Trends in Flow-based Biosensing Systems for Pesticide Assessment
Abstract
:1. Introduction
2. Flow-based immunosensing systems
3. Flow-based enzymatic sensing systems
3.1. Principle of the enzymatic-based sensor system

3.2. Electrochemical-based systems
3.2.1. Biosensor systems based on an electrochemical detection (single amperometric detection not included)
3.2.1.1. Single potentiometric-based systems
3.2.1.2. Dual potentiometric/amperometric-based systems
3.2.1.3. Dual potentiometric/conductimetric-based systems
3.2.1.4. Single conductimetric-based systems
3.2.2. Single amperometric-based systems
3.2.2.1. Use of alternative enzyme substrates
3.2.2.2. Use of membranes
3.2.2.3. Study of the counter ion effect
3.2.2.4. Use of electron mediators
3.2.2.5. Array systems
3.2.2.6. Use of novel matrices
3.2.2.7. OPH-based systems
3.2.2.8. PH-based system
3.3. Optical and thermal-based systems
4. Conclusions
Acknowledgments
References
- Skládal, P.; Nunes, G.S.; Yamanaka, H.; Ribero, M.L. Detection of carbamate pesticides in vegetable samples using cholinesterase-based biosensors. Electroanalysis 1997, 9, 1083–1087. [Google Scholar]
- Pogačnik, L.; Franko, M. Detection of organophosphate and carbamate pesticides in vegetable samples by photothermal biosensor. Biosens. Bioelectron. 2003, 18, 1–9. [Google Scholar]
- Donarski, W.J.; Dumas, D.P.; Heitmeyer, D.P.; Lewis, V.E.; Raushel, F.M. Structure-activity relationships in the hydrolysis of substrates by the phosphotriesterase from Pseudomonas diminuta. Biochemistry 1989, 28, 4650–4655. [Google Scholar]
- Tuovinen, K.; Kaliste-Korhonen, E.; Raushel, F.M.; Hanninen, O. Phosphotriesterase-A promising candidate for use in detoxification of organophosphates. Fundam. Appl. Toxicol. 1994, 23, 578–584. [Google Scholar]
- Corrigan, F.M.; Macdonald, S.; Brown, A.; Armstrong, K.; Armstrong, E.M. Neurasthenic fatigue, chemical sensitivity and GABAa receptor toxins. Med. Hypotheses 1994, 43, 195–200. [Google Scholar]
- Ashani, Y.; Rothschild, N.; Segall, Y.; Levanon, D.; Raveh, L. Prophylaxis against organophosphate poisoning by an enzyme hydrolysing organophosphorus compounds in mice. Life Sci. 1991, 49, 367–374. [Google Scholar]
- http://ecoport.org/Resources/Refs/Pesticid/Code/PM_Code.htm
- http://ecoport.org/Resources/Refs/Pesticid/residue.htm
- http://ec.europa.eu/environment/water/water-framework/index_en.html
- http://ec.europa.eu/food/plant/protection/index_en.htm
- http://www.epa.gov/pesticides/
- http://www.epa.gov/oppfod01/fqpa/
- http://www.cdc.gov/niosh/topics/pesticides/
- Aprea, C.; Colosio, C.; Mammone, T.; Minoia, C.; Maroni, M. Biological monitoring of pesticide exposure: a review of analytical methods. J. Chromatogr. B 2002, 769, 191–219. [Google Scholar]
- Diehl-Faxon, J.; Ghindilis, A.L.; Atanasov, P.; Wilkins, E. Direct electron transfer based tri-enzyme electrode for monitoring of organophosphorus pesticides. Sens. Actuat. B 1996, 36, 448–457. [Google Scholar]
- Di Corcia, A.; Marchetti, M. Multiresidue method for pesticides in drinking water using a graphitized carbon black cartridge extraction and liquid chromatographic analysis. Anal. Chem. 1991, 63, 580–585. [Google Scholar]
- Mulchandani, A.; Chen, W.; Mulchandani, P.; Wang, J.; Rogers, K.R. Biosensors for direct determination of organophosphate pesticides. Biosens. Bioelectron. 2001, 16, 225–230. [Google Scholar]
