New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities
Abstract
:1. Introduction
2. Results
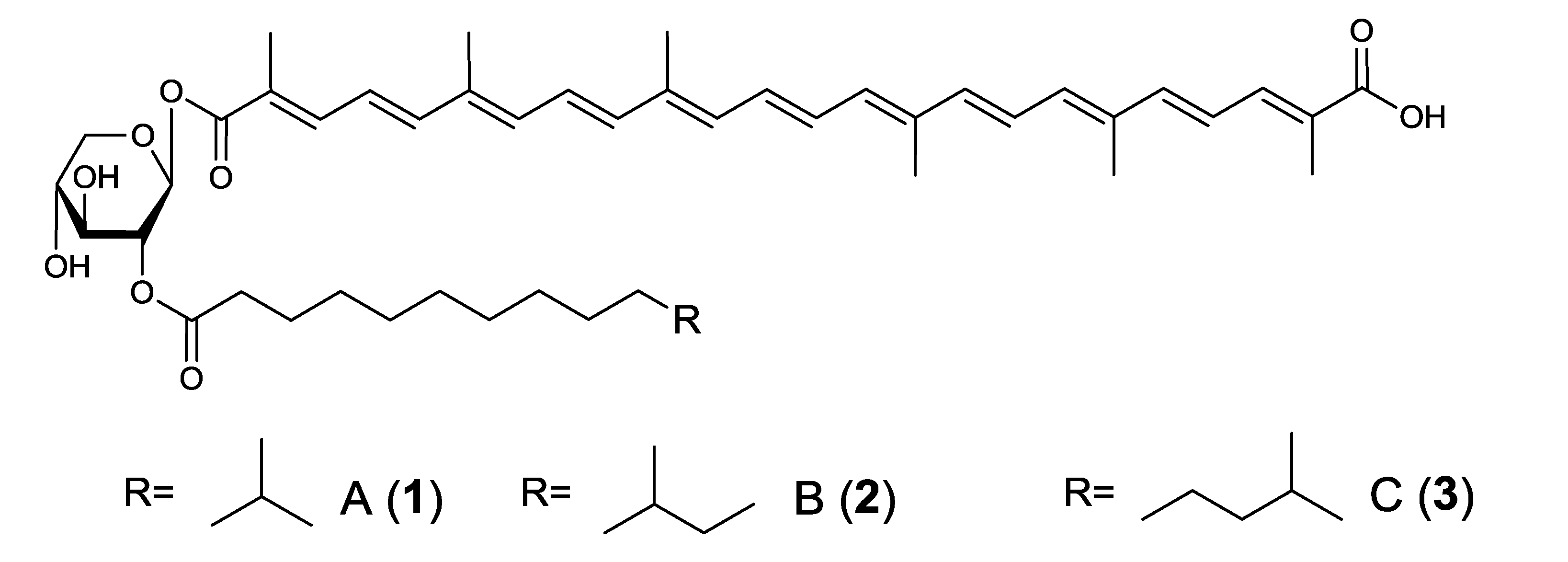
2.1. Diapolycopenedioc Acid Xylosylesters A–C from Rubritalea Squalenifaciens [27,28]
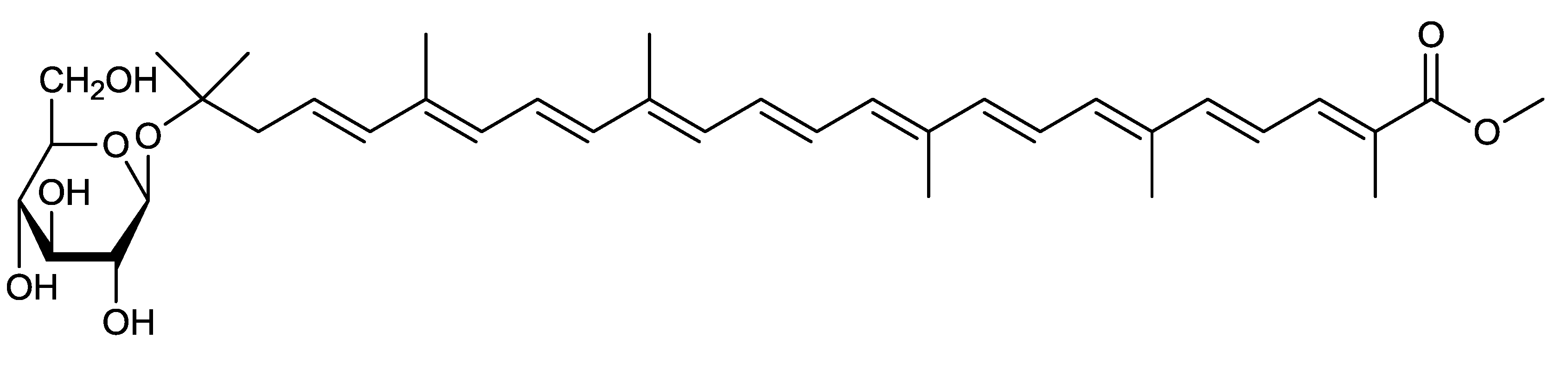
2.2. Methyl 5-Glucosyl-5,6-Dihydro-Apo-4,4′-Lycopenoate from Planococcus Maritimus [29]
2.3. (3R)-Saproxanthin and (3R,2′S)-Myxol [30]

3. Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Handbook; Birkhäuser Verlag: Basel, Switzerland, 2004. [Google Scholar]
- Nishino, H.; Tokuda, H.; Murakoshi, M.; Satomi, Y.; Masuda, M.; Onozuka, M.; Yamaguchi, S.; Takayasu, J.; Tsuruta, J.; Okuda, M.; et al. Cancer prevention by natural carotenoids. Biofactors 2000, 13, 89–94. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of low-density lipoprotein oxidation by astaxanthin. J. Atheoscler. Thomb. 2000, 7, 216–222. [Google Scholar]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biological mechanism of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Naito, Y.; Uchiyama, K.; Aoi, W.; Hasegawa, G.; Nakamura, N.; Yoshida, N.; Maoka, T.; Takahashi, J.; Yoshikawa, T. Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors 2004, 20, 49–59. [Google Scholar] [CrossRef]
- Hosokawa, M.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of PPARgamma ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta 2004, 1675, 113–119. [Google Scholar] [CrossRef]
- Talegawkar, S.A.; Johnson, E.J.; Carithers, T.C.; Taylor, H.A., Jr.; Bogle, M.L.; Tucker, K.L. Carotenoid intakes, assessed by food-frequency quesionnaires (FFQs), are associated with serum carotenoid concentrations in the Jackson Heart Study: Validation of the Jackson Heart Study Delta NIRI Adult FFQs. Public Health Nutr. 2008, 11, 989–997. [Google Scholar]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, M. High serum carotenoids associated with lower risk for bone loss and osteoporosis in post-menopausal Japanese female subjects: Prospective cohort study. PLoS One 2012, 7, e52643. [Google Scholar]
- Katsuta, A.; Adachi, K.; Matsuda, S.; Shizuri, Y.; Kasai, H. Ferrimonas marina sp. nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 1851–1855. [Google Scholar] [CrossRef]
