Magneto-Optical Relaxation Measurements of Functionalized Nanoparticles as a Novel Biosensor
Abstract
:1. Introduction
2. Experimental Section
2.1. Magnetic Nanoparticles
2.2. Magneto-optical Relaxation Measurements of Ferrofluids
2.2.1. Measurement Setup
2.2.2. Monitoring Antibody-Antigen Interactions
2.3. Kinetic Model for Immunometric Analyses with MORFF
3. Results and Discussion
3.1. Characterization of DDM128N
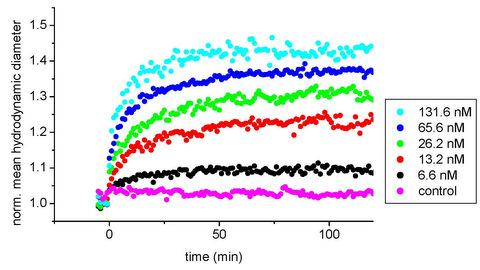
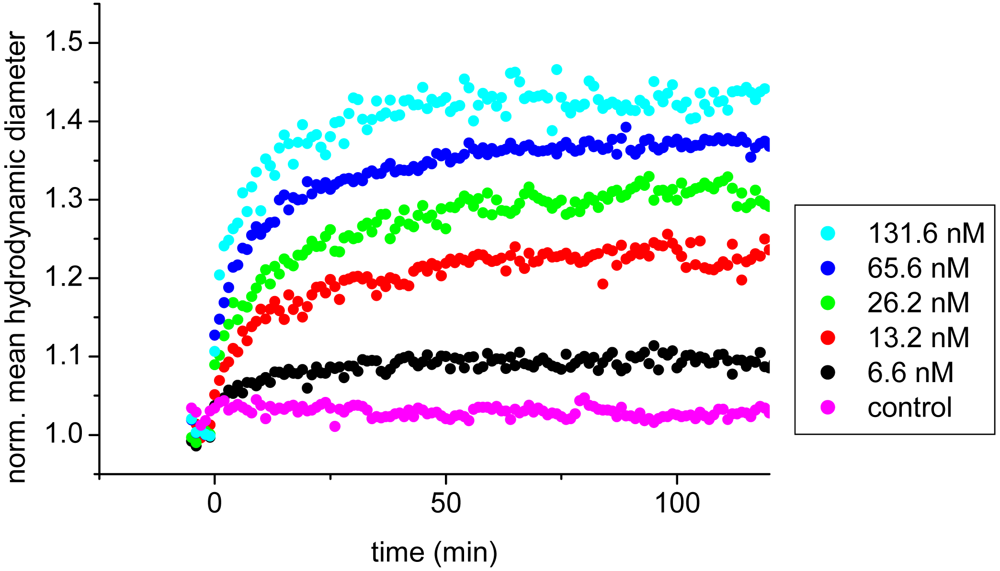
3.2. IGF-1/anti-IGF-1 Binding Assay
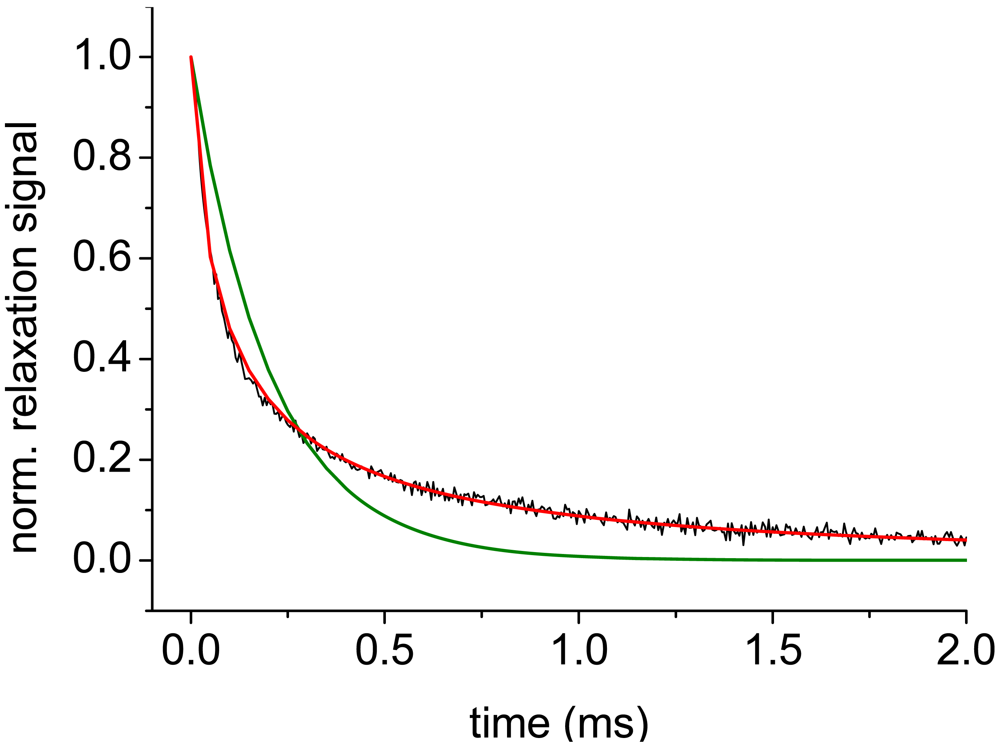
3.3. Kinetic Aspects of the IGF-1/Anti-IGF-1 Interaction
3.4. Method Validation
4. Conclusions
References
- Leuvering, J.H.; Thal, P.J.; van der Waart, M.; Schuurs, A.H. Sol particle immunoassay (SPIA). J. Immun. 1980, 1, 77–91. [Google Scholar]
- Dungchai, W.; Siangproh, W.; Lin, J.M.; Chailapakul, O.; Lin, S.; Ying, X. Development of a sensitive micro-magnetic chemiluminescence enzyme immunoassay for the determination of carcinoembryonic antigen. Anal Bioanal. Chem. 2007, 387, 1965–1971. [Google Scholar]
- Choi, J.W.; Oh, K.W.; Thomas, J.H.; Heineman, W.R.; Halsall, H.B.; Nevin, J.H.; Helmicki, A.J.; Henderson, H.T.; Ahn, C.H. An integrated microfluidic biochemical detection system for protein analysis with magnetic bead-based sampling capabilities. Lab Chip. 2002, 2, 27–30. [Google Scholar]
- Enpuku, K.; Soejima, K.; Nishimoto, T.; Kuma, H.; Hamasaki, N.; Tsukamoto, A.; Saitoh, K.; Kandori, A. Quantitative evaluation of magnetic immunoassay with remanence measurement. Supercond. Sci. Technol. 2006, 19, S257–S260. [Google Scholar]
- Weitschies, W.; Kötitz, R.; Bunte, T.; Trahms, L. Determination of relaxing or remanent nanoparticle magnetization provides a novel binding-specific technique for the evaluation of immunoassays. Pharm. Pharmacol. Lett. 1997, 7, 5–8. [Google Scholar]
- Ludwig, F.; Heim, E.; Schilling, M.; Enpuku, K. Characterization of superparamagnetic Fe3O4 nanoparticles by fluxgate magnetorelaxometry for use in biomedical applications. J. Appl. Phys. 2008, 103, 07A314. [Google Scholar]
