Solid State Gas Sensor Research in Germany – a Status Report
Abstract
:Introduction
1. Potentiometric Sensors
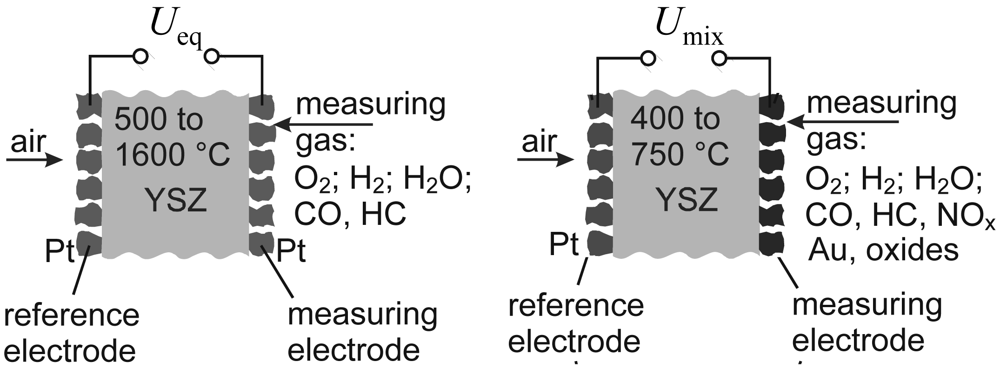
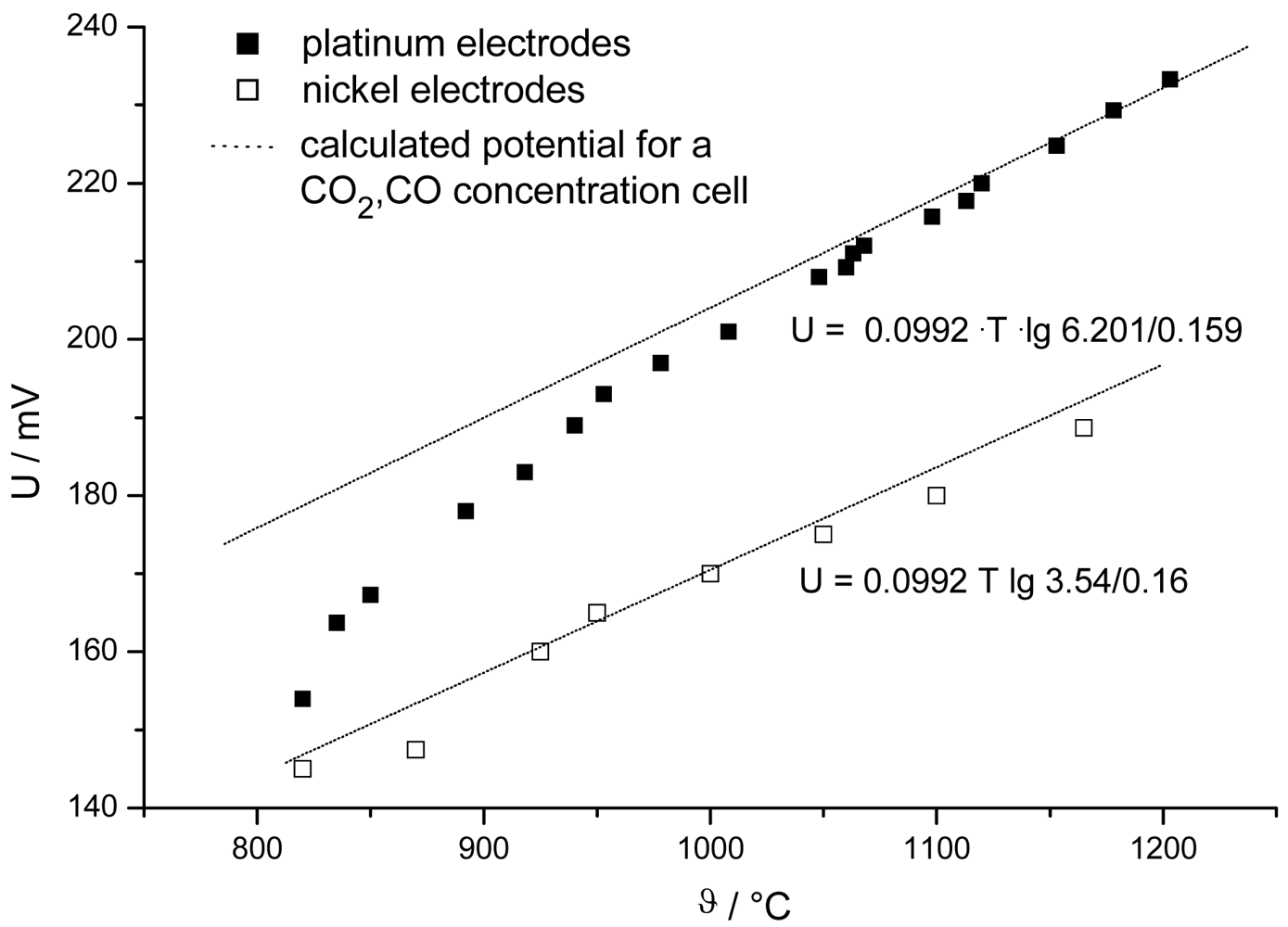
1.1. Nernst-type Sensors: Basic Considerations
- Gas mixtures that contain free oxygen beside inert gases, e.g. O2, N2
- Gas mixtures that are in chemical equilibrium, e.g. water gas
- Dissolved oxygen in molten metals (e.g. steel and copper)
1.2. Nernst-type Sensors: Novel Materials
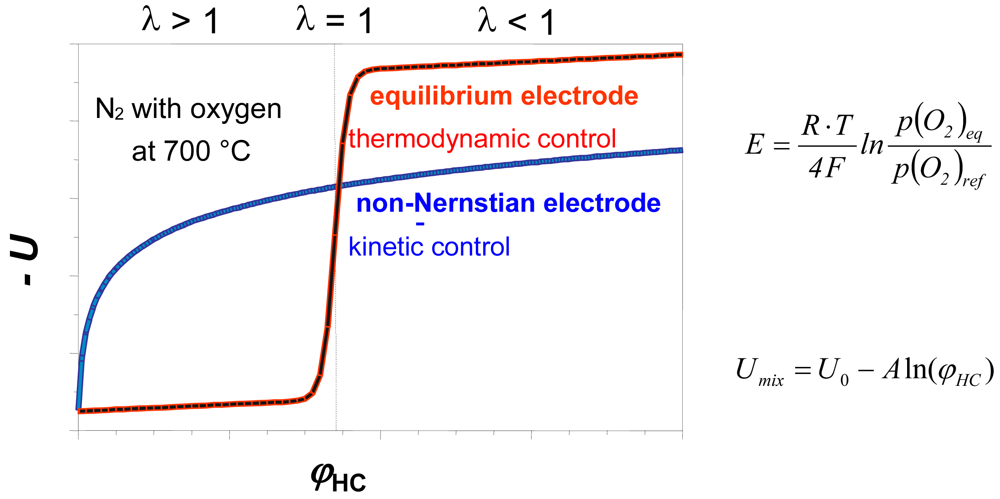
1.3. Mixed-potential Sensors: Latest Developments
- Oxygen reduction: 9/2 O2(g) + 9VO•• + 18e- ↔ 9OOx (YSZ)
- Hydrocarbon reduction: C3H6(g) + 9OOx (YSZ) → 3CO2(g) + 3H2O(g) + 9VO•• + 18e-
- Overall reaction: 9/2 O2(g) + C3H6(g) → 3CO2(g) + 3H2O(g)
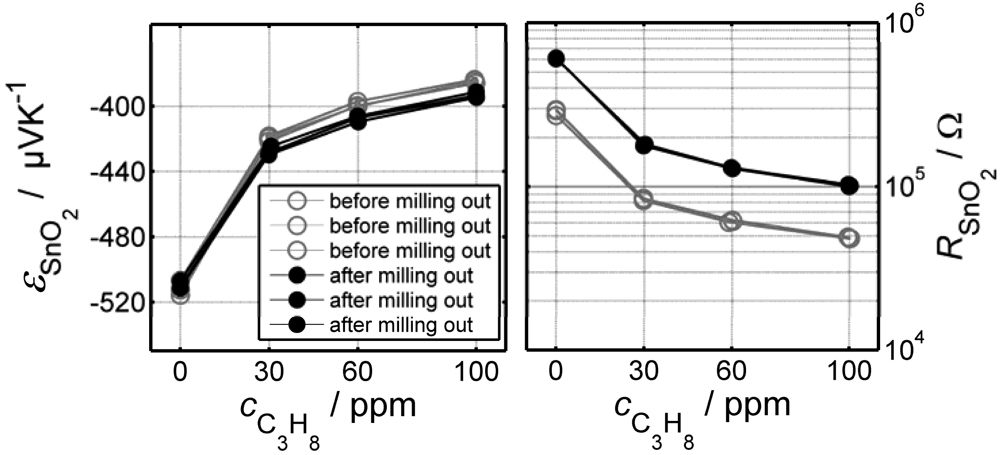
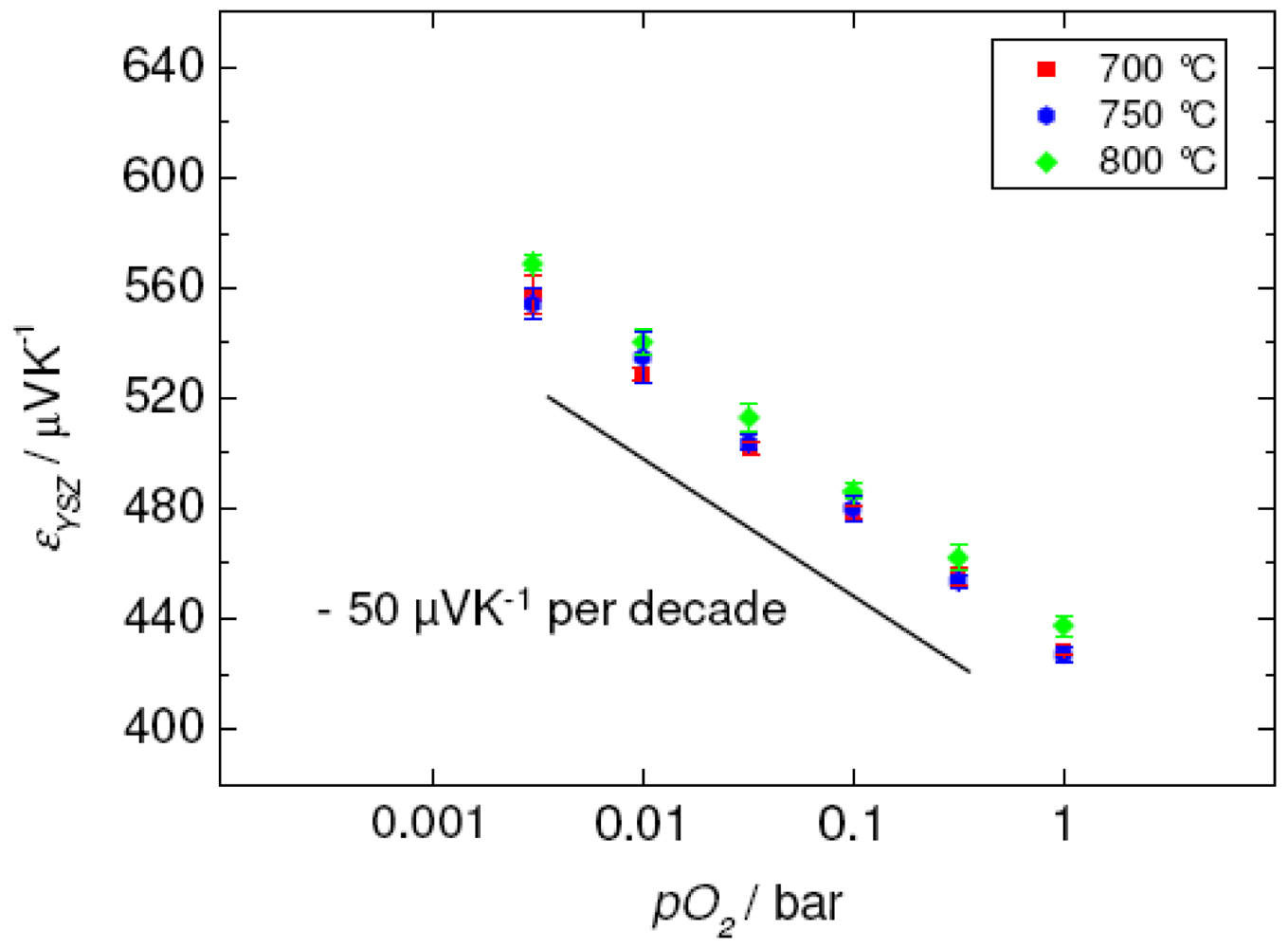
1.4. Direct Thermoelectric Sensors: a Novel Potentiometric Principle
2. Amperometric Sensors
3. Conductometric Sensors