- Tao, L.; Yao, H.; Kasai, H.; Misawa, N.; Cheng, Q. A carotenoid synthesis gene cluster from Algoriphagus sp. KK10202C with a novel fusion-type lycopene-β-cyclase gene. Mol. Genet. Genomics 2006, 276, 79–86. [Google Scholar] [CrossRef]
- Peng, X.; Adachi, K.; Chen, C.; Kasai, H.; Kanoh, K.; Shizuri, Y.; Misawa, N. Discovery of a marine bacterium producing 4-hydroxybenzoate and its alkyl esters, parabens. Appl. Environ. Microbiol. 2006, 72, 5556–5561. [Google Scholar] [CrossRef]
- Matsuo, Y.; Katsuta, A.; Matsuda, S.; Shizuri, Y.; Yokota, A.; Kasai, H. Mechercharimyces mesophilus gen. nov., sp. nov. and Mecherocharimyces asporophorigenens sp. nov., antitumor substance-producing marine bacteria, and description of Thermoactinomy cetaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 2837–2842. [Google Scholar] [CrossRef]
- Goodwin, T.M. The Biochemistry of Barotenoids, 2nd ed.; Plant Chapman and Hall: London, UK, 1980; Volume 1, pp. 291–319. [Google Scholar]
- Yokoyama, A.; Adachi, K.; Shizuri, Y. New carotenoid glucosides, astaxanthin glucoside and adonixanthin glucoside, isolated from the astaxanthin-producing marine bacterium, Agrobacterium aurantiacum. J. Nat. Prod. 1995, 58, 1929–1933. [Google Scholar] [CrossRef]
- Yokoyama, A.; Miki, W.; Izumida, H.; Shizuri, Y. New trihydroxy-keto-carotenoids isolated from an astaxanthin-producing marine bacterium. Biosci. Biotechnol. Biochem. 1996, 60, 200–203. [Google Scholar] [CrossRef]
- Yokoyama, A.; Izumida, H.; Shizuri, Y. New carotenoid sulfates isolated from a marine bacterium. Biosci. Biotechnol. Biochem. 1996, 60, 1877–1878. [Google Scholar] [CrossRef]
- Misawa, N. Carotenoids. In Comprehensive Natural Products II Chemistry and Biology; Mander, L., Liu, H.-W., Eds.; Elsevier: Oxford, UK, 2010; Volume 1, pp. 733–753. [Google Scholar]
- Misawa, N.; Satomi, Y.; Kondo, K.; Yokoyama, A.; Kajiwara, S.; Saito, T.; Ohtani, T.; Miki, W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 1995, 177, 6575–6584. [Google Scholar]
- Nishida, Y.; Adachi, K.; Kasai, H.; Shizuri, Y.; Shindo, K.; Sawabe, A.; Komemushi, S.; Miki, W.; Misawa, N. Elucidation of a carotenoid biosynthesis gene cluster encoding a novel enzyme, 2,2′-β-hydroxylase, from Brevundimonas sp. strain SD212 and combinatorial biosynthesis of new or rare xanthophylls. Appl. Environ. Microbiol. 2005, 71, 4286–4296. [Google Scholar] [CrossRef]