- Rekha, K.; Thakur, M. S.; Karanth, N.G. Biosensors for the detection of organo-phosphorous pesticides. Crit. Rev. Biotechnol. 2000, 20, 213–235. [Google Scholar]
- Schmid, R.D.; Künnecke, W. Flow injection analysis (FIA) based on enzymes or antibodies – applications in the life sciences. J. Biotechnol. 1990, 14, 3–31. [Google Scholar]
- Schmidt, H.-L. Biosensors and flow injection analysis in bioprocess control. J. Biotechnol. 1993, 31, v–vi. [Google Scholar]
- Gorton, L.; Csöregi, E.; Domínguez, E.; Emnéus, J.; Jönsson-Pettersson, G.; Marko-Varga, G.; Persson, B. Selective detection in flow analysis based on the combination of immobilized enzymes and chemically modified electrodes. Anal. Chim. Acta 1991, 250, 203–248. [Google Scholar]
- Tran-Minh, C. Biosensors in flow-injection systems for biomedical analysis, process and environmental monitoring. J. Molec. Recogn. 1996, 9, 658–663. [Google Scholar]
- Marco, M.-P.; Gee, S.; Hammock, B.D. Immunochemical techniques for environmental analysis. I. Immunosensors. Trends Anal. Chem. 1995, 14, 341–350. [Google Scholar]
- Hock, B.; Dankwardt, A.; Kramer, K.; Marx, A. Immunochemical techniques: Antibody production for pesticide analysis. A review. Anal. Chim. Acta 1995, 311, 393–405. [Google Scholar]
- González-Martínez, M.A.; Puchades, R.; Maquieira, A. On-line immunoanalysis for environmental pollutants: from batch assays to automated sensors. Trends Anal. Chem. 1999, 18, 204–218. [Google Scholar]
- Mallat, E.; Barzen, C.; Klotz, A.; Brecht, A.; Gauglitz, G.; Barceló, D. River analyzer for chlorotriazines with a direct optical immunosensor. Environ. Sci. Technol. 1999, 33, 965–971. [Google Scholar]
- González-Martínez, M.A.; Morais, S.; Puchades, R.; Maquieira, A.; Abad, A.; Montoya, A. Monoclonal antibody-based flow-through immunosensor for analysis of carbaryl. Anal. Chem. 1997, 69, 2812–2818. [Google Scholar]
- Gascón, J.; Oubiña, A.; Ballesteros, B.; Barceló, D.; Camps, F.; Marco, M.-P.; González-Martínez, M.A.; Morais, S.; Puchades, R.; Maquieira, A. Development of a highly sensitive enzyme-linked immunoassay for atrazine. Performance evaluation by flow injection immunoassay. Anal. Chem. 1997, 69, 2812–2818. [Google Scholar]
- González-Martínez, M.A.; Penalva, J.; Puchades, R.; Maquieira, A.; Ballesteros, B.; Marco, M.-P.; Barceló, D. An immunosensor for the automatic determination of the antifouling agent Irgarol 1051 in natural waters. Environ. Sci. Technol. 1998, 32, 3442–3447. [Google Scholar]
- Bauer, C.G.; Eremenko, A.V.; Ehrentreich-Foŏrster, E.; Bier, F.F.; Makower, A.; Halsall, H.B.; Heineman, W.R.; Scheller, F.W. Zeptomole-detecting biosensor for alkaline phosphatase in an electrochemical immunoassay for 2,4-dichlorophenoxyacetic acid. Anal. Chem. 1996, 68, 2453–2458. [Google Scholar]
- Aldridge W.N. Some properties of specific cholinesterase with particular reference to the mechanism of inhibition by diethyl p-nitrophenyl thiophosphate (E 605) and analogues. Biochem. J. 1950, 46, 441–460.
- Andres, R.T.; Narayanaswamy, R. Fibre-optic pesticide biosensor based on covalently immobilized acetylcholinesterase and thymol blue. Talanta 1997, 44, 1335–1352. [Google Scholar]