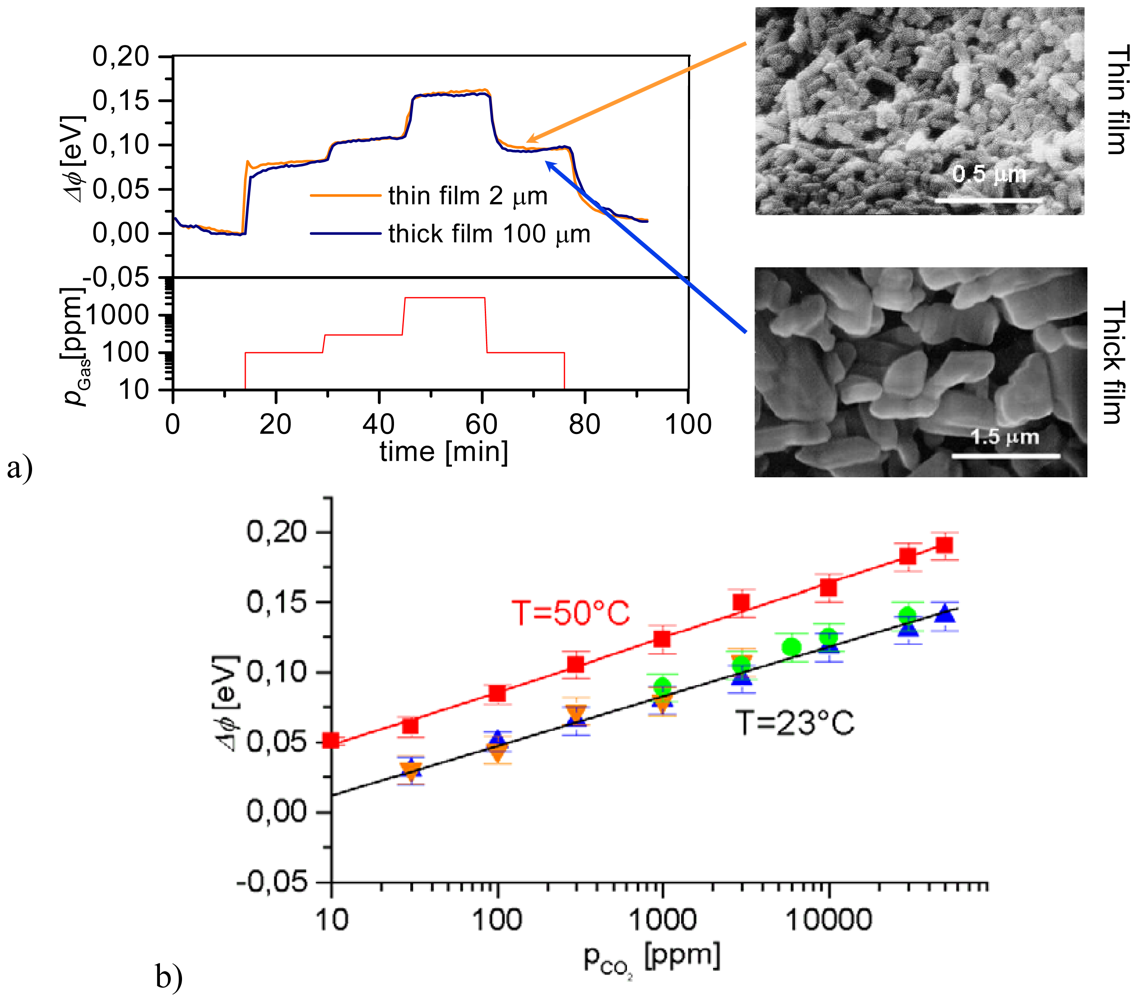
3.1. Novel Trends in n-type Semiconducting Sensors
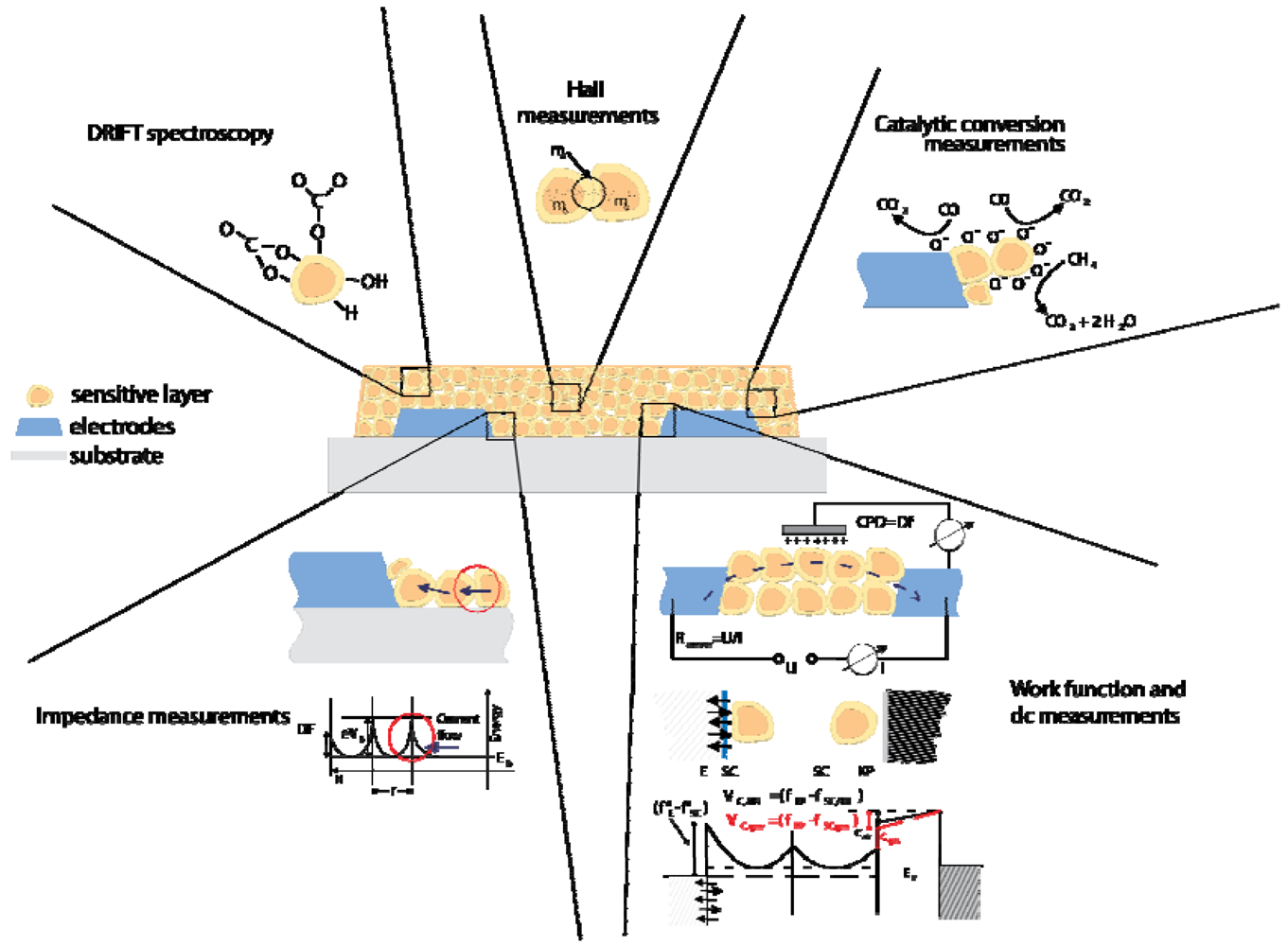
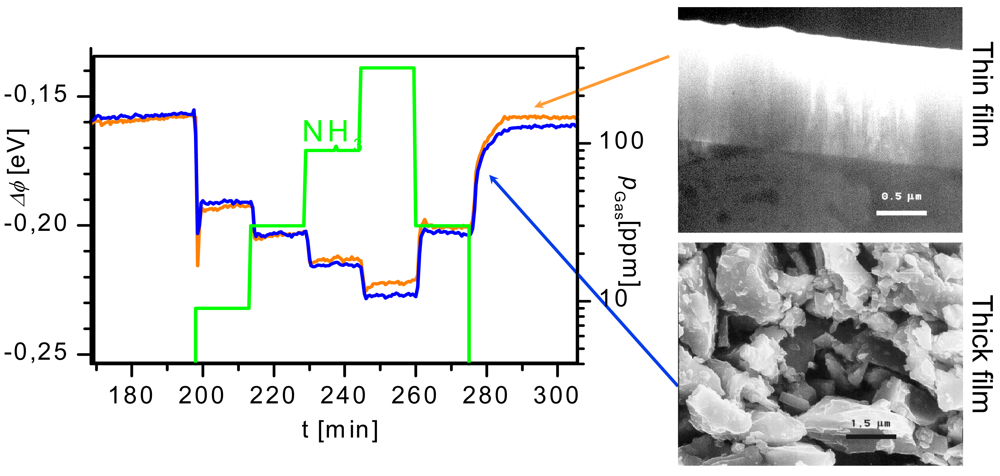
3.1.1. Operando Studies
- DC resistance measurements give information about changes in the concentration of free charge carriers induced by surface reactions.
- AC impedance spectroscopy allows for identifying charge depleted regions such as surface space charge layers or metal-metal oxide contacts and of the nature of free charge carriers (ions or electrons). The changes induced by gas reactions allow following the way in which charge transfer processes effect the free charge carrier transport and the dielectric properties.
- Hall effect measurements give information about the various contribution to the conductance changes (of mobility and/or free charge carrier concentration) when combined with the DC resistance measurements. They provide insights that help to model the conduction processes in the sensing layer.
