ± 297 | - |
| R7 | 3344 ± 57 | 4180 ± 7 | 1.2 |
| NE/R7 | 4829 ± 43 | 7684 ± 223 | 1.6 |
| R13 | 3542 ± 190 | 5064 ± 327 | 1.4 |
| NE/R13 | 8562 ± 517 | 8624 ± 487 | 1.0 |
| R15 | 3509 ± 181 | 3996 ± 328 | 1.1 |
| NE/R15 | 4942 ± 35 | 7300 ± 524 | 1.5 |
3.3. In vivo Toxicity
| Days | Control (g) | R7 (g) | R13 (g) | R15 (g) |
|---|---|---|---|---|
| 0 | 31.0 ± 1.1 | 30.8 ± 1.1 | 28.8 ± 0.8 | 31.0 ± 0.8 |
| 2 | 31.3 ± 0.9 | 31.8 ± 1.4 | 30.0 ± 0.6 | 32.7 ± 0.6 |
| 4 | 32.3 ± 0.9 * | 32.0 ± 1.1 | 29.5 ± 0.6 | 32.5 ± 0.5 |
| 6 | 32.7 ± 1.2 ** | 32.1 ± 1.1 | 29.5 ± 1.2 | 32.7 ± 0.3 |
| 8 | 32.3 ± 0.9 * | 32.3 ± 1.4 | 29.5 ± 0.9 | 32.7 ± 0.7 |
| 10 | 32.7 ± 1.2 ** | 31.8 ± 1.4 | 29.7 ± 0.8 | 32.8 ± 0.7 |
| 12 | 33.3 ± 0.9 *** | 31.8 ± 1.3 | 29.8 ± 0.5 | 32.7 ± 0.6 |
| 14 | 33.0 ± 1.1 ** | 31.7 ± 1.6 | 29.5 ± 0.7 | 32.3 ± 0.9 |
| Variation | ~2 g | ~0.9 | ~0.7 | ~1.3 |
| Days | Control (g) | NE (g) | NE/R7 (g) | NE/R13 (g) | NE/15 (g) |
|---|---|---|---|---|---|
| 0 | 29.0 ± 2.1 | 29.2 ± 3.2 | 28.2 ± 1.3 | 28.3 ± 1.2 | 28.2 ± 1.6 |
| 2 | 29.7 ± 2.3 | 29.0 ± 3.2 | 28.7 ± 1.4 | 28.8 ± 1.3 | 29.3 ± 1.4 |
| 4 | 30.7 ± 2.3 | 29.4 ± 2.5 | 29.7 ± 1.4 | 29.3 ± 1.2 | 29.3 ± 1.5 |
| 6 | 29.0 ± 1.5 | 30.2 ± 2.5 | 28.7 ± 1.0 | 29.5 ± 1.1 | 29.7 ± 1.5 |
| 8 | 30.7 ± 2.3 | 30.4 ± 2.2 | 29.2 ± 1.5 | 29.2 ± 0.9 | 30.2 ± 1.5 |
| 10 | 30.7 ± 2.3 | 30.6 ± 2.3 | 29.5 ± 1.3 | 31.2 ± 0.8 *** | 30.8 ± 1.8 * |
| 12 | 31.3 ± 1.7 * | 32.2 ± 2.2 ** | 29.8 ± 1.2 * | 31.5 ± 1.2 *** | 30.3 ± 1.0 |
| 14 | 32.7 ± 1.3 *** | 32.2 ± 2.1 * | 31.5 ± 0.8 *** | 33.8 ± 1.0 *** | 32.8 ± 1.8 *** |
| Variation | ~3.7 | ~2.2 | ~3.3 | ~5.5 | ~4.6 |
| Parameters | Control (g) | R7 (g) | R13 (g) | R15 (g) |
|---|---|---|---|---|
| Spleen | 0.18 ± 0.03 | 0.23 ± 0.03 | 0.21 ± 0.01 | 0.27 ± 0.03 |
| Liver | 2.21 ± 0.14 | 2.15 ± 0.09 | 2.02 ± 0.03 | 2.46 ± 0.16 |
| Heart | 0.23 ± 0.02 | 0.21 ± 0.01 | 0.20 ± 0.04 | 0.23 ± 0.01 |
| Lung | 0.25 ± 0.01 | 0.28 ± 0.01 | 0.26 ± 0.01 | 0.29 ± 0.01 |
| Brain | 0.42 ± 0.04 | 0.35 ± 0.02 | 0.36 ± 0.01 | 0.39 ± 0.01 |
| Kidneys | 0.65 ± 0.03 | 0.70 ± 0.06 | 0.62 ± 0.01 | 0.69 ± 0.03 |
| Stomach | 0.24 ± 0.03 | 0.30 ± 0.02 | 0.30 ± 0.01 | 0.31 ± 0.04 |
| Parameters | Control (g) | NE (g) | NE/R7 (g) | NE/R13 (g) | NE/15 (g) |
|---|---|---|---|---|---|
| Spleen | 0.17 ± 0.01 | 0.20 ± 0.02 | 0.25 ± 0.03 | 0.24 ± 0.01 | 0.20 ± 0.01 |
| Liver | 1.58 ± 0.13 | 2.15 ± 0.09 | 1.96 ± 0.06 | 2.05 ± 0.08 | 1.96 ± 0.14 |