- Traysman, R.J.; Kirsch, J.R.; Koehler, R.C. Oxygen radical mechanisms of brain injury follwing ischemia and reperfusion. J. Appl. Physiol. 1991, 71, 1185–1195. [Google Scholar]
- Palinski, W.; Rosenfeld, M.E.; Yla, H.S.; Gurtner, G.C.; Socher, S.S.; Butler, S.W.; Parthasarathy, S.; Carew, T.E.; Steinberg, D.; Witztum, J.L. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA 1989, 86, 1372–1376. [Google Scholar] [CrossRef]
- Erdogan, C.; Unlucerci, Y.; Turkmen, A.; Kuru, A.; Cetin, O.; Bekpinar, S. The evaluation of oxidative stress in patients with chronic renal failure. Clin. Chim. Acta 2002, 322, 157–161. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Forni, L.G. An investigation of the novel anti-inflammatory agents ONO-3144 and MK-447. Studies on their potential antioxidant activity. Biochem. Pharmacol. 1988, 37, 4225–4233. [Google Scholar] [CrossRef]
- Bodamyali, T.; Kanczler, J.M.; Millar, T.M.; Stevens, C.R.; Blake, D.R. Free radicals in rheumatoid arthritis: Mediators and modulators. Oxid. Stress Dis. 2004, 10, 591–610. [Google Scholar]
- Mylonas, C.; Kouretas, D. Lipid peroxidation and tissue damage. In Vivo 1999, 13, 295–309. [Google Scholar]
- Van den Berg, H.; Faulks, R.; Granado, H.F.; Hirschberg, J.; Olmedilla, B.; Sandmann, G.; Southon, S.; Stahl, W. The potential for the improvement of carotenoid levels in foods and the likely systemic effects. J. Sci. Food Agric. 2000, 80, 880–912. [Google Scholar] [CrossRef]
- Shindo, K.; Mikami, K.; Tamesada, E.; Takaichi, S.; Adachi, K.; Misawa, N.; Maoka, T. Diapolycopendioic acid xylosyl ester, a novel glycol-C30-carotenoic acid produced by a new marine bacterium Rubritalea squalenifaciens. Tetrahedron Lett. 2007, 48, 2725–2727. [Google Scholar] [CrossRef]
- Shindo, K.; Asagi, E.; Sano, A.; Hotta, E.; Minemura, N.; Mikami, K.; Tamesada, E.; Misawa, N.; Maoka, T. Diapolycopenedioic acid xlosyl esters A, B, and C, novel antioxidative glycol-C30-carotenoic acids produced by a new marine bacterium Rubritalea squalenifaciens. J. Antibiot. 2008, 61, 185–191. [Google Scholar]