- Work function changes are measured by the Kelvin method (details see Section 4). They help to evaluate the impact of surface reactions (charge transfer processes between gases and metal oxides). In combination with conductance measurements localized chemisorption and ionosorption can be discriminated.
- On-line gas analysis of the composition of the sensor ambient atmosphere allows determining the end products of solid-gas interaction and gives insight about the possible reaction paths.
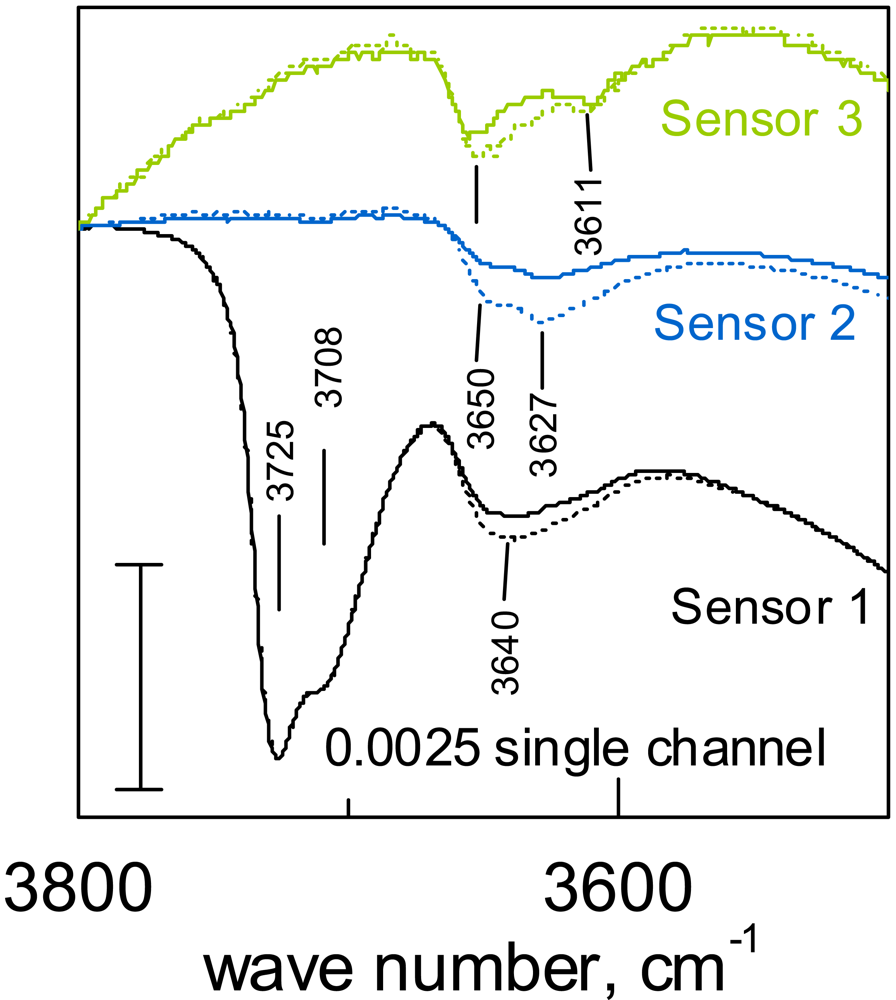
- Diffuse Reflection Infrared Fourier Transformed Spectroscopy (DRIFTS) measurements are allowing for the identification of the adsorbed surface species involved in the gas solid reaction. It is one of the few spectroscopic techniques possible to be applied in-operando and its input is essential for the identification of reaction mechanisms.
Overview of operando results
3.1.2. One Dimensional Materials
3.2. P-type Materials
3.3. Zeolites
3.4. Further Approaches for Selectivity Enhancement
3.5. Novel Deposition Techniques
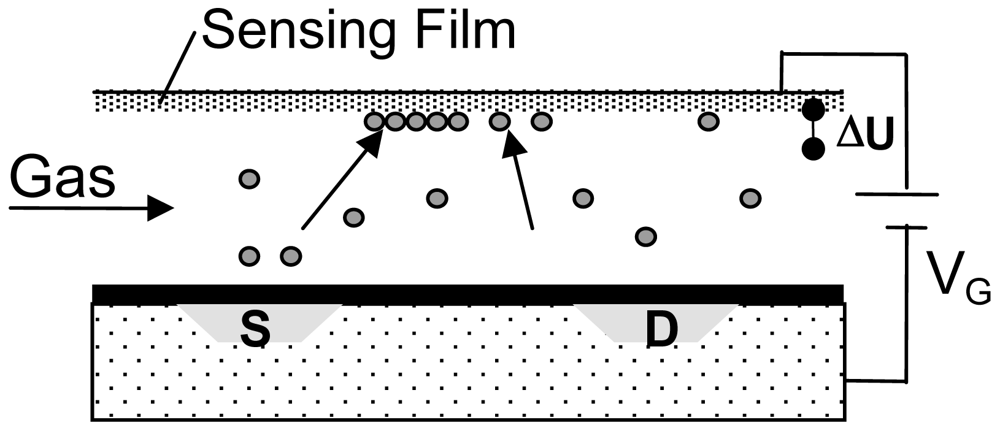
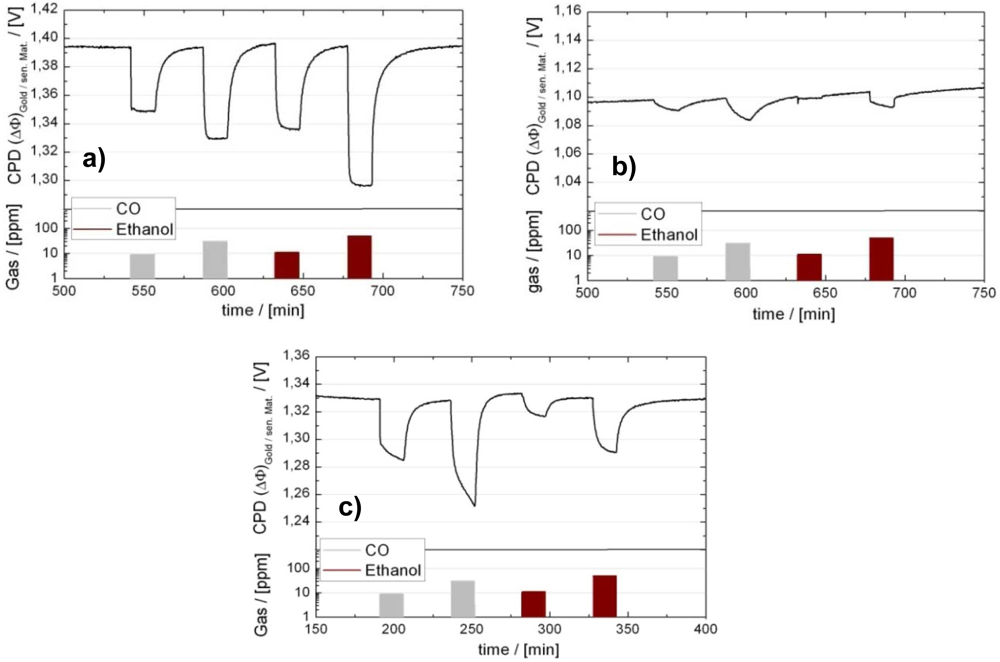
4. Field Effect Sensors
4.1. Device Technology
- An appropriate sensing material is deposited on a flat carrier substrate forming what eventually becomes the gate-electrode. The preparation conditions are not limited by any Si-electronics related constraints.
- The Si FET-chip is separately prepared in standard CMOS. Electronics for driving the sensor may be integrated in the Si-Chip
- Finally both parts are bonded together so that a defined air gap is formed.
4.2. Gas Sensing Materials for Suspended Gate FETS
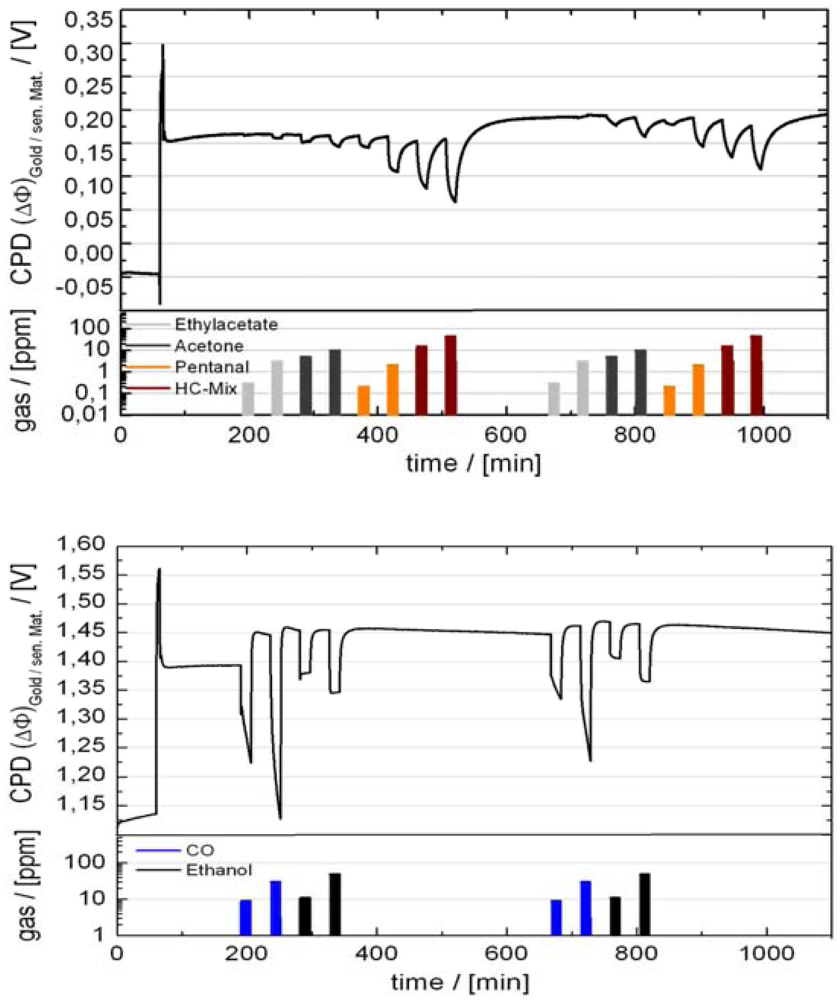
4.3. Applications of GasFETs
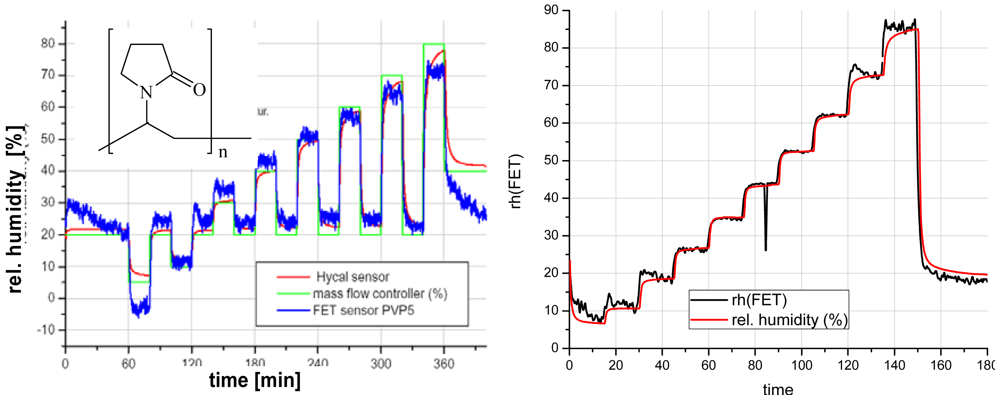
- Temperature and humidity. The relative humidity (r.h.) has to be kept optimally at a level between 40 - 60 % which is best for comfort as well as for the performance of people.