| Heart | 0.18 ± 0.01 | 0.17 ± 0.02 | 0.19 ± 0.01 | 0.20 ± 0.06 | 0.19 ± 0.01 |
| Lung | 0.21 ± 0.01 | 0.20 ±0.01 | 0.25 ± 0.01 | 0.25 ± 0.01 | 0.25 ± 0.02 |
| Brain | 0.39 ± 0.04 | 0.32 ± 0.02 | 0.38 ± 0.03 | 0.37 ± 0.01 | 0.38 ± 0.02 |
| Kidneys | 0.39 ± 0.04 | 0.23 ±0.02 | 0.48 ± 0.03 | 0.50 ± 0.03 | 0.48 ± 0.04 |
| Stomach | 0.33 ± 0.04 | 0.24 ± 0.02 | 0.26 ± 0.02 | 0.33 ± 0.02 | 0.29 ± 0.02 |
3.4. Biochemical Parameter Investigation
| Parameters | Control | R7 | R13 | R15 |
|---|---|---|---|---|
| ALT (UI/L) | 159 ± 12 | 101 ± 4 | 109 ± 4 | 111 ± 5 |
| AST (UI/L) | 161 ± 19 | 161 ± 4 | 171 ± 4 | 170 ± 3 |
| Albumin (g/dL) | 2.2 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.3 ± 0.1 |
| Total Proteins (g/dL) | 5.8 ± 0.2 | 6.0 ± 0.2 | 5.2 ± 0.1 | 5.5 ± 0.1 |
| Urea (mg/dL) | 62 ± 4 | 78 ± 4 | 74 ± 2 | 69 ± 6 |
| Creatinine (mg/dL) | 0.5 ± 0.04 | 0.5 ± 0.01 | 0.5 ± 0.01 | 0.5 ± 0.02 |
| Triglycerides (mg/dL) | 75 ± 4 | 129 ± 11 ** | 125 ± 9 * | 115 ± 11 * |
| Total Cholesterol (mg/dL) | 118 ± 9 | 77 ± 3 ** | 85 ± 3 ** | 78 ± 4 ** |
| Parameters | Control | NE | NE/R7 | NE/R13 | NE/15 |
|---|---|---|---|---|---|
| ALT (UI/L) | 159 ± 12 | 157 ± 10 | 104 ± 4 | 111 ± 4 | 100 ± 2 |
| AST (UI/L) | 161 ± 19 | 145 ± 6 | 168 ± 2.7 | 165 ± 8 | 178 ± 6 |
| Albumin (g/dL) | 2.2 ± 0.1 | 2.0 ± 0.1 | 3.4 ± 0.1 | 2.7 ± 0.2 | 2.3 ± 0.1 |
| Total Proteins (g/dL) | 5.8 ± 0.2 | 5.9 ± 0.2 | 5.9 ± 0.3 | 5.6 ± 0.3 | 5.6 ± 0.1 |
| Urea (mg/dL) | 62 ± 4 | 61 ± 5 | 74 ± 7.5 | 64 ± 3.6 | 50 ± 2.2 |
| Creatinine (mg/dL) | 0.5 ± 0.04 | 0.4 ± 0.01 | 0.4 ± 0.02 | 0.5 ± 0.03 | 0.5 ± 0.05 |
| Triglycerides (mg/dL) | 75 ± 4 | 96 ± 14 | 145 ± 9 ** | 99 ± 9 | 87 ± 10 |
| Total Cholesterol (mg/dL) | 118 ± 9 | 120 ± 6 | 94 ± 6 | 92 ± 2 * | 87 ± 7 * |
3.5. Hematological Evaluation
| Parameters | Control | R7 | R13 | R15 |
|---|---|---|---|---|
| Total RBC count (×105/mm3) | 53 ± 4 | 57 ± 2 | 57 ± 2 | 59 ± 2 |
| Leucocytes (×102/mm3) | 59 ± 13 | 46 ± 5 | 53 ± 9 | 59 ± 10 |
| MCV (fL) | 87 ± 1 | 87 ± 4 | 85 ± 2 | 86 ± 2 |
| HCM (pg) | 28 ± 1 | 26 ± 3 | 24 ± 2 | 27 ± 2 |
| MCHC (%) | 33 ± 1 | 30 ± 4 | 28 ± 2 | 31 ± 3 |
| Hemoglobin (g/dL) | 15 ± 1 | 15 ± 2 | 14 ± 3 | 16 ± 4 |
| Hematocrit (%) | 46 ± 4 | 50 ± 3 | 49 ± 2 | 51 ± 4 |
| Neutrophils (×102/mm3) | 11 ± 3 | 14 ± 2 | 17 ± 4 | 22 ± 3 |
| Band cells (×101/mm3) | 11 ± 3 | 18 ± 9 | 16 ± 11 | 24 ± 12 |
| Mononuclear (×102/mm3) | 47 ± 11 | 30 ± 2 | 32 ± 4 | 36 ± 5 |
| Eosinophils (×101/mm3) | - | - | - | - |