- Astalan, A.P.; Ahrentorp, F.; Johansson, C.; Larsson, K.; Krozer, A. Biomolecular reactions studied using changes in Brownian rotation dynamics of magnetic particles. Biosens. Bioelectron. 2004, 19, 945–951. [Google Scholar]
- Tamanaha, C.R.; Mulvaney, S.P.; Rife, J.C.; Whitman, L.J. Magnetic labeling, detection, and system integration. Biosens. Bioelectron. 2008, 24, 1–13. [Google Scholar]
- Le Roith, D.; Bondy, C.; Yakar, S.; Liu, J.L.; Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar]
- Jerome, L.; Shiry, L.; Leyland-Jones, B. Deregulation of the IGF axis in cancer: epidemiological evidence and potential therapeutic interventions. Endocr. Relat. Cancer 2003, 10, 561–578. [Google Scholar]
- Smith, G.D.; Gunnell, D.; Holly, J. Cancer and insulin-like growth factor-I. A potential mechanism linking the environment with cancer risk. BMJ 2000, 321, 847–848. [Google Scholar]
- Bidlingmaier, M. Pitfalls of insulin-like growth factor I assays. Horm Res. 2009, 71, 30–33. [Google Scholar]
- Romanus, E.; Gross, C.; Kötitz, R.; Prass, S.; Lange, J.; Weber, P.; Weitschies, W. Monitoring of biological binding reactions by magneto-optical relaxation measurements. Magnetohydrodynamics 2000, 2, 328–333. [Google Scholar]
- Aurich, K.; Nagel, S.; Heister, E.; Weitschies, W. Affinity analysis for biomolecular interactions based on magneto-optical relaxation measurements. Nanotechnology 2008, 19, 505102. [Google Scholar]
- Cotton, A.; Mouton, H. New optical property (magnetic birefringence) of certain noncolloidal organic liquids. CR Hebd Seances Acad. Sci. Paris 1907, 145, 229–230. [Google Scholar]
- Büscher, K.; Helm, C.A.; Gross, C.; Glöckl, G.; Romanus, E.; Weitschies, W. Nanoparticle composition of a ferrofluid and its effects on the magnetic properties. Langmuir. 2004, 20, 2435–2444. [Google Scholar]