- The CO2-content: human breath enriches the air with CO2. At a level of 1,000 ppm, the first physiological reactions occur. Above 2,000 ppm people tend to become tired.
- The overall smell level. Smell arising from human sources as well as from building components has a distracting effect, lowering the comfort level as well as the effectiveness of people. Some components have direct unhealthy effects.
4.4. Field Effect Sensors: Discussion and Outlook
- They are able to work at room temperature. This reduces the energy demand for operation and avoids thermal decomposition of instable gases at the sensing surface.
- Due to their functional principle they make direct use of surface properties, thus facilitating the preparation of materials with reproducible sensing properties.
- They enable the application of various classes of sensing materials. This enhances the chances to generate a sensing surface that has a surface chemistry that allows the direct and selective reaction with the target gas to be detected.
Conclusions
References and Notes
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuat. B-Chem. 2005, 108, 2–14. [Google Scholar]
- Williams, D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuat. B-Chem. 1999, 57, 1–16. [Google Scholar]
- Chen, Z.; Lu, C. Humidity sensors: a review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar]
- Moos, R. A brief overview on automotive exhaust gas sensors based on electroceramics. Int. J. Appl. Ceram. Technol. 2005, 2, 401–413. [Google Scholar]
- Janke, D. A new immersion sensor for the rapid electrochemical determination of dissolved oxygen in metallic melts. Solid State Ionics 1981, 3-4, 599–604. [Google Scholar]
- Moos, R.; Schönauer, D. Review: recent developments in the field of automotive exhaust gas ammonia sensing. Sens. Lett. 2008, 6, 821–825. [Google Scholar]
- Zosel, J.; Müller, R.; Vashook, V.; Guth, U. Response behaviour of perovskites and Au/oxide composites as HC-electrodes in different combustibles. Solid State Ionics 2004, 175, 531–533. [Google Scholar]
- Azad, A.M.; Akbar, S.A.; Mhaisalkar, S.G.; Birkefeld, L.D.; Goto, K.S. Solid-state gas sensors: a review. J. Electrochem. Soc. 1992, 139, 3690–3704. [Google Scholar]
- Moseley, P. Solid state gas sensors. Meas. Sci. Technol. 1997, 8, 223–237. [Google Scholar]
- Riegel, J.; Neumann, H.; Wiedenmann, H.-M. Exhaust gas sensors for automotive emission control. Solid State Ionics 2002, 152-153, 783–800. [Google Scholar]
- Fergus, J.W. Solid electrolyte based sensors for the measurement of CO and hydrocarbon gases. Sens. Actuat. B-Chem. 2007, 122, 683–693. [Google Scholar]
- Guth, U.; Zosel, J. Electrochemical solid electrolyte gas sensors - hydrocarbon and NOx analysis in exhaust gases. Ionics 2004, 10, 366–377. [Google Scholar]
- Park, CO.; Akbar, S.A.; Weppner, W. Ceramic electrolytes and electrochemical sensors. J. Mater. Sci. 2003, 38, 4639–4660. [Google Scholar]
- Möbius, H.-H. Solid-state electrochemical potentiometric sensors for gas analysis. In Sensors; Göpel, W., Hesse, J., Zemel, J.N., Eds.; Wiley-VCH: Weinheim, Germany, 1991; pp. 1105–1124. [Google Scholar]
- Möbius, H.; Sandow, H; Hartung, R; Jakobs, S; Guth, U.; Buhrow, J. Entwicklung neuer sensorsysteme mit galvanischen hochtemperatur-festelektrolytzellen. DECHEMA Monographie 1992, 126, 329. [Google Scholar]
- Harbeck, W.; Guth, U. Ermittlung der ausbrandgrenze von gasflammen mit hilfe gaspotentiometrischer bestimmungsmethoden. Die Industriefeuerung 1990, 49, 43–57. [Google Scholar]
- Möbius, H.-H.; Zhuk, P.P.; Jakobs, S.; Hartung, R.; Guth, U.; Sandow, H.; Vecher, A.A. Investigation of electrochemical oxygen sensors with solid electrolytes and oxide powder electrodes. Sov. Electrochem. Tranl. 1991, 26, 1235–1243. [Google Scholar]
- Guth, U.; Hartung, R.; Jakobs, S.; Sandow, K.-P.; Westphal, D. Oxides as an electrode material for solid electrolyte gas sensors. Proceedings of the 1997 Joint International Meeting of The Electrochemical Society and The International Society of Electrochemistry, Paris, France, August 31-September 5 1997.Proceedings of Electrochemical Society 97-24 (Ionic and Mixed Conducting Ceramics), Pennington, NJ, USA; 1998; pp. 231–250.
- Shuk, P.; Vecher, A.; Kharton, V.; Tichonova, L.; Wiemhöfer, H.D.; Göpel, W.; Guth, U. Electrodes for oxygen sensors based on rate earth manganites or cobaltites. Sens. Actuat. B-Chem. 1993, 15, 401–405. [Google Scholar]
- Sandow, K.-P.; Jakobs, S.; Thiemann, S.; Hartung, R.; Guth, U.; Schönauer, U. Dotierte lanthan-chrom-mischoxide als elektrodenmaterialien für ZrO2-Festelektrolyte. GDCH-Monographie. 1996, 3, 377–389. [Google Scholar]
- Shuk, P.; Bailey, E.; Guth, U. Zirconia oxygen sensors for process application: state-of-the-art sensors. Transducers 2008, 90, 174–184. [Google Scholar]
- Guth, U. Gas sensors. In Electrochemical Dictionary; Bard, A.J., Inzelt, G., Scholz, F., Eds.; Springer: Berlin Heidelberg, Germany, 2008; pp. 294–299. [Google Scholar]
- Westphal, D.; Jakobs, S.; Guth, U. Gold-composite electrodes for hydrocarbon sensors based on YSZ solid electrolytes. Ionics 2001, 7, 182–186. [Google Scholar]
- Miura, N.; Raisen, T.; Lu, G.; Yamazoe, N. Highly selective CO sensor using stabilized zirconia and a couple of oxide electrodes. Sens. Actuat. B-Chem. 1998, 47, 84–91. [Google Scholar]
- Hartung, R.; Schröder, R.; Möbius, H.-H. Brenngas-sensitive gassymmetrische galvanische Zellen mit oxidionenleitenden Festelektrolyten. Z. Phys. Chem. 1981, 262, 961–966. [Google Scholar]
- Guth, U.; Zosel, J.; Jakobs, S.; Westphal, D.; Müller, R. Au–oxide composites as HC-sensitive electrode material for mixed potential gas sensors. Solid State Ionics 2002, 152-153, 525–529. [Google Scholar]
- Wang, J.; Elumalai, P.; Terada, D.; Hasei, M.; Miura, N. Mixed-potential-type zirconia-based NOx sensor using Rh-loaded NiO sensing electrode operating at high temperatures. Solid State Ionics 2006, 177, 2305–2311. [Google Scholar]
- Chevallier, L.; Di Bartolomeo, E.; Grilli, ML.; Mainas, M.; White, B.; Wachsman, E.D.; Traversa, E. Non-Nernstian planar sensors based on YSZ with a Nb2O5 electrode. Sens. Actuat. B-Chem. 2008, 129, 591–598. [Google Scholar]
- Morata, A.; Viricelle, J.P.; Tarancon, A.; Dezanneau, G.; Pijolat, C.; Peiro, F.; Morante, J.R. Development and characterisation of a screen-printed mixed potential gas sensor. Sens. Actuat. B-Chem. 2008, 130, 561–566. [Google Scholar]
- Miura, N.; Nakatou, M.; Zhuiykov, S. Impedancemetric gas sensor based on stabilised zirconia solid electrolyte and oxide sensing electrode for detecting total NOx at high temperature. Sens. Actuat. B-Chem. 2003, 93, 221–228. [Google Scholar]
- Nakatou, M.; Miura, N. Detection of propene by using new-type impedancemetric zirconia based sensor attached with oxide sensing-electrode. Sens. Actuat. B-Chem. 2006, 120, 57–62. [Google Scholar]
- Zosel, J.; Franke, D.; Ahlborn, K.; Gerlach, F.; Vashook, V.; Guth, U. Perovskite related electrode materials with enhanced NO sensitivity for mixed potential sensors. Solid State Ionics 2008, 179, 1628–1631. [Google Scholar]
- Di Bartolomeo, E.; Grilli, ML.; Traversa, E. Sensing mechanism of potentiometric gas sensors based on stabilized zirconia with oxide electrodes. J. Electrochem. Soc. 2004, 151, H133–H139. [Google Scholar]
- Zosel, J.; Tuchtenhagen, D.; Ahlborn, K.; Guth, U. Mixed potential gas sensor with short response time. Sens. Actuat. B-Chem. 2008, 130, 326–329. [Google Scholar]
- Plashnitsa, V.V.; Ueda, T.; Elumalai, P.; Miura, N. NO2 sensing performances of planar sensor using stabilized zirconia and thin-NiO sensing electrode. Sens. Actuat. B-Chem. 2008, 130, 231–239. [Google Scholar]
- Nakatou, M.; Miura, N. Detection of combustible hydrogen-containing gases by using impedancemetric zirconia-based water-vapor sensor. Solid State Ionics 2005, 176, 2511–2515. [Google Scholar]
- Miura, N.; Wang, J.; Nakatou, M.; Elumalai, P.; Hasei, M. NOx sensing characteristics of mixed-potential-type zirconia sensor using NiO sensing electrode at high temperatures. Electrochem. Solid-State Lett. 2005, 8, H9–H11. [Google Scholar]
- Available on line at www.zirox.de.
- Zosel, J.; Opitz, T.; Bley, T.; Guth, U. Application of highly sensitive gas sensors for monitoring of mould culture. (Einsatz hochempfindlicher Gassensoren für das Monitoring der Aktivität von Schimmelpilzkulturen (in German)). Der Versuchs- und Forschungsingenieur 2006, 39, 31–37. [Google Scholar]
- Rettig, F.; Moos, R. Direct thermoelectric gas sensors: design aspects and first gas sensors. Sens. Actuat. B-Chem. 2007, 123, 413–419. [Google Scholar]
- Rettig, F.; Moos, R. Temperature modulated direct thermoelectric gas sensors: thermal modeling and results for fast hydrocarbon sensors. Meas. Sci. Technol. 2009, 20, 065205. [Google Scholar]
- Moos, R.; Gnudi, A.; Härdtl, K.H. Thermopower of Sr1-xLaxTiO3 ceramics. J. Appl. Phys. 1995, 78, 5042–5047. [Google Scholar]
- Jonker, G.H. Application of combined conductivity and seebeck-effect plots for analysis of semiconductor properties. Philips Res. Rep. 1968, 23, 131–138. [Google Scholar]
- Moos, R. Verfahren und Meßwandler zur Detektion des Sauerstoffgehaltes in einem Gas. German Patent Specification, DE 19853595 C1 1998. [Google Scholar]Method and Apparatus for Detecting the Oxygen Content of a gas. US Patent Specification, US 6,368,868, 2002.