- Xavier, M.P.; Vallejo, B.; Marazuela, M.D.; Moreno-Bondi, M.C.; Baldini, F.; Falai, A. Fiber optic monitoring of carbamate pesticides using porous glass with covalently bound chlorophenol red. Biosens. Bioelectron. 2000, 14, 895–905. [Google Scholar]
- Navas Díaz, A.; Ramos Peinado, M.C. Sol-gel cholinesterase biosensor for organophosphonus pesticide fluorimetric analysis. Sens. Actuat. B 1997, 38-39, 426–431. [Google Scholar]
- Doong, R.-A.; Tsai, H.-C. Immobilization and characterization of sol–gel-encapsulated acetylcholinesterase fiber-optic biosensor. Anal. Chim. Acta 2001, 434, 239–246. [Google Scholar]
- Lee, H.-S.; Kim, Y.A.; Cho, Y.A.; Lee, Y.T. Oxidation of organophosphorus pesticides for the sensitive detection by a cholinesterase-based biosensor. Chemosphere 2002, 46, 571–576. [Google Scholar]
- Simonian, A.L.; Rainina, E.I.; Wild, J.R. A new approach for discriminative detection fo organophosphate neurotoxins in the presence of other cholinesterase inhibitors. Anal. Lett. 1997, 30, 2453–2468. [Google Scholar]
- Evtugyn, G.A.; Ivanov, A.N.; Gogol, E.V.; Marty, J.-L.; Budnikov, H.C. Amperometric flow-through biosensor for the determination of cholinesterase inhibitors. Anal. Chim. Acta 1999, 385, 13–21. [Google Scholar]
- Khayyami, M.; Pérez Pita, M.T.; Peña Garcia, N.; Johansson, G.; Danielsson, B.; Larsson, P.-O. Development of an amperometric biosensor based on acetylcholine esterase covalently bound to a new support material. Talanta 1998, 45, 557–563. [Google Scholar]
- Neufeld, T.; Eshkenazi, I.; Cohen, E.; Rishpon, J. A micro flow injection electrochemical biosensor for organophosphorus pesticides. Biosens. Bioelectron. 2000, 15, 323–329. [Google Scholar]
- Bucur, B.; Dondoi, M.; Dănet, A.; Marty, J.-L. Insecticide identification using a flow injection analysis system with biosensors based on various cholinesterases. Anal. Chim. Acta 2005, 539, 195–201. [Google Scholar]
- La Rosa, C.; Pariente, F.; Hernández, L.; Lorenzo, E. Amperometric flow-through biosensor for the determination of pesticides. Anal. Chim. Acta 1995, 308, 129–136. [Google Scholar]
- Pariente, F.; La Rosa, C.; Galan, F.; Hernández, L.; Lorenzo, E. Enzyme support systems for biosensor applications based on gold-coated nylon meshes. Biosens. Bioelectron. 1996, 11, 1115–1128. [Google Scholar]
- Jeanty, G.; Marty, J.-L. Detection of paraoxon by continuous flow system based enzyme sensor. Biosens. Bioelectron. 1998, 13, 213–218. [Google Scholar]
- Rainina, E.I.; Efremenco, E.N.; Varfolomeyev, S.D.; Simonian, A.L.; Wild, J.R. The development of a new biosensor based on recombinant E. coli for the direct detection of organophosphorus neurotoxins. Biosens. Bioelectron. 1996, 11, 991–1000. [Google Scholar]
- Mulchandani, P.; Chen, W.; Mulchandani, A. Flow injection amperometric enzyme biosensor for direct determination of organophosphate nerve agents. Environ. Sci. Technol. 2001, 35, 2562–2565. [Google Scholar]
- Wang, J.; Krause, R.; Block, K.; Musameh, M.; Mulchandani, A.; Schöning, M.J. Flow injection amperometric detection of OP nerve agents based on an organophosphorus-hydrolase biosensor detector. Biosens. Bioelectron. 2003, 18, 255–260. [Google Scholar]
- Wang, J.; Krause, R.; Block, K.; Musameh, M.; Mulchandani, A.; Mulchandani, P.; Chen, W.; Schöning, M.J. Dual amperometric-potentiometric biosensor detection system for monitoring organophosphorus neurotoxins. Anal. Chim. Acta 2002, 469, 197–203. [Google Scholar]