- Randolph, T.W.; Jones, L.S. Surfactant-protein interactions. In Rational design of stable protein formulations-theory and practice; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 159–175. [Google Scholar]
- Leckband, D.E.; Schmitt, F.J.; Israelachvili, J.N.; Knoll, W. Direct force measurements of specific and nonspecific protein interactions. Biochemistry 1994, 33, 4611–4624. [Google Scholar]
- Jaenicke, R. Stability and stabilization of globular proteins in solution. J. Biotechnol. 2000, 79, 193–203. [Google Scholar]
- de Wildt, R.M.; Mundy, C.R.; Gorick, B.D.; Tomlinson, I.M. Antibody arrays for high-throughput screening of antibody-antigen interactions. Nat. Biotechnol. 2000, 18, 989–994. [Google Scholar]
- Chantrell, R.W.; Bradbury, A.; Popplewell, J.; Charles, S.W. Agglomerate formation in a magnetic fluid. J. Appl. Phys. 1982, 53, 2742–2744. [Google Scholar]
- Klokkenburg, M.; Erné, B.H. Comparison of reversible and irreversible dipolar assemblies in a ferrofluid. J. Magn. Magn. Mater. 2006, 306, 85–91. [Google Scholar]
- Manes, S.; Kremer, L.; Albar, J.P.; Mark, C.; Llopis, R.; Martinez, C. Functional epitope mapping of insulin-like growth factor I (IGF-I) by anti-IGF-I monoclonal antibodies. Endocrinology 1997, 138, 905–915. [Google Scholar]
- Gajanandana, O.; Irvine, K.; Grant, P.A.; Francis, G.L.; Knowles, S.E.; Wrin, J.; Wallace, J.C.; Owens, P.C. Measurement of an analog of insulin-like growth factor-I in blood plasma using a novel enzyme-linked immunosorbent assay. J. Endocrinol. 1998, 156, 407–414. [Google Scholar]
- Day, Y.S.; Baird, C.L.; Rich, R.L.; Myszka, D.G. Direct comparison of binding equilibrium, thermodynamic, and rate constants determined by surface- and solution-based biophysical methods. Protein Sci. 2002, 11, 1017–1025. [Google Scholar]
- Myszka, D.G. Improving biosensor analysis. J. Mol. Recognit. 1999, 12, 279–284. [Google Scholar]
- Guidance for Industry - Bioanalytical Method Validation; U.S. Department of Health and Human Services - Food and Drug Administration: Washington, DC, USA, May 2001; pp. 1–25.