| Basophils (×101/mm3) | - | 14 ± 5 | 27 ± 10 * | - |
| Parameters | Control | NE | NE/R7 | NE/R13 | NE/R15 |
|---|---|---|---|---|---|
| Total RBC count (×105/mm3) | 53 ± 4 | 68 ± 4 | 45 ± 2 | 54 ± 7 | 57 ± 4 |
| Leucocytes (×102/mm3) | 59 ± 13 | 91 ± 3 ** | 85 ± 2 * | 66 ± 2 | 64 ± 3 |
| MCV (fL) | 87 ± 1 | 81 ± 8 | 92 ± 7 | 88 ± 4 | 85 ± 3 |
| HCM (pg) | 28 ± 1 | 26 ± 4 | 33 ± 4 | 27 ± 3 | 26 ± 5 |
| MCHC (%) | 33 ± 1 | 32 ± 3 | 33 ± 2 | 31 ± 4 | 31 ± 2 |
| Hemoglobin (g/dL) | 15 ± 1 | 18 ± 3 | 15 ± 3 | 15 ± 2 | 15 ± 1 |
| Hematocrit (%) | 46 ± 4 | 55 ± 5 | 45 ± 2 | 48 ± 4 | 48 ± 2 |
| Neutrophils (×102/mm3) | 11 ± 3 | 21 ± 2 ** | 10 ± 2 | 10 ± 1 | 10 ± 1 |
| Band cells (×101/mm3) | 11 ± 3 | 9 ± 9 | 25 ± 8 | 20 ± 13 | 6 ± 6 |
| Mononuclear (×102/mm3) | 47 ± 11 | 69 ± 4 * | 72 ± 3 * | 50 ± 3 | 54 ± 2 |
| Eosinophils (×101/mm3) | - | 9 ± 9 | - | - | - |
| Basophils (×101/mm3) | - | 9 ± 9 | 17 ± 17 | 26 ± 13 | - |
3.6. Oxidative Stress Evaluation
| Parameters | R7 (%) | R13 (%) | R15 (%) |
|---|---|---|---|
| TBARS | 213 ± 22 *** | 148 ± 18 | 139 ± 4 |
| ROS | 158 ± 11 * | 181 ± 6 ** | 228 ± 20 ** |
| GSH | 57 ± 8 ** | 29 ± 3 *** | 45 ± 5 *** |
| Catalase | 160 ± 23 | 135 ± 11 | 130 ± 16 |
| GR | 92 ± 10 | 103 ± 7 | 79 ± 8 |
| GPx | 96 ± 3 | 101 ± 1 | 80 ± 9 |
| GST | 99 ± 12 | 108 ± 9 | 81 ± 10 |
| Parameters | NE (%) | NE/R7 (%) | NE/R13 (%) | NE/15 (%) |
|---|---|---|---|---|
| TBARS | 144 ± 10 ** | 109 ± 3 | 125 ± 5 | 109 ± 7 |
| ROS | 95 ± 14 | 129 ± 8 | 147 ± 17 | 107 ± 10 |
| GSH | 118 ± 6 | 100 ± 3 | 46 ± 7 ** | 46 ± 7 ** |
| Catalase | 84 ± 4 | 61 ± 9 * | 31 ± 8 ** | 77 ± 5 |
| GR | 106 ± 4 | 111 ± 6 | 102 ± 14 | 109 ± 21 |
| GPx | 89 ± 3 | 100 ± 3 | 95 ± 4 | 103 ± 4 |
| GST | 113 ± 14 | 99 ± 13 | 101 ± 15 | 113 ± 9 |
3.7. Histopathological Analysis
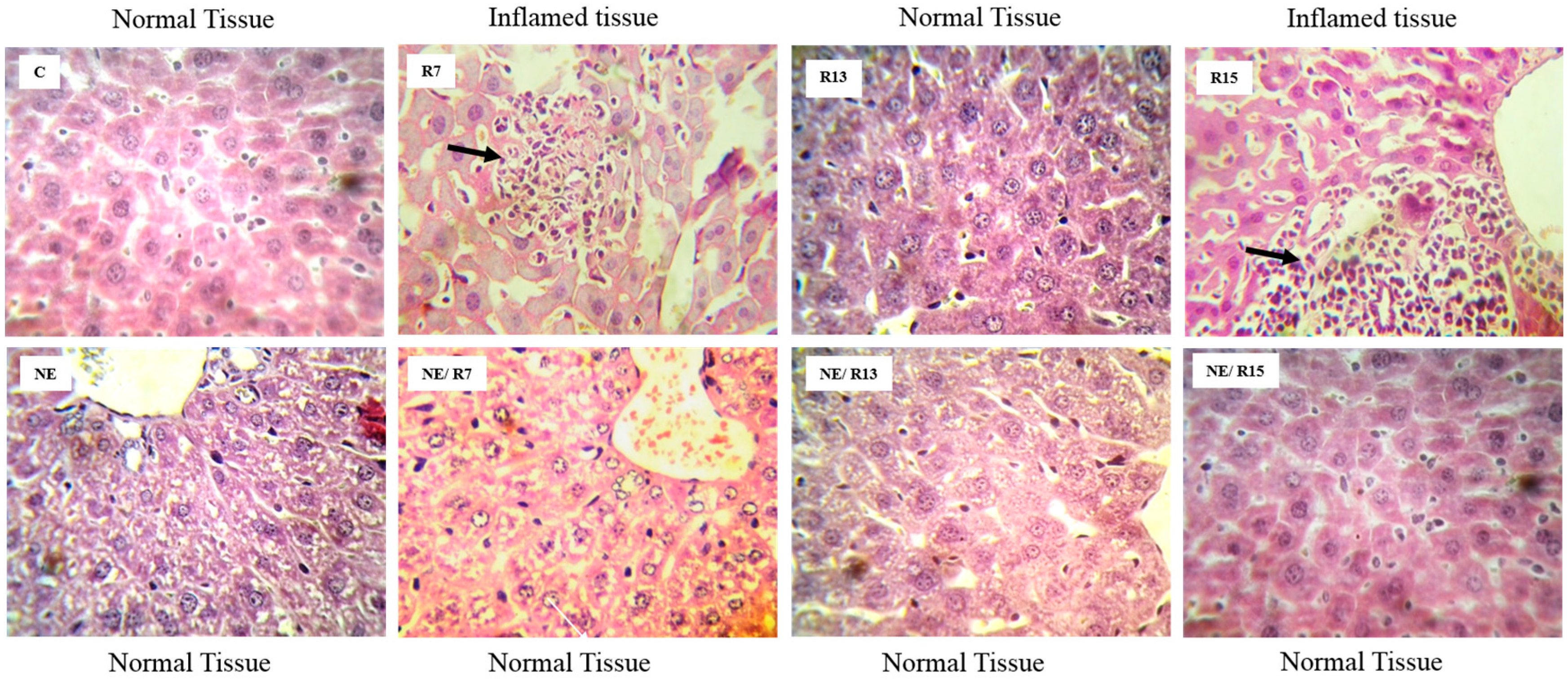
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children's oncology group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef]
- Diller, L. Adult Primary Care after Childhood Acute Lymphoblastic Leukemia. N. Eng. J. Med. 2011, 365, 1417–1424. [Google Scholar] [CrossRef]
- Reulen, R.C.; Winter, D.L.; Frobisher, C.; Lancashire, E.R.; Stiller, C.A.; Jenney, M.E.; Skinner, R.; Stevens, M.C.; Hawkins, M.M.; British Childhood Canc, S. Long-term Cause-Specific Mortality Among Survivors of Childhood Cancer. J. Am. Med. Ass. 2010, 304, 172–179. [Google Scholar] [CrossRef]