- Schöning, M.J.; Krause, R.; Block, K.; Musahmeh, M.; Mulchandani, A.; Wang, J. A dual amperometric/potentiometric FIA-based biosensor for the distinctive detection of organophosphorus pesticides. Sens. Actuat. B 2003, 95, 291–296. [Google Scholar]
- Sacks, V.; Eshkenazi, I.; Neufeld, T.; Dosoretz, C.; Rishpon, J. Immobilized parathion hydrolase: An amperometric sensor for parathion. Anal. Chem. 2000, 72, 2055–2058. [Google Scholar]
- Trojanowicz, M. Determination of pesticides using electrochemical enzymatic biosensors. Electroanalysis 2002, 14, 1311–1328. [Google Scholar]
- Solé., S.; Merkoci, A.; Alegret, S. Determination of toxic substances based on enzyme inhibition. Part II. Electrochemical biosensors for the determination of pesticides using flow systems. Crit. Rev. Anal. Chem. 2003, 33, 127–143. [Google Scholar]
- Lee, H.-S.; Kim, Y.-A.; Chung, D.H.; Lee, Y.-T. Determination of carbamate pesticides by a cholinesterase-based flow injection biosensor. Int. J. Food Sci. Technol. 2001, 36, 263–270. [Google Scholar]
- Chung, M.-S.; Lee, Y.-T.; Lee, H.-S. Flow injection biosensor for the detection of anti-cholinesterases. J. Biochem. Mol. Biol. 1998, 31, 296–302. [Google Scholar]
- Kumaran, S.; Tran-Minh, C. Determination of organophosphorous and carbamate insecticides by flow injection analysis. Anal. Biochem. 1992, 200, 187–194. [Google Scholar]
- Aaron, J.-J.; Tran-Minh, C.; Colliss, J.S.; Smart, N.A. The analyses of fungicides, herbicides and insecticides. Anal. Proc. 1993, 30, 72–77. [Google Scholar]
- Ivnitskii, D.M.; Rishpon, J. A potentiometric biosensor for pesticides based on the thiocholine hexacyanoferrate (III) reaction. Biosens. Bioelectron. 1994, 9, 569–576. [Google Scholar]
- Nikolelis, D.P.; Simantiraki, M.G.; Siontorou, C.G.; Toth, K. Flow injection analysis of carbofuran in foods using air stable lipid film based acetylcholinesterase biosensor. Anal. Chim. Acta 2005, 537, 169–177. [Google Scholar]
- Suwansa-ard, S.; Kanatharana, P.; Asawatreratanakul, P.; Limsakul, C.; Wongkittisuksa, B.; Thavarungkul, P. Semi disposable reactor biosensors for detecting carbamate pesticides in water. Biosens. Bioelectron. 2005, 21, 445–454. [Google Scholar]
- Rodrigues, T.C.; Tubino, M.; Godinho, O.E.S.; de Oliveira Neto, G. An immobilized acetylcholinesterase flow-injection conductimetric system for the determination of paraoxon. Anal. Sci. 1997, 13, 423–427. [Google Scholar]
- Botrè, F.; Lorenti, G.; Mazzei, F.; Simonetti, G.; Porcelli, F.; Botrè, C.; Scibona, G. Cholinesterase based bioreactor for determination of pesticides. Sens. Actuat. B 1994, 19, 689–693. [Google Scholar]
- Pariente, F.; Hernández, L.; Lorenzo, E. 4-Aminophenyl acetate as a substrate for amperometric esterase sensors. Anal. Chim. Acta 1993, 273, 399–407. [Google Scholar]
- La Rosa, C.; Pariente, F.; Hernández, L.; Lorenzo, E. Determination of organophosphorus and carbamic pesticides with an acetylcholinesterase amperometric biosensor using 4-aminophenyl acetate as substrate. Anal. Chim. Acta 1995, 295, 273–282. [Google Scholar]
- Günther, A.; Bilitewski, U. Characterisation of inhibitors of acetylcholinesterase by an automated amperometric flow-injection system. Anal. Chim. Acta 1995, 300, 117–125. [Google Scholar]