- Ionescu, R. Combined seebeck and resistive SnO2 gas sensors, a new selective device. Sens. Actuat. B-Chem. 1998, 48, 392–394. [Google Scholar]
- Liess, M.; Steffes, H. The modulation of thermoelectric power by chemisorption - a new detection principle for microchip chemical sensors. J. Electrochem. Soc. 2000, 147, 3151–3153. [Google Scholar]
- Moos, R.; Härdtl, K.H. Dependence of the intrinsic conductivity minimum of SrTiO3 ceramics on the sintering atmosphere. J. Amer. Ceram. Soc. 1995, 78, 2569–2571. [Google Scholar]
- Choi, G.M.; Tuller, H.L. Defect structure and electrical properties of single-crystal Ba0.03Sr0.97TiO3. J. Amer. Ceram. Soc. 1988, 71, 201–205. [Google Scholar]
- Moos, R.; Menesklou, W.; Schreiner, H.J.; Härdtl, K.H. Materials for temperature independent resistive oxygen sensors for combustion exhaust gas control. Sens. Actuat. B-Chem. 2000, 67, 178–183. [Google Scholar]
- Sahner, K.; Schönauer, D.; Matam, M.; Post, M.; Moos, R. Selectivity enhancement of p-type semiconducting hydrocarbon sensors - The use of sol precipitated nano-powders. Sens. Actuat. B-Chem. 2008, 130, 470–476. [Google Scholar]
- Rettig, F.; Moos, R. Morphology dependence of thermopower and resistance in semiconducting oxides with space charge regions. Solid State Ionics 2008, 179, 2299–2307. [Google Scholar]
- Rettig, F.; Moos, R. Direct thermoelectric hydrocarbon gas sensors based on SnO2. IEEE Sensors J. 2007, 7, 1490–1496. [Google Scholar]
- Ahlgren, E.O.; Poulsen, F.W. Thermoelectric power of stabilized zirconia. Solid State Ionics 1995, 82, 193–201. [Google Scholar]
- Röder-Roith, U.; Rettig, F.; Röder, T.; Janek, J.; Moos, R.; Sahner, K. Thick-film solid electrolyte oxygen sensors using the direct ionic thermoelectric effect. Sens. Actuat. B-Chem. 2009, 136, 530–535. [Google Scholar]
- Stetter, J.R.; Li, J. Amperometric gas sensors - A review. Chem. Rev. 2008, 108, 352–366. [Google Scholar]
- Turner, A.P.F.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications; Oxford University Press: Oxford, U.K., 1987. [Google Scholar]
- Mitsubayashi, K.; Yokoyama, K.; Takeuchi, T.; Karube, I. Gas-phase biosensor for ethanol. Anal. Chem. 1994, 66, 3297–3302. [Google Scholar]
- Mitsubayashi, K.; Nishio, G.; Sawai, M.; Saito, T.; Kudo, H.; Saito, H.; Otsuka, K.; Noguer, T.; Marty, J.L. A bio-sniffer stick with FALDH (formaldehyde dehydrogenase) for convenient analysis of gaseous formaldehyde. Sens. Actuat. B-Chem. 2008, 130, 32–37. [Google Scholar]
- Achmann, S.; Hämmerle, M.; Moos, R. Amperometric enzyme-based biosensor for direct detection of formaldehyde in the gas phase: Dependence on electrolyte composition. Electroanalysis 2008, 20, 410–417. [Google Scholar]
- Hämmerle, M.; Achmann, S.; Moos, R. Gas diffusion electrodes for use in an amperometric enzyme biosensor. Electroanalysis 2008, 20, 2279–2286. [Google Scholar]
- Achmann, S.; Hämmerle, M.; Moos, R. Amperometric enzyme-based gas sensor for formaldehyde: Impact of possible interferences. Sensors 2008, 8, 1351–1365. [Google Scholar]
- Achmann, S.; Hämmerle, M.; Kita, J.; Moos, R. Miniaturized low temperature co-fired ceramics (LTCC) biosensor for amperometric gas sensing. Sens. Actuat. B-Chem. 2008, 135, 89–95. [Google Scholar]
- Heiland, G. Zum Einfluss von Wasserstoff auf die elektrische Leitfähigkeit an der Oberfläche von Zinkoxydkristallen. Z. Phys. 1957, 148, 15–27. [Google Scholar]
- Seiyama, T.; Kato, A.; Fujiishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502. [Google Scholar]
- Taguchi, N. Gas-detecting device. US Patent Specification, US 3,631,436, 1956. [Google Scholar]
- Eranna, G.; Joshi, B.; Runthala, D.; Gupta, R. Oxide materials for development of integrated gas sensors - a comprehensive review. CRC Crit. Rev. Sol. St. Mat. Sci. 2004, 29, 111–188. [Google Scholar]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B-Solid State M. 2007, 139, 1–23. [Google Scholar]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; the example of CO sensing with SnO2 sensing in the presence of humidity. J. Phys. Condens. Matter 2003, 15, R813–R839. [Google Scholar]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide based gas sensor research: how to? Sens. Actuat. B-Chem. 2007, 121, 18–35. [Google Scholar]
- Kappler, J.; Tomescu, A.; Barsan, N.; Weimar, U. CO consumption of Pd doped SnO2 based sensors. Thin Solid Films 2001, 391, 186–91. [Google Scholar]
- Emiroglu, S.; Barsan, N.; Weimar, U.; Hoffmann, V. In situ diffuse reflectance infrared spectroscopy study of CO adsorption on SnO2. Thin Solid Films 2001, 391, 176–185. [Google Scholar]
- Harbeck, S.; Szatvanyi, A.; Barsan, N.; Weimar, U.; Hoffmann, V. DRIFT studies of thick film un-doped and Pd-doped SnO2 sensors: temperature changes effect and CO detection mechanism in the presence of water vapour. Thin Solid Films 2003, 436, 76–83. [Google Scholar]
- Sahm, T.; Gurlo, A.; Barsan, N.; Weimar, U.; Madler, L. Fundamental studies on SnO2 by means of simultaneous work function change and conduction measurements. Thin Solid Films. 2005, 490, 43–47. [Google Scholar]
- Koziej, D.; Barsan, N.; Weimar, U.; Szuber, J.; Shimanoe, K.; Yamazoe, N. Water-oxygen interplay on tin dioxide surface: Implication on gas sensing. Chem. Phys. Lett. 2005, 410, 321–323. [Google Scholar]
- Sahm, T.; Mädler, L.; Gurlo, A.; Barsan, N.; Pratsinis, S.E.; Weimar, U. Flame spray synthesis of tin dioxide nanoparticles for gas sensing. Sens. Actuat. B-Chem. 2004, 98, 148–153. [Google Scholar]
- Schmid, W.; Barsan, N.; Weimar, U. Sensing of hydrocarbons and CO in low oxygen conditions with tin dioxide sensors: possible conversion paths. Sens. Actuat. B-Chem. 2004, 103, 362–368. [Google Scholar]
- Schmid, W.; Barsan, N.; Weimar, U. Sensing of hydrocarbons with tin oxide sensors: possible reaction path as revealed by consumption measurements. Sens. Actuat. B-Chem. 2003, 89, 232–236. [Google Scholar]
- Bertrand, J.; Koziej, D.; Barsan, N.; Viricelle, J.P.; Pijolat, C.; Weimar, U. Influence of the nature of the electrode on the sensing performance of SnO2 sensors; Impedance spectroscopy studies. Eurosensors XX 2006, 100–101. [Google Scholar]
- Bertrand, J.; Viricelle, J.P.; Pijolat, C.; Haensch, A.; Koziej, D.; Barsan, N.; Weimar, U. Metal/SnO2 interface effects on CO sensing; operando studies. In IEEE Sensors, Proceedings of the 6th IEEE Sensors Conference, Atlanta, GA, USA, October 2007; pp. 492–495.
- Gurlo, A.; Barsan, N.; Oprea, A.; Sahm, M.; Sahm, T.; Weimar, U. An n- to p-type conductivity transition induced by oxygen adsorption on alpha-Fe2O3. Appl. Phys. Lett. 2004, 85, 2280–2282. [Google Scholar]
- Pokhrel, S.; Simion, C.E.; Quemener, V.; Barsan, N.; Weimar, U. Investigations of conduction mechanism in Cr2O3 gas sensing thick films by ac impedance spectroscopy and work function changes measurements. Sens. Actuat. B-Chem. 2008, 133, 78–83. [Google Scholar]
- Hernandez-Ramirez, F.; Prades, J.D.; Tarancon, A.; Barth, S.; Casals, O.; Jiménez-Diaz, R.; Pellicer, E.; Rodriguez, J.; Juli, M.A.; Romano-Rodriguez, A.; Morante, J.R.; Mathur, S.; Helwig, A.; Spannhake, J.; Mueller, G. Portable microsensors based on individual SnO2 nanowires. Nanotechnology 2007, 18, 495501. [Google Scholar]
- Hernandez-Ramirez, F.; Prades, J.D.; Tarancon, A.; Barth, S.; Casals, O.; Jiménez-Diaz, R.; Pellicer, E.; Rodriguez, J.; Morante, J.R.; Juli, M.A.; Mathur, S.; Romano-Rodriguez, A. Insight into the role of oxygen diffusion in the sensing mechanisms of SnO2 nanowires. Adv. Funct. Mater. 2008, 18, 2990–2994. [Google Scholar]
- Polleux, J.; Gurlo, A.; Barsan, N.; Weimar, U.; Antonietti, M.; Niederberger, M. Template-free synthesis and assembly of single-crystalline tungsten oxide nanowires and their gas-sensing properties. Angew. Chem. Int. Ed. 2006, 45, 261–265. [Google Scholar]
- Yannopoulos, L.N. A p-type semiconducting thick film gas sensor. Sens. Actuat. 1987, 12, 263–273. [Google Scholar]
- Carotta, M.C.; Martinelli, G.; Sadaoka, Y.; Nunziante, P.; Traversa, E. Gas-sensitive electrical properties of perovskite-type SmFeO3 thick films. Sens. Actuat. B-Chem. 1998, 48, 270–276. [Google Scholar]
- Morrison, S. R. Selectivity in semiconductor sensors. Proceedings of the 2nd International Meeting on Chemical Sensors (IMCS), Bordeaux, France, July 1986.