- Shindo, K.; Endo, M.; Miyake, Y.; Wakasugi, K.; Morritt, D.; Bramley, M.P.; Fraser, D.P.; Kasai, H.; Misawa, N. Methyl glucosyl-3,4-dehydro-apo-8′-lycopenoate, a novel antioxidative glycol-C30-carotenoic acid produced by a marine bacterium Planococcus maritimus. J. Antibiot. 2008, 61, 729–735. [Google Scholar] [CrossRef]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef]
- Kasai, H.; Katsuta, A.; Sekiguchi, H.; Matsuda, S.; Adachi, K.; Shindo, K.; Yoon, J.; Yokota, A.; Shizuri, Y. Rubritalea squalnifaciens sp. nov., a squalene-producing marine bacterium belonging to subdivision 1 of the phylum “Verrucomicrobia”. Int. J. Syst. Evol. Microbiol. 2007, 57, 1630–1634. [Google Scholar] [CrossRef]
- Kleing, H.; Schmitt, R.; Meister, W.; Englert, G.; Thommen, H. New carotenoic acid glucosyl esters from Pseudomonas rhodos. Z. Naturforsch C 1979, 34, 181–185. [Google Scholar]
- Osawa, A.; Iki, K.; Sandmann, G.; Shindo, K. Isolation and identification of 4,4′-diapolycopene-4,4′-dioic acids produced by Bacillus firmus GB1 and its singlet oxygen quenching activity. J. Oleo Sci. 2013, 62, 955–960. [Google Scholar] [CrossRef]
- Tao, L.; Schenzle, A.; Odom, J.M.; Cheng, Q. Novel carotenoid oxidase involved in biosynthesis of 4,4′-diapolycopene dialdehyde. Appl. Environ. Microbiol. 2005, 71, 3294–3301. [Google Scholar] [CrossRef]
- Aasen, A.J.; Liaaen-Jensen, S. The carotenoids of flexibacteria: II. A new xanthophyll from Saprospira grandis. Acta Chem. Scand. 1966, 20, 811–819. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M.; Maoka, T. Presence of free myxol and 4-hydroxymyxol and absence of myxol glycosides in Anabaena variabilis ATCC 29413, and proposal of biosynthetic pathway of carotenoids. Plant Cell Physiol. 2006, 47, 211–216. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shindo, K.; Misawa, N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs 2014, 12, 1690-1698. https://doi.org/10.3390/md12031690
Shindo K, Misawa N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Marine Drugs. 2014; 12(3):1690-1698. https://doi.org/10.3390/md12031690
Chicago/Turabian StyleShindo, Kazutoshi, and Norihiko Misawa. 2014. "New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities" Marine Drugs 12, no. 3: 1690-1698. https://doi.org/10.3390/md12031690