- Mertens, A.C.; Liu, Q.; Neglia, J.P.; Wasilewski, K.; Leisenring, W.; Armstrong, G.T.; Robison, L.L.; Yasui, Y. Cause-specific late mortality among 5-year survivors of childhood cancer: The childhood cancer survivor study. J. Nat. Cancer Inst. 2008, 100, 1368–1379. [Google Scholar] [CrossRef]
- Kaste, S.C.; Rai, S.N.; Fleming, K.; McCammon, E.A.; Tylavsky, F.A.; Danish, R.K.; Rose, S.R.; Sitter, C.D.; Pui, C.H.; Hudson, M.M. Changes in bone mineral density in survivors of childhood acute lymphoblastic leukemia. Ped. Blood Cancer 2006, 46, 77–87. [Google Scholar] [CrossRef]
- Thomas, I.H.; Donohue, J.E.; Ness, K.K.; Dengel, D.R.; Baker, K.S.; Gurney, J.G. Bone Mineral Density in Young Adult Survivors of Acute Lymphoblastic Leukemia. Cancer 2008, 113, 3248–3256. [Google Scholar] [CrossRef]
- Pui, C.H. Toward optimal central nervous system-directed treatment in childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2003, 21, 179–181. [Google Scholar] [CrossRef]
- Bassan, R.; Hoelzer, D. Modern therapy of acute lymphoblastic leukemia. J. Clin. Oncol. 2011, 29, 532–543. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomedicine 2007, 3, 20–31. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M.M. Nanoemulsions in Translational Research-Opportunities and Challenges in Targeted Cancer Therapy. AAPS PharmSciTech 2014, 15, 694–708. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Z.; Bu, H.; Huang, Y.; Gao, Z.; Shen, J.; Zhao, C.; Li, Y. Nanoemulsion improves the oral absorption of candesartan cilexetil in rats: Performance and mechanism. J. Control. Release 2011, 149, 168–174. [Google Scholar] [CrossRef]
- Winter, E.; Chiaradia, L.D.; de Cordova, C.A.; Nunes, R.J.; Yunes, R.A.; Creczynski-Pasa, T.B. Naphthylchalcones induce apoptosis and caspase activation in a leukemia cell line: The relationship between mitochondrial damage, oxidative stress, and cell death. Bioorg. Med. Chem. 2010, 18, 8026–8034. [Google Scholar] [CrossRef]
- Winter, E.; Chiaradia, L.D.; Silva, A.H.; Nunes, R.J.; Yunes, R.A.; Creczynski-Pasa, T.B. Involvement of extrinsic and intrinsic apoptotic pathways together with endoplasmic reticulum stress in cell death induced by naphthylchalcones in a leukemic cell line: Advantages of multi-target action. Toxicol. In Vitro 2014, 28, 769–777. [Google Scholar] [CrossRef]
- Chiaradia, L.D.; Santos, R.; Vitor, C.E.; Vieira, A.A.; Leal, P.C.; Nunes, R.J.; Calixto, J.B.; Yunes, R.A. Synthesis and pharmacological activity of chalcones derived from 2,4,6-trimethoxyacetophenone in RAW 264.7 cells stimulated by LPS: Quantitative structure—activity relationships. Bioorg. Med. Chem. 2008, 16, 658–667. [Google Scholar]