- Rippeth, J.J.; Gibson, T.D.; Hart, J.P.; Hartley, I.C.; Nelson, G. Flow-injection detector incorporating a screen-printed disposable amperometric biosensor for monitoring organophosphate pesticides. Analyst 1997, 122, 1425–1429. [Google Scholar]
- Law, K.A.; Higson, S.P.J. Sonochemically fabricated acetylcholinesterase micro-electrode arrays within a flow injection analyzer for the determination of organophosphate pesticides. Biosens. Bioelectron. 2005, 20, 1914–1924. [Google Scholar]
- Solná, R.; Sapelnikova, S.; Skládal, P.; Winther-Nielsen, M.; Carlsson, C.; Emnéus, J.; Ruzgas, T. Multienzyme electrochemical array sensor for determination of phenols and pesticides. Talanta 2005, 65, 349–357. [Google Scholar]
- Sotiropoulou, S.; Chaniotakis, N.A. Lowering the detection limit of the acetylcholinesterase biosensor using a nanoporous carbon matrix. Anal. Chim. Acta 2005, 530, 199–204. [Google Scholar]
- Liu, G.; Riechers, S.L.; Mellen, M.C.; Lin, Y. Sensitive electrochemical detection of enzymatically generated thiocholine at carbon nanotube modified glassy carbon electrode. Electrochem. Commun. 2005, 7, 1163–1169. [Google Scholar]
- Liu, G.; Lin, Y. Biosensor based on self-assembling acetylcholinesterase on carbon nanotubes for flow injection/amperometric detection of organophosphate pesticides and nerve agents. Anal. Chem. 2006, 78, 835–843. [Google Scholar]
- Kandimalla, V.B.; Ju, H.X. Binding of acetylcholinesterase to multiwall carbon nanotube-cross-linked chitosan composite for flow-injection amperometric detection of an organophosphorous insecticide. Chem. Eur. J. 2006, 12, 1074–1080. [Google Scholar]
- Shi, M.; Xu, J.; Zhang, S.; Liu, B.; Kong, J. A mediator-free screen-printed amperometric biosensor for screening of organophosphorus pesticides with flow-injection analysis (FIA) system. Talanta 2006, 68, 1089–1095. [Google Scholar]
- Marty, J.-L.; Mionetto, N.; Lacorte, S.; Barceló, D. Validation of an enzymatic biosensor with various liquid chromatographic techniques for determining organophosphorus pesticides and carbaryl in freeze-dried waters. Anal. Chim. Acta 1995, 311, 265–271. [Google Scholar]
- Jeanty, G.; Ghommidh, Ch.; Marty, J.-L. Automated detection of chlorpyrifos and its metabolites by a continuous flow system-based enzyme sensor. Anal. Chim. Acta 2001, 436, 119–128. [Google Scholar]
- Andres, R.T.; Narayanaswamy, R. Fibre-optic pesticide biosensor based on covalently immobilized acetylcholinesterase and thymol blue. Talanta 1997, 44, 1335–1352. [Google Scholar]
- Xavier, M.P.; Vallejo, B.; Marazuela, M.D.; Moreno-Bondi, M.C.; Baldini, F.; Falai, A. Fiber optic monitoring of carbamate pesticides using porous glass with covalently bound chlorophenol red. Biosens. Bioelectron. 2000, 14, 895–905. [Google Scholar]
- Dănet, A.F.; Bucur, B.; Cheregi, M.-C.; Badea, M.; Şerban, S. Spectrophotometric determination of organophosphoric insecticides in a FIA system based on AChE inhibition. Anal. Lett. 2003, 36, 59–73. [Google Scholar]
- Shi, R.; Stein, K. Flow injection analysis of paraoxon with the use of an immobilized acetylcholinesterase reactor. Anal. Chim. Acta 1996, 324, 21–27. [Google Scholar]
- Doong, R.-A.; Tsai, H.-C. Immobilization and characterization of sol–gel-encapsulated acetylcholinesterase fiber-optic biosensor. Anal. Chim. Acta 2001, 434, 239–246. [Google Scholar]