- Chemla, Y.R.; Grossman, H.L.; Poon, Y.; McDermott, R.; Stevens, R.; Alper, M.D.; Clarke, J. Ultrasensitive magnetic biosensor for homogeneous immunoassay. Proc Natl Acad Sci USA 2000, 97, 14268–14272. [Google Scholar]
- Enpuku, K.; Minotani, T.; Gima, T.; Kuroki, Y.; Itoh, Y.; Yamashita, M.; Katakura, Y.; Kuhara, S. Detection of magnetic nanoparticles with superSconducting quantum interference device (SQUID) magnetometer and application to immunoassays. Jpn. J. Appl. Phys. 1999, 38, L1102–L1105. [Google Scholar]
- Clayton, P.E.; Hall, C.M. Insulin-like growth factor I levels in healthy children. Horm Res. 2004, 62, 2–7. [Google Scholar]
- Landin-Wilhelmsen, K.; Lundberg, P.A.; Lappas, G.; Wilhelmsen, L. Insulin-like growth factor I levels in healthy adults. Horm Res. 2004, 62, 8–16. [Google Scholar]
- Xu, H.; Ma, X.; Fan, X.; Zeng, L. Plasma level of IGF-1 in type 2 diabetic patients with neuropathy. Guangdong Yixue 2001, 22, 298–300. [Google Scholar]
- Rockich, K.T.; Hatton, J.C.; Kryscio, R.J.; Young, B.A.; Blouin, R.A. Effect of recombinant human growth hormone and insulin-like growth factor-1 administration on IGF-1 and IGF-binding protein-3 levels in brain injury. Pharmacotherapy 1999, 19, 1432–1436. [Google Scholar]
- Shi, R.; Huang, Y.; Wang, D.; Zhao, M.; Li, Y. Expression and characterization of insulin growth factor-1-enhanced green flourescent protein fused protein as a tracer for immunoassay. Anal. Chim. Acta 2006, 578, 131–136. [Google Scholar]
- Nelson, R.W.; Nedelkov, D.; Tubbs, K.A.; Kiernan, U.A. Quantitative mass spectrometric immunoassay of insulin like growth factor 1. J. Proteome Res. 2004, 3, 851–855. [Google Scholar]



| Physiological concentration | Pathological concentration | LOD of MORFF |
|---|---|---|
| Children (before puberty): 130 – 485 ng/mL [30] | 100 – 150 μg/mL Type 2 diabetes [32] | 24.2 ng/mL |
| Adults: 95 – 250 ng/mL [31] | 1 – 3 μg/mL traumatic brain injuries [33] |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aurich, K.; Glöckl, G.; Nagel, S.; Weitschies, W. Magneto-Optical Relaxation Measurements of Functionalized Nanoparticles as a Novel Biosensor. Sensors 2009, 9, 4022-4033. https://doi.org/10.3390/s90604022
Aurich K, Glöckl G, Nagel S, Weitschies W. Magneto-Optical Relaxation Measurements of Functionalized Nanoparticles as a Novel Biosensor. Sensors. 2009; 9(6):4022-4033. https://doi.org/10.3390/s90604022
Chicago/Turabian StyleAurich, Konstanze, Gunnar Glöckl, Stefan Nagel, and Werner Weitschies. 2009. "Magneto-Optical Relaxation Measurements of Functionalized Nanoparticles as a Novel Biosensor" Sensors 9, no. 6: 4022-4033. https://doi.org/10.3390/s90604022