- Fontana, G.; Maniscalco, L.; Schillaci, D.; Cavallaro, G.; Giammona, G. Solid lipid nanoparticles containing tamoxifen characterization and in vitro antitumoral activity. Drug Deliv. 2005, 12, 385–392. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Stochastic Problems in Physics and Astronomy. Rev. Modern Physics 1943, 15, 1–89. [Google Scholar] [CrossRef]
- ICH Expert Working Group. Validation of Analytical Procedures: Text and Methodology Q2(R1). Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 15 July 2014).
- Michalowski, C.B.; Guterres, S.S.; Dalla Costa, T. Microdialysis for evaluating the entrapment and release of a lipophilic drug from nanoparticles. J. Pharm. Biomed. Anal. 2004, 35, 1093–1100. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Morton, D.B.; Jennings, M.; Buckwell, A.; Ewbank, R.; Godfrey, C.; Holgate, B.; Inglis, I.; James, R.; Page, C.; Sharman, I.; et al. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab. Anim. 2001, 35, 1–41. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 265–275. [Google Scholar]
- Cathcart, R.; Schwiers, E.; Ames, B.N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal. Biochem. 1983, 134, 111–116. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar]
- Keen, J.H.; Habig, W.H.; Jakoby, W.B. Mechanism for the several activities of the glutathione S-transferases. J. Biol. Chem. 1976, 251, 6183–6188. [Google Scholar]
- Bird, R.P.; Draper, H.H. Comparative studies on different methods of malonaldehyde determination. Methods Enzymol. 1984, 105, 299–305. [Google Scholar]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Garg, D.K.; Goyal, R.N. Haematological and hepatotoxic effects of silken styles of corn in albino rats. J. Appl. Toxicol. 1992, 12, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Murugavel, P. Role of diallyl tetrasulfide in ameliorating the cadmium induced biochemical changes in rats. Environ. Toxicol. Pharmacol. 2005, 20, 493–500. [Google Scholar] [CrossRef]
- Ahsan, F.; Rivas, I.P.; Khan, M.A.; Torres Suárez, A.I. Targeting to macrophages: Role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J. Control. Rel. 2002, 79, 29–40. [Google Scholar] [CrossRef]
- Galindo-Rodriguez, S.; Allemann, E.; Fessi, H.; Doelker, E. Physicochemical parameters associated with nanoparticle formation in the salting-out, emulsification-diffusion, and nanoprecipitation methods. Pharm. Res. 2004, 21, 1428–1439. [Google Scholar] [CrossRef]