- Ayyagari, M.S.; Kamtekar, S.; Pande, R.; Marx, K.A.; Kumar, J.; Tripathy, S.K.; Kaplan, D.L. Biosensors for pesticide detection based on alkaline phosphatase-catalyzed chemiluminescence. Mat. Sci. Eng. C 1995, 2, 191–196. [Google Scholar]
- Pogačnik, L.; Franko, M. Determination of organophosphate and carbamate pesticides in spiked samples of tap water and fruit juices by a biosensor with photothermal detection. Biosens. Bioelectron. 1999, 14, 569–578. [Google Scholar]
- Zheng, Y.-H.; Hua, T.-C.; Sun, D.-W.; Xiao, J.-J.; Xu, F.; Wang, F.-F. Detection of dichlorvos residue by flow injection calorimetric biosensor based on immobilized chicken liver esterase. J. Food Eng. 2006, 74, 24–29. [Google Scholar]

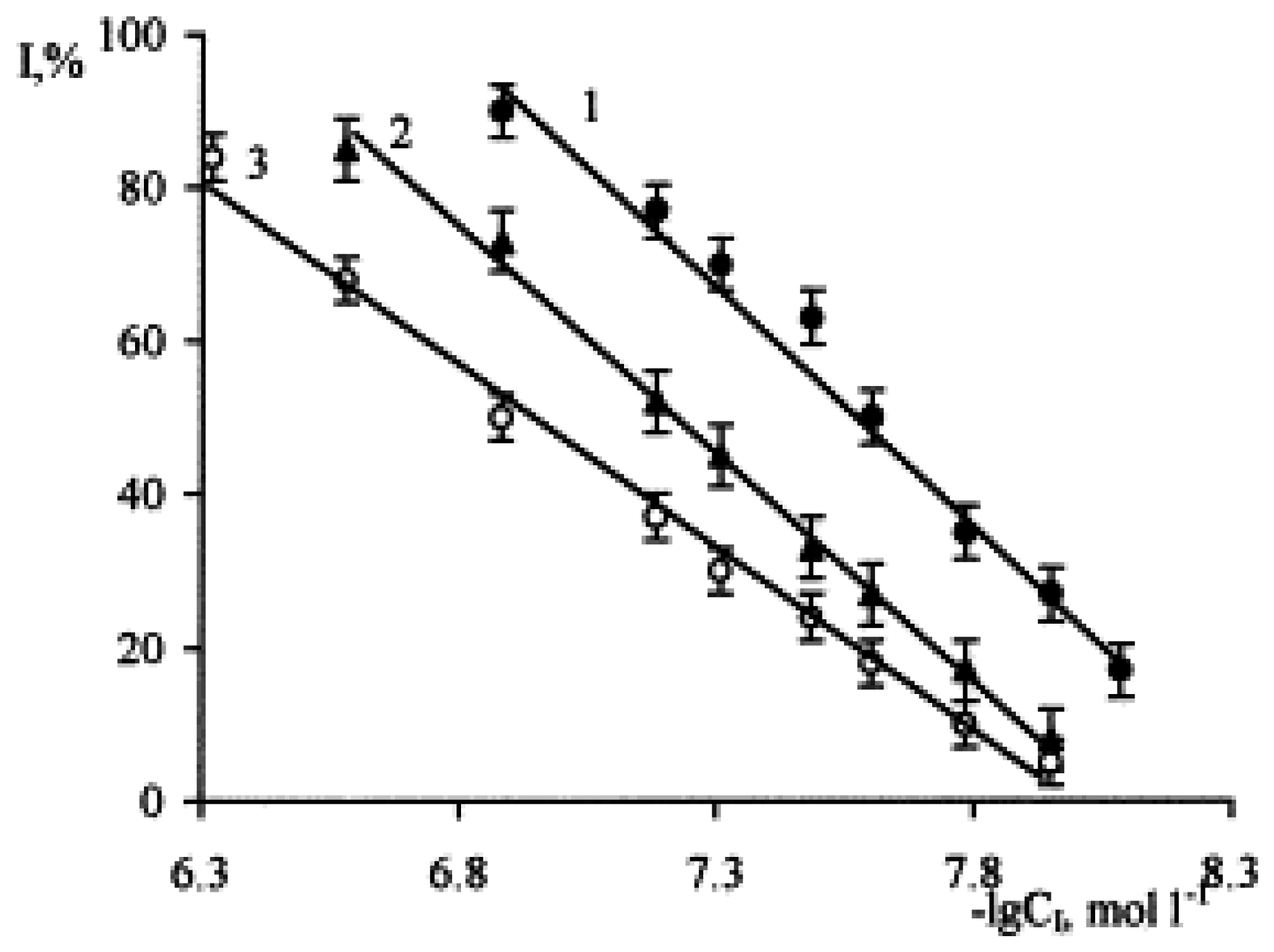

| Biosensor systems based on electrochemical detection (single amperometric detection not included) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme | Immobilisation method, support and electrode | Measurement | Pesticides | Analysis time | Working range | LOD | Inhibition time | Enzyme reactivation | RSD | Stability | Real samples | Ref. |
| AChE V-type from Electric Eel | Covalent binding on activated CPG beads (reactor), tubular H+-selective membrane electrode | Potentiometry | Diazinon Parathion-ethyl (previously oxidized with bromine) | ---- | 10-5 - 100 μM | 0.2 nM 1 nM | 30 min | 20 μM 2-PAM (20 min) and working buffer (20 min) | ---- | ---- | ---- | 36 |
| (IC10) | ||||||||||||
| AChE VI-S-type from Electric Eel | Incorporation in air stable lipid films supported on a methylacrylate polymer, glass electrode | Ion current transients | Carbofuran | ---- | 1 - 100 nM | 1 nM | 6 min | Substrate injections | ---- | 90% activity after 30 days | Fruits, vegetables and dairy products | 58 |
| OPH from recombinant E. coli | Cryoimmobilisation by PVA entrapment in a mini-reactor, glass pH micro-electrode | Potentiometry | Paraoxon | 20 min | 0.001 - 1 mM | ---- | Not needed | Not needed | 3.5% (n=10, 1 mM) | 90% activity after 60 days | ---- | 45 |
| OPH from recombinant E. coli | Cystamine-glutaraldehyde coupling on a thin-film gold electrode / Glutaraldehyde cross-linking on a pH-sensitive Ta2O5 / silane-modified EIS | Amperometry (+0.75 V vs. Ag/AgCl) / Potentiometry (constant-capacitance mode, 22 nF) | Paraoxon (amp./pot.) Parathion (amp./pot.) Dichlorvos (pot.) Diazinon (pot.) | < 1 min | 1 - 100 μM ---- 2 - 100 μM ---- | 70 nM / 2 μM ---- 6 μM ---- | Not needed | Not needed | 1.6% / 3.8% (n= 15, 100 μM paraoxon) 8% (n=15, 100 μM dichlorvos) | 100% activity after 30 days (1 or 2 cycles/day) | ---- | 48 49 |
| (s/n = 3) | ||||||||||||
| AChE VI-S-type from Electric Eel | Covalent binding on silica gel, conductivity or pH electrode | Conductimetry / Potentiometry | Carbofuran Carbaryl | 37 and 31 min (1st and 2nd meas.) / 45 and 35 min (1st and 2nd meas.) | 0.09 - 36 μM 1.5 - 50 μM | 0.09 μM 1.5 μM | 5 min /10 min | ---- | 2.4% / 4.0% | ---- | Water | 59 |
| (IC10) | ||||||||||||
| Biosensor systems based on amperometric detection | |||||||||||
| Enzyme | Immobilisation method, support and electrode | Pesticides | Working potential | Working range (WR) | LOD | Inhibition time | Enzyme reactivation | RSD | Stability | Real samples | Ref. |