- Moseley, P.; Williams, D.E. Gas sensors based on oxides of early transition metals. Polyhedron. 1989, 8, 1615–1618. [Google Scholar]
- Niemeyer, D.; Williams, D.E.; Smith, P.; Pratt, K.F.; Slater, B.; Catlow, C.R.A.; Stoneham, A.M. Experimental and computational study of the gas-sensor behaviour and surface chemistry of the solid-solution Cr2-xTixO3 (x < 0.5). J. Mater. Chem. 2002, 12, 666–675. [Google Scholar]
- Aono, H.; Traversa, E.; Sakamoto, M.; Sadaoka, Y. Crystallographic characterization and NO2 gas sensing property of LnFeO3 prepared by thermal decomposition of Ln-Fe hexacyanocomplexes, Ln[Fe(CN)6]nH2O, Ln = La, Nd, Sm, Gd, and Dy. Sens. Actuat. B-Chem. 2003, 94, 132–139. [Google Scholar]
- Moos, R.; Rettig, F.; Hürland, A.; Plog, C. Temperature-independent resistive oxygen exhaust gas sensors for lean-burn engines in thick-film technology. Sens. Actuat. B-Chem. 2003, 93, 43–50. [Google Scholar]
- Rothschild, A.; Litzelmann, S.J.; Tuller, H.L.; Menesklou, W.; Schneider, T.; Ivers-Tiffée, E. Temperature-independent resistive oxygen sensors based on SrTi1-xFexO3-δ solid solutions. Sens. Actuat. B-Chem. 2005, 108, 223–230. [Google Scholar]
- Rettig, F.; Moos, R.; Plog, C. Sulfur adsorber for thick-film exhaust gas sensors. Sens. Actuat. B-Chem. 2003, 93, 36–42. [Google Scholar]
- Rettig, F.; Moos, R.; Plog, C. Poisoning of temperature independent resistive oxygen sensors by sulfur dioxide. J. Electroceram. 2004, 13, 733–738. [Google Scholar]
- Blase, R.; Härdtl, K.H.; Schönauer, U. Oxygen Sensor based on non-doped cuprate. United States Patent Specification, US 5,792,666, 1997. [Google Scholar]
- Moos, R.; Rettig, F. Resistiver sauerstoffsensor (Resistive Oxygen Sensor). German Patent Specification, DE 10114645 C1, 2003. [Google Scholar]
- Sahner, K.; Moos, R.; Izu, N.; Shin, W.; Murayama, N. Response kinetics of temperature independent resistive oxygen sensor formulations: a comparative study. Sens. Actuat. B-Chem. 2006, 113, 112–119. [Google Scholar]
- Sahner, K.; Straub, J.; Moos, R. Cuprate-ferrate compositions for temperature independent resistive oxygen sensors. J. Electroceram. 2006, 16, 179–186. [Google Scholar]
- Sahner, K.; Moos, R.; Matam, M.; Tunney, J.; Post, M. Hydrocarbon sensing with thick and thin film p-type conducting perovskite materials. Sens. Actuat. B-Chem. 2005, 108, 102–112. [Google Scholar]
- Sahner, K.; Schönauer, D.; Moos, R.; Matam, M.; Post, M.L. Effect of electrodes and zeolite cover layer on hydrocarbon sensing with p-type perovskite SrTi0.8Fe0.2O3-δ thick and thin films. J. Mater. Sci. 2006, 41, 5828–5835. [Google Scholar]
- Sahner, K.; Gouma, P.; Moos, R. Electrodeposited and sol-gel precipitated p-type SrTi1-xFexO3-δ semiconductors for gas sensing. Sensors 2007, 7, 1871–1886. [Google Scholar]
- Sahner, K.; Moos, R. Modeling of hydrocarbon sensors based on p-type semiconducting perovskites. Phys. Chem. Chem. Phys. 2007, 9, 635–642. [Google Scholar]
- Sahner, K.; Moos, R. P-type semiconducting perovskite sensors for reducing gases – model description. Sens. Lett. 2008, 6, 808–811. [Google Scholar]
- Weitkamp, J. Zeolites and catalysis. Solid State Ionics 2000, 131, 175–188. [Google Scholar]
- Sahner, K.; Hagen, G.; Schönauer, D.; Reiß, S.; Moos, R. Zeolites - Versatile materials for gas sensors. Solid State Ionics 2008, 179, 2416–2423. [Google Scholar]
- Xu, X.; Wang, J.; Long, Y. Zeolite-based materials for gas sensors. Sensors 2006, 6, 1751–1764. [Google Scholar]
- Moos, R.; Sahner, K.; Hagen, G.; Dubbe, A. Zeolites for sensors for reducing gases. Rare Metal Mat. Eng. 2006, 35 Suppl. 3, 447–451. [Google Scholar]
- Vilaseca, M.; Coronas, J.; Cirera, A.; Cornet, A.; Morante, J.; Santamaría, J. Use of zeolite films to improve the selectivity of reactive gas sensors. Catal Today 2003, 82, 179–185. [Google Scholar]
- Hugon, O.; Sauvan, M.; Benech, P.; Pijolat, C.; Lefebvre, F. Gas separation with a zeolite filter, application to the selectivity enhancement of chemical sensors. Sens. Actuat. B-Chem. 2000, 67, 235–243. [Google Scholar]
- Sahner, K.; Schönauer, D.; Kuchinke, P.; Moos, R. Zeolite cover layer for selectivity enhancement of p-type semiconducting hydrocarbon sensors. Sens. Actuat. B-Chem. 2008, 133, 502–508. [Google Scholar]
- Meier, B.; Werner, T.; Klimant, I.; Wolfbeis, O.S. Novel oxygen sensor material based on a ruthenium bipyridil complex encapsulated in zeolite Y - dramatic differences in the efficiency of luminescence quenching by oxygen on going from surface-adsorbed to zeolite-encapsulated flurophores. Sens. Actuat. B-Chem. 1995, 29, 240–245. [Google Scholar]
- Nischwitz, P.; Amels, P.; Fetting, F. Studies on the ionic conductivity of zeolitic solids. Solid State Ionics 1994, 73, 105–118. [Google Scholar]
- Plog, C.; Maunz, W.; Kurzweil, P.; Obermeier, E.; Scheibe, C. Combustion gas sensitivity of zeolite layers on thin-film capacitors. Sens. Actuat. B-Chem. 1995, 24-25, 403–406. [Google Scholar]
- Schäf, O.; Ghobarkar, H.; Guth, U. Sensors for combustible gas components using modified single crystal zeolites. Ionics 1997, 3, 282–288. [Google Scholar]
- Schäf, O.; Ghobarkar, H.; Steinbach, A.C.; Guth, U. Basic investigations on zeolite application for electrochemical analysis. Fresenius J. Anal. Chem. 2000, 367, 388–392. [Google Scholar]
- Neumeier, S.; Echterhof, T.; Bölling, R.; Pfeifer, H.; Simon, U. Zeolite based trace humidity sensor for high temperature applications in hydrogen atmosphere. Sens. Actuat. B-Chem. 2008, 134, 171–174. [Google Scholar]
- Simon, U.; Flesch, U.; Maunz, W.; Müller, R.; Plog, C. The effect of NH3 on the ionic conductivity of dehydrated zeolites Na beta and H beta. Micropor. Mesopor. Mat. 1998, 21, 111–116. [Google Scholar]
- Franke, M.; Simon, U.; Moos, R.; Knezevic, A.; Müller, R.; Plog, C. Development and working principle of an ammonia gas sensor based on a refined model for solvate supported proton transport in zeolites. Phys. Chem. Chem. Phys. 2003, 5, 5195–5198. [Google Scholar]
- Moos, R.; Müller, R.; Plog, C.; Knezevic, A.; Leye, H.; Irion, E.; Braun, T.; Marquardt, K.; Binder, K. Selective ammonia exhaust gas sensor for automotive applications. Sens. Actuat. B-Chem. 2002, 83, 181–189. [Google Scholar]
- Rodriguez-Gonzalez, L.; Franke, M.; Simon, U. Electrical detection of different amines with proton-conductive H-ZSM-5. Molecular Sieves: from basic research to industrial applications 2005, Pts. A and B 158, 2049–2056. [Google Scholar]
- Schönauer, D.; Sichert, I.; Moos, R. Zeolithe zur Ammoniakdetektion in Abgasen. Z. Anorg. Allg. Chem. 2008, 634, 2077. [Google Scholar]
- Tennison, P.; Lambert, C.; Levin, M. NOx control development with urea SCR on a diesel passenger car; SAE 2004-01-1291; SAE: Miami, 2004. [Google Scholar]
- Hagen, G.; Dubbe, A.; Rettig, F.; Jerger, A.; Birkhofer, T.; Müller, R.; Plog, C.; Moos, R. Selective impedance based gas sensors for hydrocarbons using ZSM-5 zeolite films with chromium(III)oxide interface. Sens. Actuat. B-Chem. 2006, 119, 441–448. [Google Scholar]
- Hagen, G.; Schulz, A.; Knörr, M.; Moos, R. Four-wire impedance spectroscopy on planar zeolite/chromium oxide based hydrocarbon gas sensors. Sensors 2007, 7, 2681–2692. [Google Scholar]
- Reiß, S.; Hagen, G.; Moos, R. Zeolite-based Impedimetric Gas Sensor Device in Low-cost Technology for Hydrocarbon Gas Detection. Sensors 2008, 8, 7904–7916. [Google Scholar]
- Fischerauer, A.; Gollwitzer, A.; Thalmayr, F.; Hagen, G.; Moos, R.; Fischerauer, G. An initial physics-based model for the impedance spectrum of a hydrocarbon sensor with a zeolite/Cr2O3 interface. Sens. Lett. 2008, 6, 1019–1022. [Google Scholar]
- Achmann, S.; Hagen, G.; Kita, J.; Malkowsky, I.M.; Kiener, C.; Moos, R. Metal-organic frameworks for sensing applications in the gas phase. Sensors 2009, 9, 1574–1589. [Google Scholar]
- Sauerwald, T.; Skiera, D.; Kohl, C.-D. Selectivity enhancement of gas sensors using non-equilibrium polarisation effects in metal oxide films. Appl. Phys. A 2007, 87, 525–529. [Google Scholar]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.-D.; Tiemann, M. Ordered mesoporous In2O3: synthesis by structure replication and application as a methane gas sensor. Adv. Funct. Mater. 2009, 19, 653–661. [Google Scholar]