- Santos, L.; Curi Pedrosa, R.; Correa, R.; Cechinel Filho, V.; Nunes, R.J.; Yunes, R.A. Biological evaluation of chalcones and analogues as hypolipidemic agents. Arch. Pharm. (Weinheim) 2006, 339, 541–546. [Google Scholar] [CrossRef]
- Sarker, D.K. Engineering of nanoemulsions for drug delivery. Curr. Drug Deliv. 2005, 2, 297–310. [Google Scholar] [CrossRef]
- Xiang, G.; Wu, J.; Lu, Y.; Liu, Z.; Lee, R.J. Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. Int. J. Pharm. 2008, 356, 29–36. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, J.; Wu, J.; Gonit, M.; Yang, X.; Lee, A.; Xiang, G.; Li, H.; Liu, S.; Marcucci, G.; et al. Role of formulation composition in folate receptor-targeted liposomal doxorubicin delivery to acute myelogenous leukemia cells. Mol. Pharm. 2007, 4, 707–712. [Google Scholar] [CrossRef]
- Salazar, M.D.; Ratnam, M. The folate receptor: What does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007, 26, 141–152. [Google Scholar] [CrossRef]
- Basha, R.; Sabnis, N.; Heym, K.; Bowman, W.P.; Lacko, A.G. Targeted nanoparticles for pediatric leukemia therapy. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef]
- Satake, N.; Lee, J.; Xiao, K.; Luo, J.; Sarangi, S.; Chang, A.; McLaughlin, B.; Zhou, P.; Kenney, E.; Kraynov, L.; et al. Nanoparticle targeted therapy against childhood acute lymphoblastic leukemia. In SPIE 8031, Micro- and Nanotechnology Sensors, Systems, and Applications III; 2011; 2011, pp. 80311U1–80311U5. [Google Scholar]
- Chen, W.C.; Sigal, D.S.; Saven, A.; Paulson, J.C. Targeting B lymphoma with nanoparticles bearing glycan ligands of CD22. Leuk. Lymphoma 2012, 53, 208–210. [Google Scholar] [CrossRef]
- Chen, W.C.; Completo, G.C.; Sigal, D.S.; Crocker, P.R.; Saven, A.; Paulson, J.C. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood 2010, 115, 4778–4786. [Google Scholar] [CrossRef]
- Krishnan, V.; Xu, X.; Barwe, S.P.; Yang, X.; Czymmek, K.; Waldman, S.A.; Mason, R.W.; Jia, X.; Rajasekaran, A.K. Dexamethasone-loaded block copolymer nanoparticles induce leukemia cell death and enhance therapeutic efficacy: A novel application in pediatric nanomedicine. Mol. Pharm. 2013, 10, 2199–2210. [Google Scholar] [CrossRef]
- Memisoglu-Bilensoy, E.; Vural, I.; Bochot, A.; Renoir, J.M.; Duchene, D.; Hıncal, A.A. Tamoxifen citrate loaded amphiphilic β-cyclodextrin nanoparticles: In vitro characterization and cytotoxicity. J. Control. Rel. 2005, 104, 489–496. [Google Scholar] [CrossRef]