| AChE III-type from Electric Eel | Glutaraldehyde/BSA crosslinking onto nylon grids on a GC electrode | Paraoxon Carbaryl | +0.25 V vs. Ag/AgCl | 0.5 - 10 μM 0.5 - 50 μM | 0.1 μM 0.1 μM (IC5) | ---- | 1.4 mM 2-PAM (4 h) and working buffer (2 h) | 3.7% (n= 5, 40 μM) 4% (n= 5, 8 μM) | 90% activity after 30 days | Lagoon water and kiwis | 42 |
| AChE III-type from Electric Eel | Coupling through a cystamine SAM onto a gold-coated nylon mesh attached to a GC electrode | Carbaryl Paraoxon | +0.25 V vs. Ag/AgCl | 0.01 - 10 μM 0.01 - 10 μM | 0.05 μM 0.05 μM | ---- | 1.4 mM 2-PAM (1 h) and working buffer (2 h) | <5% (10 μM) <5% (10 μM) | ---- | ---- | 43 |
| (IC5) | |||||||||||
| BChE from horse serum | Glutaraldehyde crosslinking on nylon, cellulose nitrate or white tracing paper membranes, covering epoxy carbon-paste electrodes | Diazinon | +0.61 V vs. Ag/AgCl | ---- | 5 nM (in solution) 4 nM (nylon) 1.5 nM (cellulose) | 10 min | 0.1% TMB-4 (10 min) | ---- | 100% after 10 uses and subsequent regeneration | ---- | 38 |
| AChE from bovine erythrocytes | Glutaraldehyde crosslinking on aminated magnetic particles (magnetic reactor), Pt thick-film electrode | Carbofuran Paraoxon-ethyl Malaoxon Paraoxon-methyl | +0.6 V vs. Ag/AgCl | 4.5 - 271 nM 3.8 - 230 nM 3.2 - 191 nM 4.0 - 243 nM | 14 nM 12 nM 22 nM 28 nM | 10 min | Release of the magnetic particles by switching off the electromagnet | ---- | ---- | Drinking and brook water | 64 |
| AChE V-S-type from Electric Eel | Immobilisation onto activated nylon membrane covering SP electrodes | Dichlorvos | +0.3 V vs. Ag/AgCl | 0.9 - 90 μM | 0.9 μM | 3 min | 10 mM substrate or 0.1 mM 2-PAM (3 min) | ---- | ---- | ---- | 40 |
| AChE VI-S-type from Electric Eel | Carbodiimide immobilisation onto pretreated RVC or immobilisation by cyanogen bromide activation onto RVC-agarose composite | Paraoxon | +0.25 V vs. Ag/AgCl | 5 - 2000 μM | 5 μM | Competitive | ---- | ---- | 60% activity after 30 days | ---- | 39 |
| (s/n = 3) | |||||||||||
| AChE III- type from Electric Eel | Carbodiimide immobilisation, with dextran sulfate and lactitol as stabilisers, on CoPC-modified SP electrodes | Dichlorvos Paraoxon | 0 V vs. Ag/AgCl | ---- | 6 nM 0.04 nM | 20 min | ---- | 11.0% (n= 5) 6.2% (n= 5) | ---- | River water | 65 |
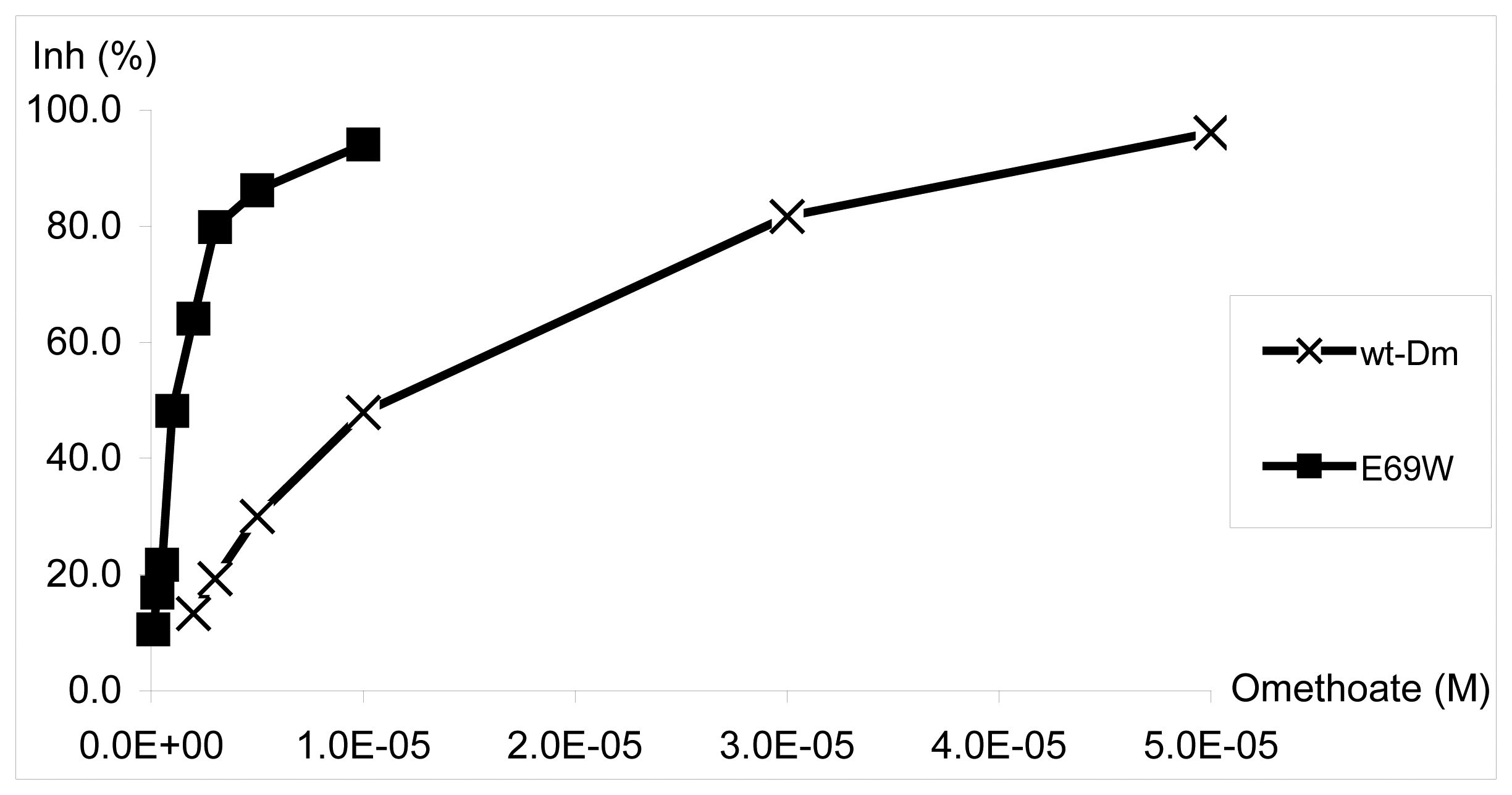
| Wt AChE / E69W Dm AChE mutant | PVA-SbQ entrapment on CoPC-modified SP electrodes | Omethoate | ---- | 0.1 - 10 μM | 2 μM / 0.1 μM | 15 min | Disposable electrodes | 8% (n= 6, 1 μM with E69W) | ---- | Water | 41 |
| (IC10) | |||||||||||
| B4-21 / B4-27 / B3 Dm AChE mutants | Co-entrapment during aniline electropolymerisation on sonicated poly(o-PDA)-coated CoPC-modified SP carbon electrodes | Dichlorvos Parathion methyl Azinphos methyl | +0.2 V vs. Ag/AgCl | 10-17 – 10-8 M 10-16 – 10-8 M 10-16 – 10-8 M | 10 aM 100 aM 100 aM | 20 min | ---- | 14% (n= 3, WR) 19% (n= 3, WR) 8% (n= 3, WR) | 65% activity after 92 days with stabilisers | ---- | 66 |
| (IC20) | |||||||||||
| AChE VI-S-type from Electric Eel / BChE from horse serum | Glutaraldehyde/BSA crosslinking on SP Pt electrodes | Carbaryl Heptenophos | +0.35 V vs. pseudoref. Ag | 0.005 - 50 μM 0.004 - 40 μM | 1.0 μM / 0.48 μM 2.5 μM / 0.47 μM | No pre-incubation | ---- | 4 % 4 % | 60-40% activity after 150 meas. during 5 days | ---- | 67 |
| (IC10) | |||||||||||