- Wagner, T.; Sauerwald, T.; Kohl, C.-D.; Waitz, T.; Weidmann, C.; Tiemann, M. Gas sensor based on ordered mesoporous In2O3. Thin Solid Films 2009. In press. [Google Scholar] [CrossRef]
- Wessels, J.M.; Nothofer, H.-G.; Ford, W.E.; von Wrochem, F.; Scholz, F.; Vossmeyer, T.; Schroedter, A.; Weller, H.; Yasuda, A. Optical and electrical properties of three-dimensional interlinked gold nanoparticle assemblies. J. Amer. Chem. Soc. 2004, 126, 3349–3356. [Google Scholar]
- Joseph, Y.; Guse, B.; Yasuda, A.; Vossmeyer, T. Chemiresistor coatings from Pt- and Au-nanoparticle/nonanedithiol films: sensitivity to gases and solvent vapors. Sens. Actuat. B-Chem. 2005, 98, 188–195. [Google Scholar]
- Krasteva, N.; Fogel, Y.; Bauer, R.E.; Müllen, K.; Joseph, Y.; Matsuzawa, N.; Yasuda, A.; Vossmeyer, T. Vapor sorption and electrical response of Au-nanoparticle-dendrimer composites. Adv. Funct. Mater. 2007, 17, 881–888. [Google Scholar]
- Sahner, K.; Tuller, H.L. Novel deposition techniques for metal oxides: prospects for gas sensing. J. Electroceram. 2009. In press. [Google Scholar] [CrossRef]
- Mädler, L.; Sahm, T.; Gurlo, A.; Barsan, N.; Grunwald, J.D.; Weimar, U.; Pratsinis, S.E. Sensing low concentrations of CO using flame-spray-made Pt/SnO2 nanoparticles. J. Nanoparticle Res. 2006, 8, 783–796. [Google Scholar]
- Sahm, T.; Rong, W.; Barsan, N.; Mädler, L.; Weimar, U. Sensing of CH4, CO and ethanol with in situ nanoparticle aerosol-fabricated multilayer sensors. Sens. Actuat. B-Chem. 2007, 127, 63–68. [Google Scholar]
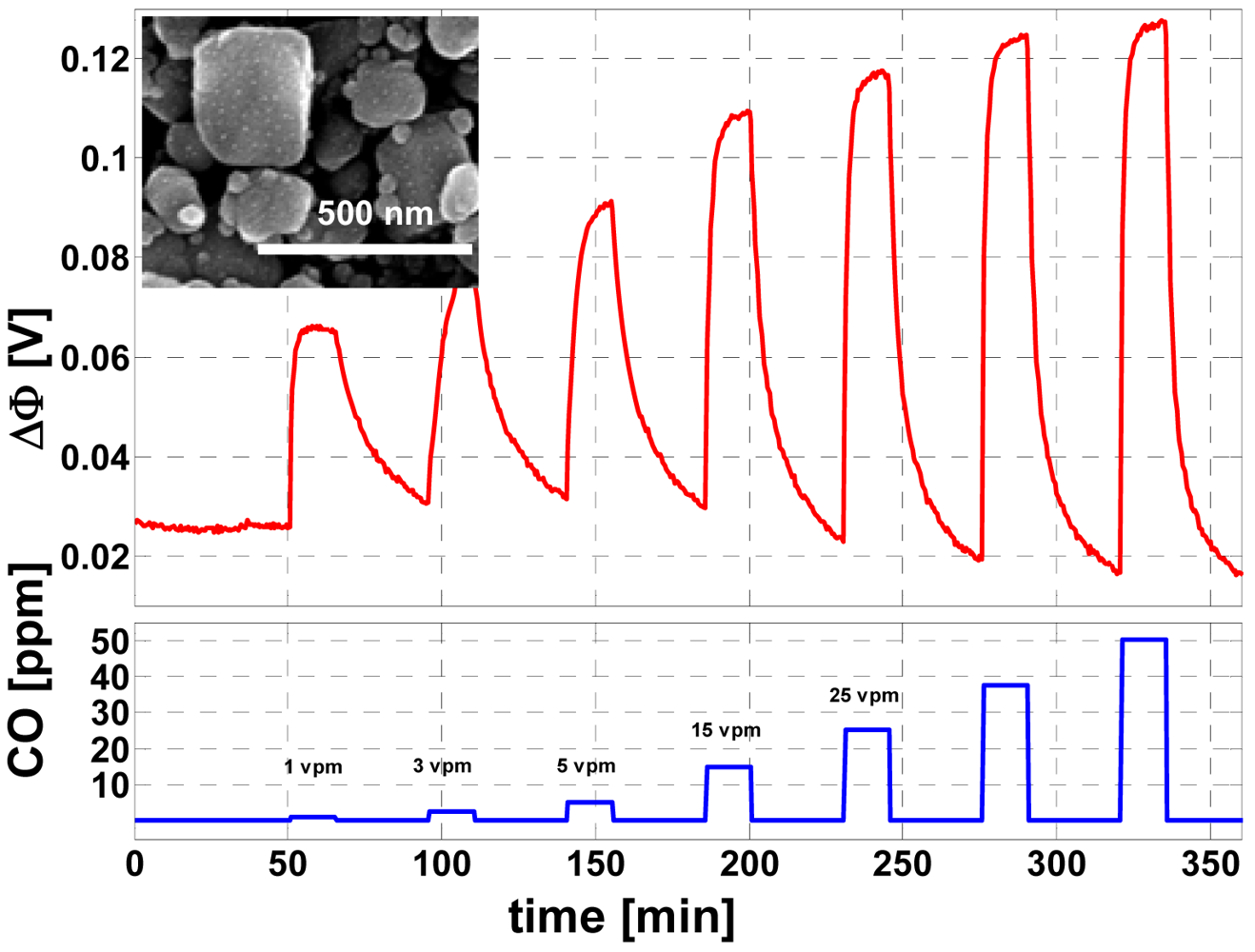
- Sahner, K.; Kaspar, M.; Moos, R. Assessment of the aerosol deposition method for preparing metal oxide gas sensors at room temperature. Sens. Actuat. B-Chem. 2009, 139, 394–399. [Google Scholar]
- Akedo, J.; Lebedev, M. Microstructure and electrical properties of lead zirconate titanate (Pb(Zr0.52Ti0.48)O3) thick film deposited with aerosol deposition method. Jpn. J. Appl. Phys. 1999, 38, 5397–5401. [Google Scholar]
- Koplin, T.J.; Siemons, M.; Océn-Valéntin, C.; Sanders, D.; Simon, U. Workflow for high throughput screening of gas sensing materials. Sensors 2006, 6, 298–307. [Google Scholar]
- Frenzer, G.; Frantzen, A.; Sanders, D.; Simon, U.; Maier, W.F. Wet chemical synthesis and screening of thick porous oxide films for resistive gas sensing applications. Sensors 2006, 6, 1568–1586. [Google Scholar]
- Siemons, M.; Leifert, A.; Simon, U. Preparation and gas sensing characteristics of nanoparticulate p-type semiconducting LnFeO3 and LnCrO3 materials. Adv. Funct. Mater. 2007, 17, 2189–2197. [Google Scholar]
- Siemons, M.; Koplin, T.J.; Simon, U. Advances in high throughput screening of gas sensing materials. Appl. Surf. Sci. 2007, 254, 669–676. [Google Scholar]
- Lundtström, I.; Shivaraman, M.S.; Svenson, C.M. Hydrogen-sensitive Pd-gate MOS-transistor. J. Appl. Phys. 1975, 46, 3876–3881. [Google Scholar]
- Zhang, T.H.; Petelenz, D.; Janata, J. Temperature-controlled Kelvin microprobe. Sens. Actuat. B-Chem. 1993, 12, 175–180. [Google Scholar]
- Lorenz, H.; Perschke, M.; Riess, H.; Janata, J.; Eisele, I. New suspended gate FET technology, for physical deposition of chemically sensitive layers. Sens. Actuat. A-Phys. 1990, 23, 1023–1026. [Google Scholar]
- Flietner, B.; Doll, T.; Eisele, I. Reliable hybrid GasFETs for work-function measurements with arbitrary materials. Sens. Actuat. B-Chem. 1994, 22, 109–113. [Google Scholar]
- Gergintschew, Z.; Kornetzky, P.; Schipanski, D. The capacitively controlled field effect transistor (CCFET) as a new low power gas sensor. Sens. Actuat. B-Chem. 1996, 35-36, 285–289. [Google Scholar]
- Pohle, R.; Simon, E.; Fleischer, M.; Meixner, H.; Frerichs, H.-P.; Lehmann, M.; Verhoeven, H. Realisation of a new sensor concept: improved CCFET and SGFET type gas sensors in hybrid flip-chip technology. Actuators and Microsystems, Proceedings of the 12th International Conference on Solid-State Sensors, Actuators and Microsystems, TRANSDUCERS 2003, June 2003; pp. 135–138.
- Fleischer, M.; Ostrick, B.; Pohle, R.; Simon, E.; Meixner, H.; Bilger, C.; Daeche, F. Low-power gas sensors based on work function measurement in low-cost hybrid flip-chip technology. Sens. Actuat. B-Chem. 2001, 80, 169–173. [Google Scholar]
- Schneider, R.; Fleischer, M.; Lampe, U.; Pohle, R.; Simon, E. Integrated sensor array based on field effect transistors for the detection of different gas components. Proceedings of Eurosensors, Barcelona, Spain, September 2005.
- Ostrick, B.; Pohle, R.; Fleischer, M.; Meixner, H. TiN in Workfunction Type Sensors: A stable ammonia sensitive material for room temperature operation with low humidity cross sensitivity. Sens. Actuat. B-Chem. 2000, 68, 234–239. [Google Scholar]
- Oprea, A.; Simon, E.; Fleischer, M.; Lehmann, M.; Frerichs, H.-P.; Weimar, U. Flip-chip suspended gate field effect transistor for ammonia detection. Sens. Actuat. B-Chem. 2005, 111-112, 582–586. [Google Scholar]
- Eisele, I.; Doll, T.; Burgmair, M. Low power gas detection with FET sensors. Sens. Actuat. B-Chem. 2001, 78, 19–25. [Google Scholar]
- Scharnagl, K.; Eriksson, M.; Karthigeyan, A.; Burgmair, M.; Zimmer, M.; Eisele, I. Hydrogen detection at high concentrations with stabilised palladium. Sens. Actuat. B-Chem. 2001, 78, 138–143. [Google Scholar]
- Senft, C.; Galonska, T.; Widanarto, W.; Frerichs, H-P.; Wilbertz, C.; Eisele, I. Stability of FET - based hydrogen sensors at high temperatures. Proceedings of IEEE Sensors 2007 Conference, Atlanta, Georgia, October 2007; pp. 189–192. [CrossRef]
- Zimmer, M.; Burgmair, M.; Scharnagl, K.; Karthigeyan, A.; Doll, T.; Eisele, I. Gold and platinum as ozone sensitive layer in work-function gas sensors. Sens. Actuat. B-Chem. 2001, 80, 174–178. [Google Scholar]
- Sulima, T.; Knittel, T.; Freitag, G.; Widanarto, W.; Eisele, I. A gas FET for chlorine detection. Proceedings of IEEE Sensors 2005 Conference, Irvine, California, October–November 2005. [CrossRef]
- Stegmeier, S. Nanotechnologische Schichten zur Detektion von flüchtigen Kohlenwasserstoffen mit SGFET- Gassensoren. Bachelor Thesis, University of Applied Science, Munich, 2007. [Google Scholar]
- Stegmeier, S.; Hauptmann, P.; Fleischer, M. Room temperature detection of VOCs with activated platinum and supported platinum sensing layers by the change of work function. Proceedings of Eurosensors 2008, Dresden, Germany, September 2008.
- Kiss, G.; Josepovits, V.K.; Kovacs, K.; Ostrick, B.; Fleischer, M.; Meixner, H.; Reti, F. CO Sensitivity of the PtO/SnO2 and PdO/SnO2 layer structures. Kelvin probe and XPS analysis. Proceedings of Eurosensors XVI, Prague, September 2002.
- Lampe, U.; Simon, E.; Pohle, R.; Fleischer, M.; Meixner, H.; Frerichs, H.-P.; Lehmann, M.; Kiss, G. GasFET for the detection of reducing gases. Sens. Actuat. B-Chem. 2005, 111-112, 106–110. [Google Scholar]
- Ostrick, B.; Mühlsteff, J.; Fleischer, M.; Meixner, H.; Doll, T.; Kohl, C.-D. Adsorbed water as key to room temperature gas-sensitive reactions in work function type gas sensors: the carbonate carbon dioxide system. Sens. Actuat. B-Chem. 1999, 57, 115–119. [Google Scholar]
- Ostrick, B.; Fleischer, M.; Meixner, H.; Kohl, C.-D. Investigation of the reaction mechanisms, in work function type gas sensors at room temperature by studies of the cross sensitivity to oxygen and water: the carbonate-carbon dioxide system. Sens. Actuat. B-Chem. 2000, 68, 197–202. [Google Scholar]
- Ostrick, B.; Fleischer, M.; Meixner, H. The influence of interfaces and interlayers on the gas sensitivity in work function type sensors. Sens. Actuat. B-Chem. 2003, 95, 271–274. [Google Scholar]
- Simon, E.; Lampe, U.; Pohle, R.; Fleischer, M.; Meixner, H.; Frerichs, H.-P.; Lehmann, M.; Verhoeven, H. Novel carbon dioxide gas sensors based on field effect transistors. Proceedings of the 12th Transducers 2003, Boston, USA, June 2003.