- Yadav, K.S.; Jacob, S.; Sachdeva, G.; Chuttani, K.; Mishra, A.K.; Sawant, K.K. Long circulating PEGylated PLGA nanoparticles of cytarabine for targeting leukemia. J. Microencapsul. 2011, 28, 729–742. [Google Scholar] [CrossRef]
- Jin, Y.J.; Termsarasab, U.; Ko, S.H.; Shim, J.S.; Chong, S.; Chung, S.J.; Shim, C.K.; Cho, H.J.; Kim, D.D. Hyaluronic acid derivative-based self-assembled nanoparticles for the treatment of melanoma. Pharm. Res. 2012, 29, 3443–3454. [Google Scholar] [CrossRef]
- Jain, V.; Swarnakar, N.K.; Mishra, P.R.; Verma, A.; Kaul, A.; Mishra, A.K.; Jain, N.K. Paclitaxel loaded PEGylated gleceryl monooleate based nanoparticulate carriers in chemotherapy. Biomaterials 2012, 33, 7206–7220. [Google Scholar] [PubMed]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood–brain barrier by nanoparticles. J. Control. Rel. 2012, 161, 264–273. [Google Scholar] [CrossRef]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Rel. 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Pui, C.H. Central nervous system disease in acute lymphoblastic leukemia: Prophylaxis and treatment. Hemat. Am. Soc. Hematol. Educ. Program. 2006, 1, 142–146. [Google Scholar] [CrossRef]
- Ng, K.K.; Lovell, J.F.; Zheng, G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc. Chem. Res. 2011, 44, 1105–1113. [Google Scholar] [CrossRef]
- Hammel, M.; Laggner, P.; Prassl, R. Structural characterisation of nucleoside loaded low density lipoprotein as a main criterion for the applicability as drug delivery system. Chem. Phys. Lipids 2003, 123, 193–207. [Google Scholar] [CrossRef]
- Ho, Y.K.; Smith, R.G.; Brown, M.S.; Goldstein, J.L. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood 1978, 52, 1099–1114. [Google Scholar]
- Teixeira, R.S.; Valduga, C.J.; Benvenutti, L.A.; Schreier, S.; Maranhao, R.C. Delivery of daunorubicin to cancer cells with decreased toxicity by association with a lipidic nanoemulsion that binds to LDL receptors. J. Pharm. Pharmacol. 2008, 60, 1287–1295. [Google Scholar] [CrossRef]
- Graziani, S.R.; Igreja, F.A.; Hegg, R.; Meneghetti, C.; Brandizzi, L.I.; Barboza, R.; Amancio, R.F.; Pinotti, J.A.; Maranhao, R.C. Uptake of a cholesterol-rich emulsion by breast cancer. Gynecol. Oncol. 2002, 85, 493–497. [Google Scholar] [CrossRef]