| AChE VI-S-type from Electric Eel | Adsorption into a nanostructured carbon matrix | Dichlorvos | +0.1 V vs. Ag/AgCl | 10-6 - 1 μM | 1 pM | 10 min | Buffer or 10 mM choline (30 min – 3 h) | ---- | 70% activity after 30 days | ---- | 68 |
| (IC20) | |||||||||||
| AChE | LbL immobilisation on CNT-modified GC electrodes | Paraoxon | +0.15 V vs. Ag/AgCl | 0.001 - 10 nM | 0.4 pM | 6 min | 200 μl of 0.1 mM 2-PAM and 10 mM ATCh | < 5.6% (n= 6, WR) | 85% activity after 21 days | ---- | 70 |
| (s/n = 3) | |||||||||||
| AChE VI-S-type | Al2O3 sol-gel entrapment on SP electrodes | Dichlorvos | +0.25 V vs. Ag/AgCl | 0.1 - 80 μM | 10 nM | 15 min | ATCh substrate | 2.9% (n= 6, 1 μM) | 93% activity after 25 meas., 90% activity after storage for 5 months | Seawater and river water | 71 |
| (s/n = 3) | |||||||||||
| AChE V-S-type from Electric Eel | PVA-SbQ entrapment on a Pt electrode | Paraoxon | +0.41 V vs. SCE | ---- | 1 nM | ---- | 1 mM 2-PAM (7 min) | ---- | 70% activity after 21 days | ---- | 44 |
| (IC10) | |||||||||||
| AChE | PVA-SbQ entrapment on a Pt electrode | Paraoxon Carbaryl | +0.41 V vs. Ag/AgCl | 10-10 - 10-5 M 10-10 - 10-5 M | nM | ---- | 0.5 mM 2-PAM (15 min) | ---- | ---- | Water | 72 |
| (IC10) | |||||||||||
| AChE V-S-type from Electric Eel | PVA-SbQ entrapment on a Pt wire electrode | Chlorpyrifos Chlorpyrifos-oxon Methyl-chlorpyrifos-oxon | +0.41 V vs. Ag/AgCl | 0.1 - 10 μM 0.1 - 10 μM 0.1 - 10 μM | 3.14 nM 73 nM 0.88 μM | ---- 16 min 8 min | 0.4 mM 2-PAM (3 min), partial with buffer when inhibited with MeCPO | ---- 6%(n= 3, 1 μM) 40-50% (n= 3, 1μM) | ---- | ---- | 73 |
| (IC10) | |||||||||||
| OPH from recombinant E. coli | Covalent immobilisation on activated aminopropyl CPG beads (reactor), carbon paste electrode | Paraoxon Methyl parathion | +0.9 V vs. Ag/AgCl | ---- | 20 nM 20 nM | Not needed | Not needed | 2% (n= 35, 1 μM) ---- | ---- | Spiked water and simulated well water | 46 |
| (s/n = 3) | |||||||||||
| OPH from recombinant E. coli | Cystamine-glutaraldehyde coupling on a thin-film gold electrode | Paraoxon Methyl parathion | +0.75 V vs. Ag/AgCl | 1 - 10 μM 1 - 10 μM | 0.1 μM | Not needed | Not needed | 3.6% (n= 20, 1 μM) ---- | 100% activity after 4 weeks | ---- | 47 |
| (s/n = 3) | |||||||||||
| PH from Pseudomonas sp. | Polyethyleneimine-glutaraldehyde coupling on a SP carbon electrode | Parathion | +0.7 (2 s) and +0.85 (1 s) V vs. Ag/AgCl | 0.03 - 0.3 μM | 0.5 nM | Not needed | Not needed | ---- | ---- | Tap water and spiked river water | 50 |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Prieto-Simón, B.; Campàs, M.; Andreescu, S.; Marty, J.-L. Trends in Flow-based Biosensing Systems for Pesticide Assessment. Sensors 2006, 6, 1161-1186. https://doi.org/10.3390/s6101161
Prieto-Simón B, Campàs M, Andreescu S, Marty J-L. Trends in Flow-based Biosensing Systems for Pesticide Assessment. Sensors. 2006; 6(10):1161-1186. https://doi.org/10.3390/s6101161
Chicago/Turabian StylePrieto-Simón, Beatriz, Mònica Campàs, Silvana Andreescu, and Jean-Louis Marty. 2006. "Trends in Flow-based Biosensing Systems for Pesticide Assessment" Sensors 6, no. 10: 1161-1186. https://doi.org/10.3390/s6101161
APA StylePrieto-Simón, B., Campàs, M., Andreescu, S., & Marty, J.-L. (2006). Trends in Flow-based Biosensing Systems for Pesticide Assessment. Sensors, 6(10), 1161-1186. https://doi.org/10.3390/s6101161