- Stegmaier, S.; Fleischer, M.; Hauptmann, P. Detection of VOC with activated Pt and supported Pt sensing layers by the change of work function at room temperature. Proceedings of Eurosensors 2009, Lausanne, Switzerland, September 2009.
- Simon, E; Fleischer, M.; Meixner, H. Polyvinylpyrrolidone, a new material for humidity sensors using workfunction readout. Proceedings of the 8th International Meeting on Chemical Sensors, Basel, Switzerland, July 2000.
- Jones, S.L.; Kittelson, J.; Cowan, J.O.; Flannery, E.M.; Hancox, R.J.; Mclachlan, C.R.; Taylor, D.R. The predictive value of exhaled nitric oxide measurements in assessing changes in asthma control. Am. J. Respir. Crit. Care Med. 2001, 164, 738–43. [Google Scholar]
- Simon, E.; Fleischer, M.; Meixner, H. Porhin-dyes-high potential NO2-layers for asthma detection in breath in workfunction type gas sensors. Proceedings of Eurosensors XVI, Prague, September 2002.
- Fleischer, M.; Simon, E.; Rumpel, E.; Ulmer, H.; Harbeck, M.; Wandel, M.; Fietzek, C.; Weimar, U.; Meixner, H. Detection of volatile compounds correlated to human diseases through breath analysis with chemical sensors. Sens. Actuat. B-Chem. 2002, 83, 245–249. [Google Scholar]
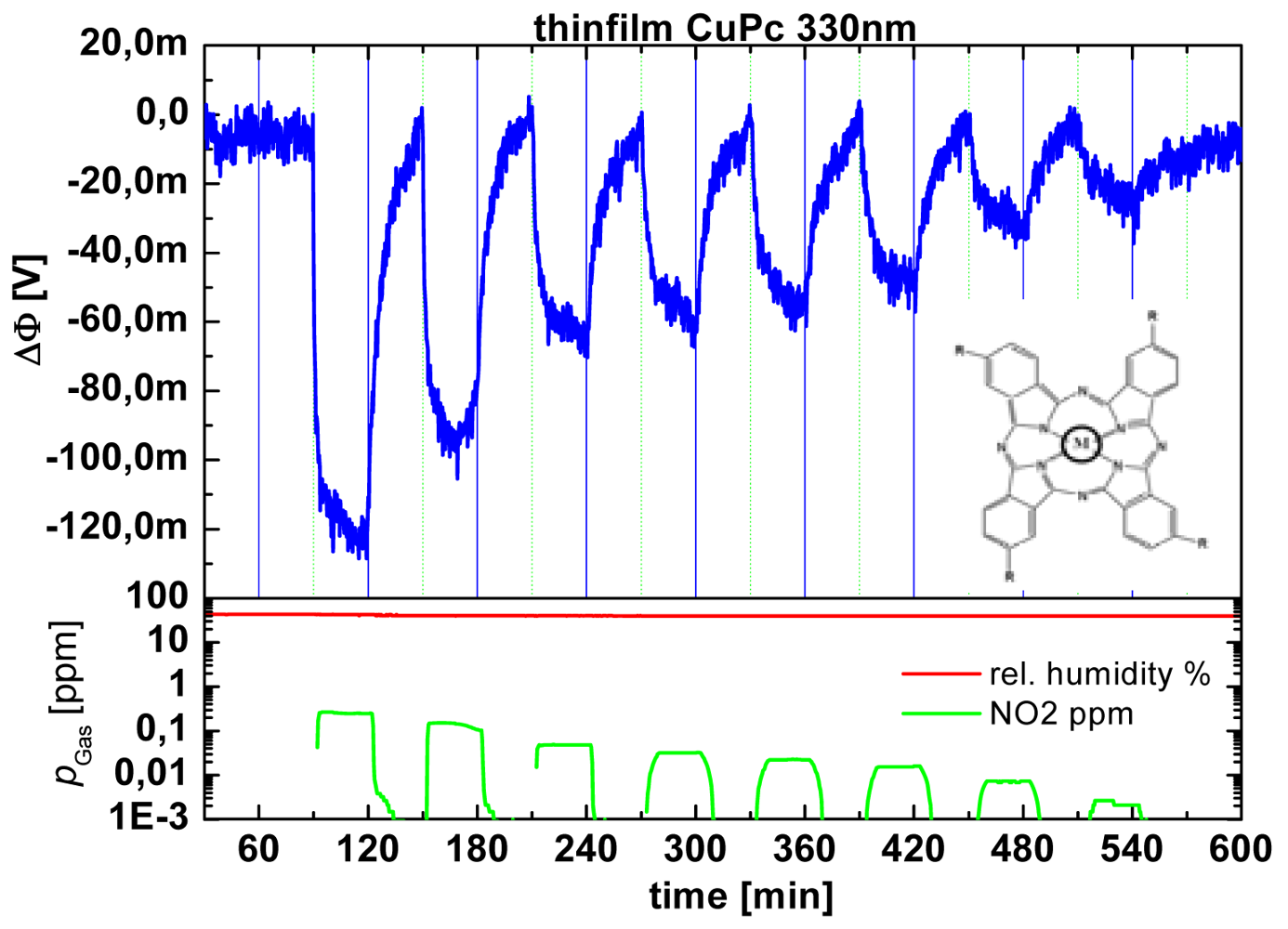
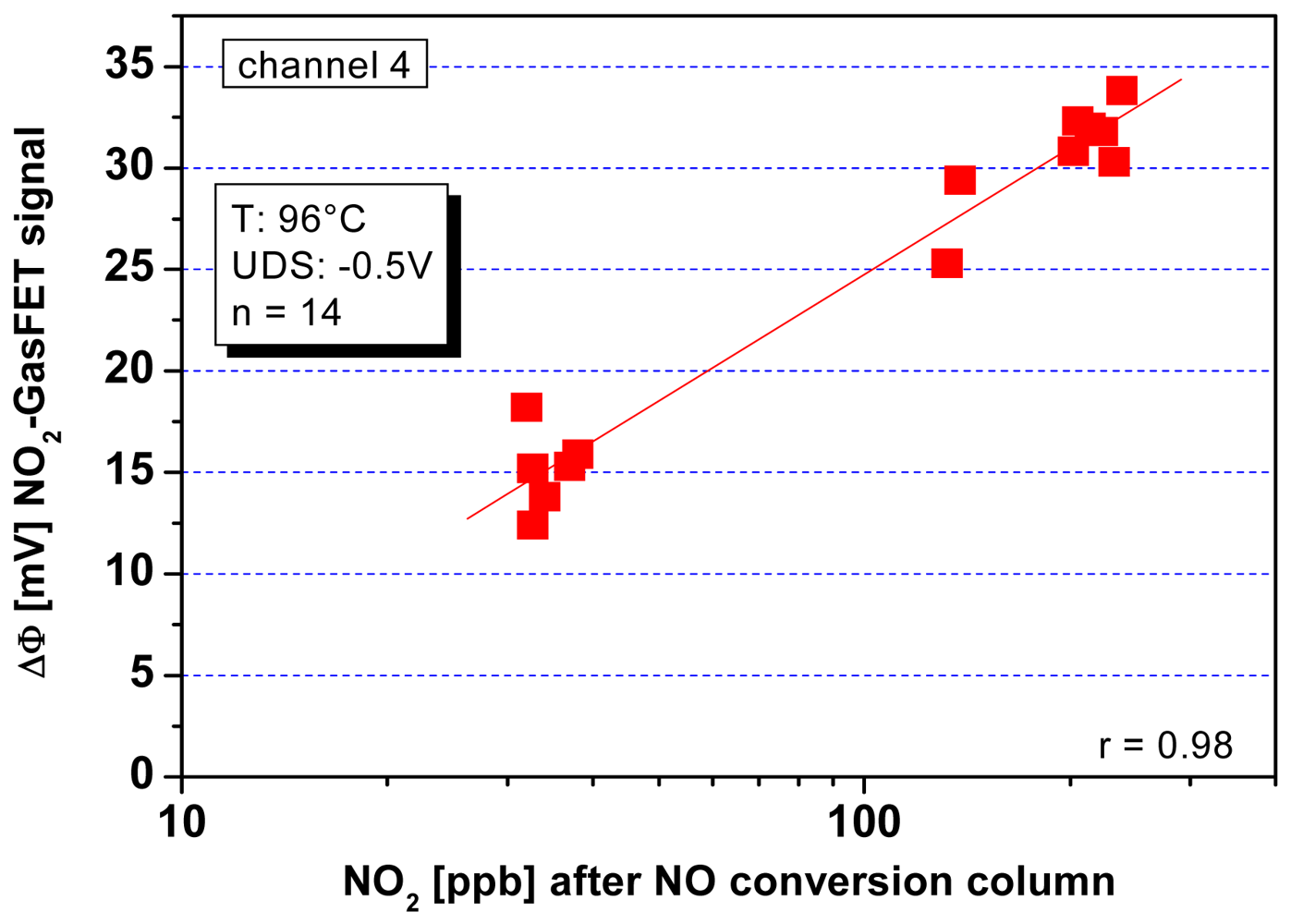
- Weimar, U.; Simon, E.; Fleischer, M.; Frerichs, H.-P.; Wilbertz, C.; Lehmann, M. Copper phthalocyanine suspended gate field effect transistors for NO2 detection. Sens. Actuat. B-Chem. 2006, 118, 249–254. [Google Scholar]
- Waitz, T.; Wagner, T.; Sauerwald, T.; Kohl, C.-D.; Tiemann, M. Ordered mesoporous In2O3: synthesis by structure replication and application as a methane gas sensor. Adv. Funct. Mater. 2009, 19, 653–661. [Google Scholar]
- Iskra, P.; Senft, C.; Kulaga-Egger, D.; Sulima, T.; Eisele, I. A concept for a GasFET for high temperature operation. Proceedings of IEEE Sensors 2008 Conference, Lecce, Italy, October 2008; pp. 1301–1304. [CrossRef]






© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moos, R.; Sahner, K.; Fleischer, M.; Guth, U.; Barsan, N.; Weimar, U. Solid State Gas Sensor Research in Germany – a Status Report. Sensors 2009, 9, 4323-4365. https://doi.org/10.3390/s90604323
Moos R, Sahner K, Fleischer M, Guth U, Barsan N, Weimar U. Solid State Gas Sensor Research in Germany – a Status Report. Sensors. 2009; 9(6):4323-4365. https://doi.org/10.3390/s90604323
Chicago/Turabian StyleMoos, Ralf, Kathy Sahner, Maximilian Fleischer, Ulrich Guth, Nicolae Barsan, and Udo Weimar. 2009. "Solid State Gas Sensor Research in Germany – a Status Report" Sensors 9, no. 6: 4323-4365. https://doi.org/10.3390/s90604323
APA StyleMoos, R., Sahner, K., Fleischer, M., Guth, U., Barsan, N., & Weimar, U. (2009). Solid State Gas Sensor Research in Germany – a Status Report. Sensors, 9(6), 4323-4365. https://doi.org/10.3390/s90604323