- Dias, M.L.; Carvalho, J.P.; Rodrigues, D.G.; Graziani, S.R.; Maranhao, R.C. Pharmacokinetics and tumor uptake of a derivatized form of paclitaxel associated to a cholesterol-rich nanoemulsion (LDE) in patients with gynecologic cancers. Cancer Chemother. Pharmacol. 2007, 59, 105–111. [Google Scholar]
- Almeida, C.P.; Vital, C.G.; Contente, T.C.; Maria, D.A.; Maranhao, R.C. Modification of composition of a nanoemulsion with different cholesteryl ester molecular species: Effects on stability, peroxidation, and cell uptake. Int. J. Nanomedicine 2010, 5, 679–686. [Google Scholar]
- Versluis, A.J.; van Geel, P.J.; Oppelaar, H.; van Berkel, T.J.; Bijsterbosch, M.K. Receptor-mediated uptake of low-density lipoprotein by B16 melanoma cells in vitro and in vivo in mice. Br. J. Cancer 1996, 74, 525–532. [Google Scholar] [CrossRef]
- Landesman-Milo, D.; Peer, D. Altering the immune response with lipid-based nanoparticles. J. Control. Rel. 2012, 161, 600–608. [Google Scholar] [CrossRef]
- Lopez, A.M.; Hendrickson, R.G. Toxin-Induced hepatic injury. Emerg. Med. Clin. North. Am. 2014, 32, 103–125. [Google Scholar] [CrossRef]
- Field, K.M.; Dow, C.; Michael, M. Part I: Liver function in oncology: Biochemistry and beyond. Lancet Oncol. 2008, 9, 1092–1101. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, E.; Pizzol, C.D.; Locatelli, C.; Silva, A.H.; Conte, A.; Chiaradia-Delatorre, L.D.; Nunes, R.J.; Yunes, R.A.; Creckzynski-Pasa, T.B. In vitro and in vivo Effects of Free and Chalcones-Loaded Nanoemulsions: Insights and Challenges in Targeted Cancer Chemotherapies. Int. J. Environ. Res. Public Health 2014, 11, 10016-10035. https://doi.org/10.3390/ijerph111010016
Winter E, Pizzol CD, Locatelli C, Silva AH, Conte A, Chiaradia-Delatorre LD, Nunes RJ, Yunes RA, Creckzynski-Pasa TB. In vitro and in vivo Effects of Free and Chalcones-Loaded Nanoemulsions: Insights and Challenges in Targeted Cancer Chemotherapies. International Journal of Environmental Research and Public Health. 2014; 11(10):10016-10035. https://doi.org/10.3390/ijerph111010016
Chicago/Turabian StyleWinter, Evelyn, Carine Dal Pizzol, Claudriana Locatelli, Adny H. Silva, Aline Conte, Louise D. Chiaradia-Delatorre, Ricardo J. Nunes, Rosendo A. Yunes, and Tânia B. Creckzynski-Pasa. 2014. "In vitro and in vivo Effects of Free and Chalcones-Loaded Nanoemulsions: Insights and Challenges in Targeted Cancer Chemotherapies" International Journal of Environmental Research and Public Health 11, no. 10: 10016-10035. https://doi.org/10.3390/ijerph111010016





