A New Overpotential — Capacitance Mechanism for H2 Electrode
Abstract
:1. Introduction
- Evolution of H2 at a cathode is opposed.
- It should be a requirement that the second step of the electrochemical description of hydrogen atoms occur in one cooperative act.
- No H+ production from H2 has been confirmed.
- The theoreticians made the redox assumption at the beginning to calculate the kinetics before ascertaining the mechanism [2]. Reaction mechanisms must be confirmed experimentally. In summary, the earlier work was deficient experimentally. Any theory of how the H2 electrode works must be judged by its usefulness and experimental evidence.
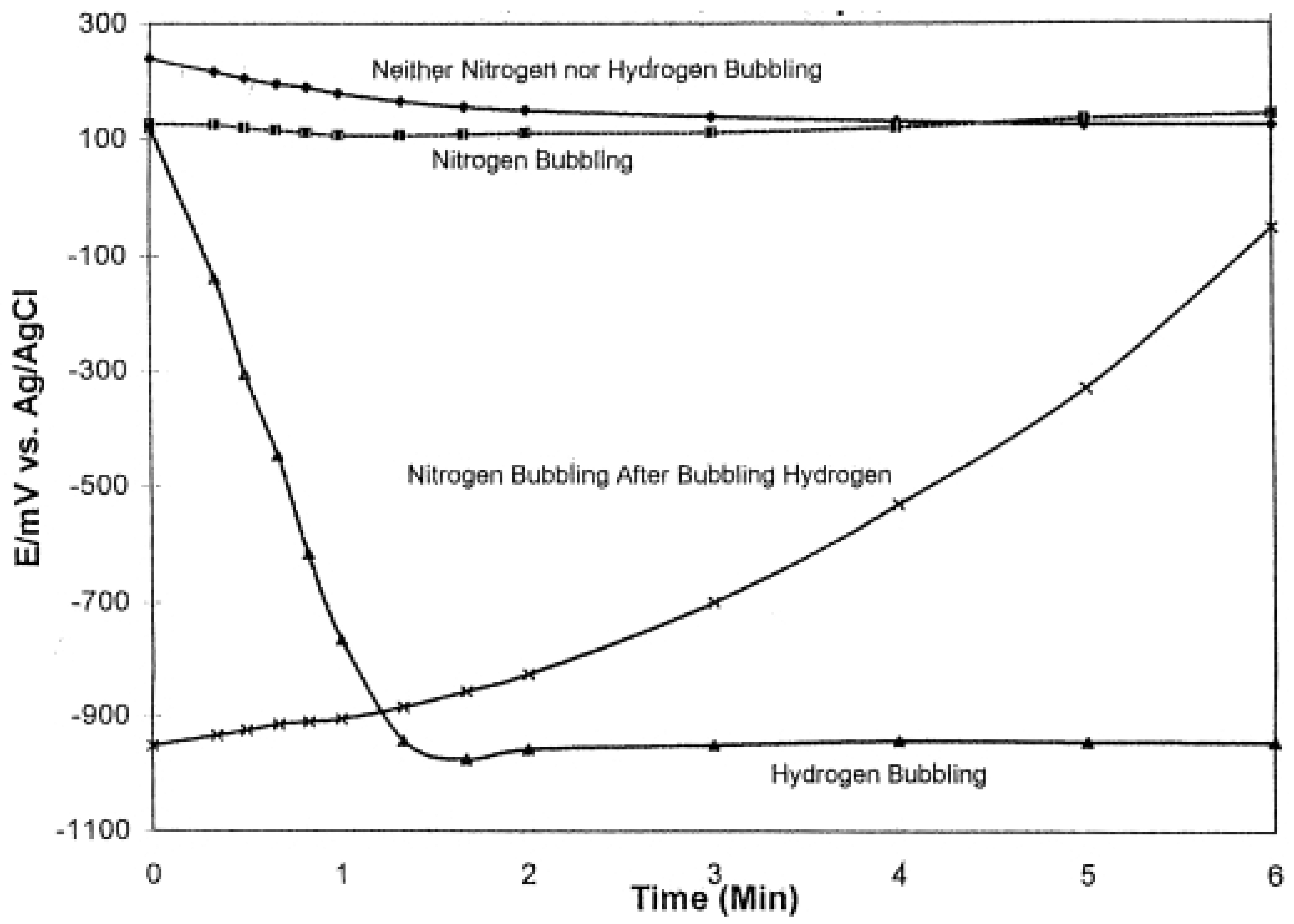
2. Results and Discussion
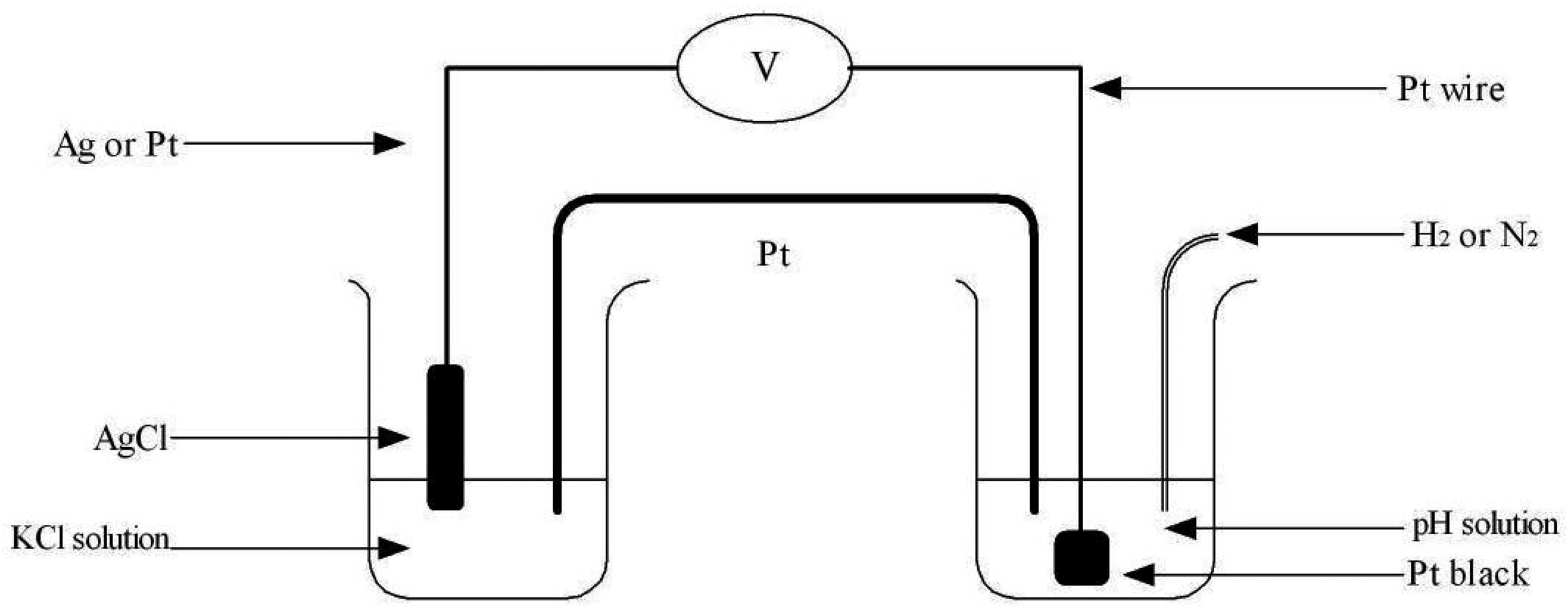
2.1 Use of Pt Wire Instead of Salt Bridge
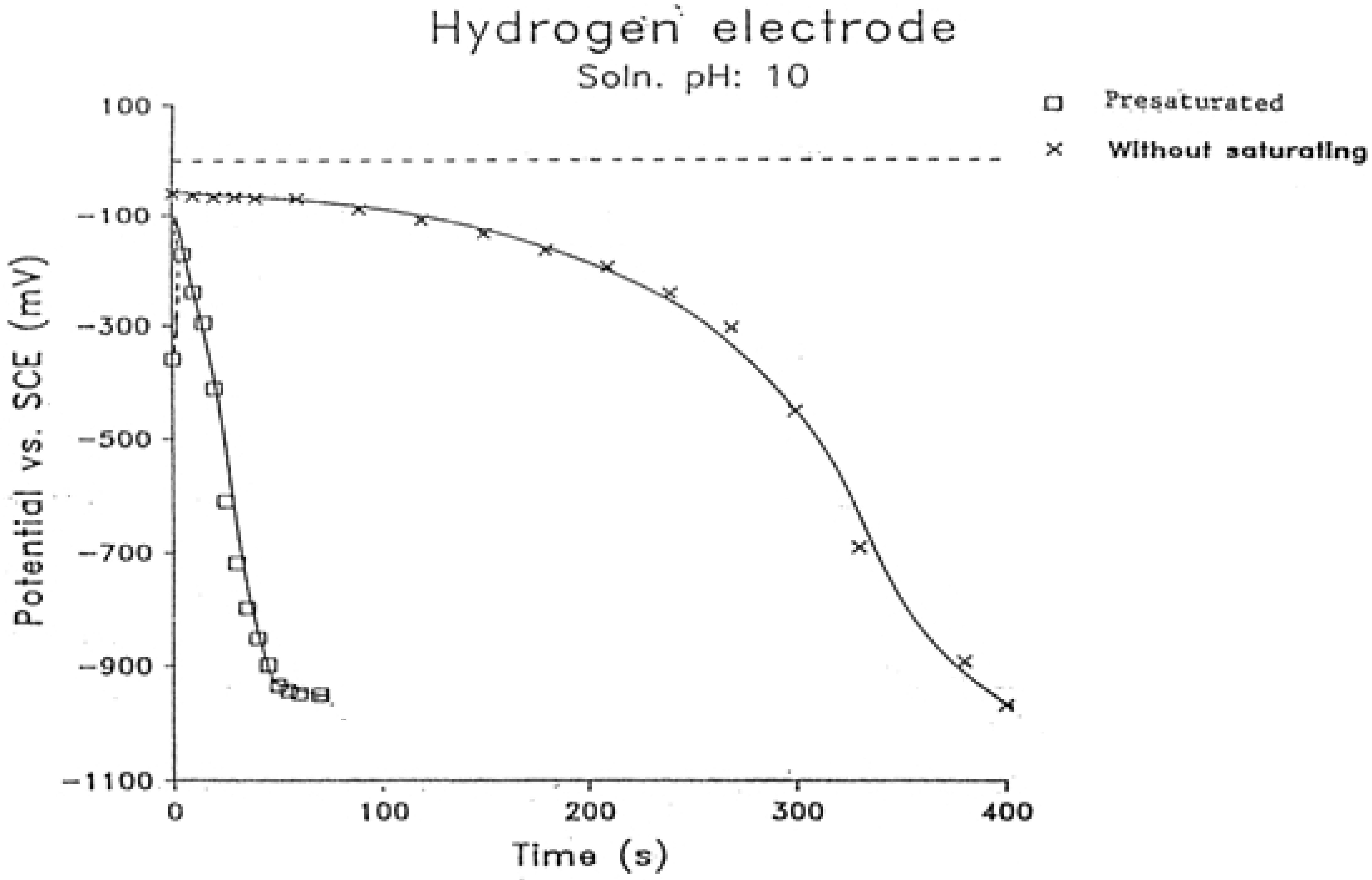
2.2 Nernst Hiatuses
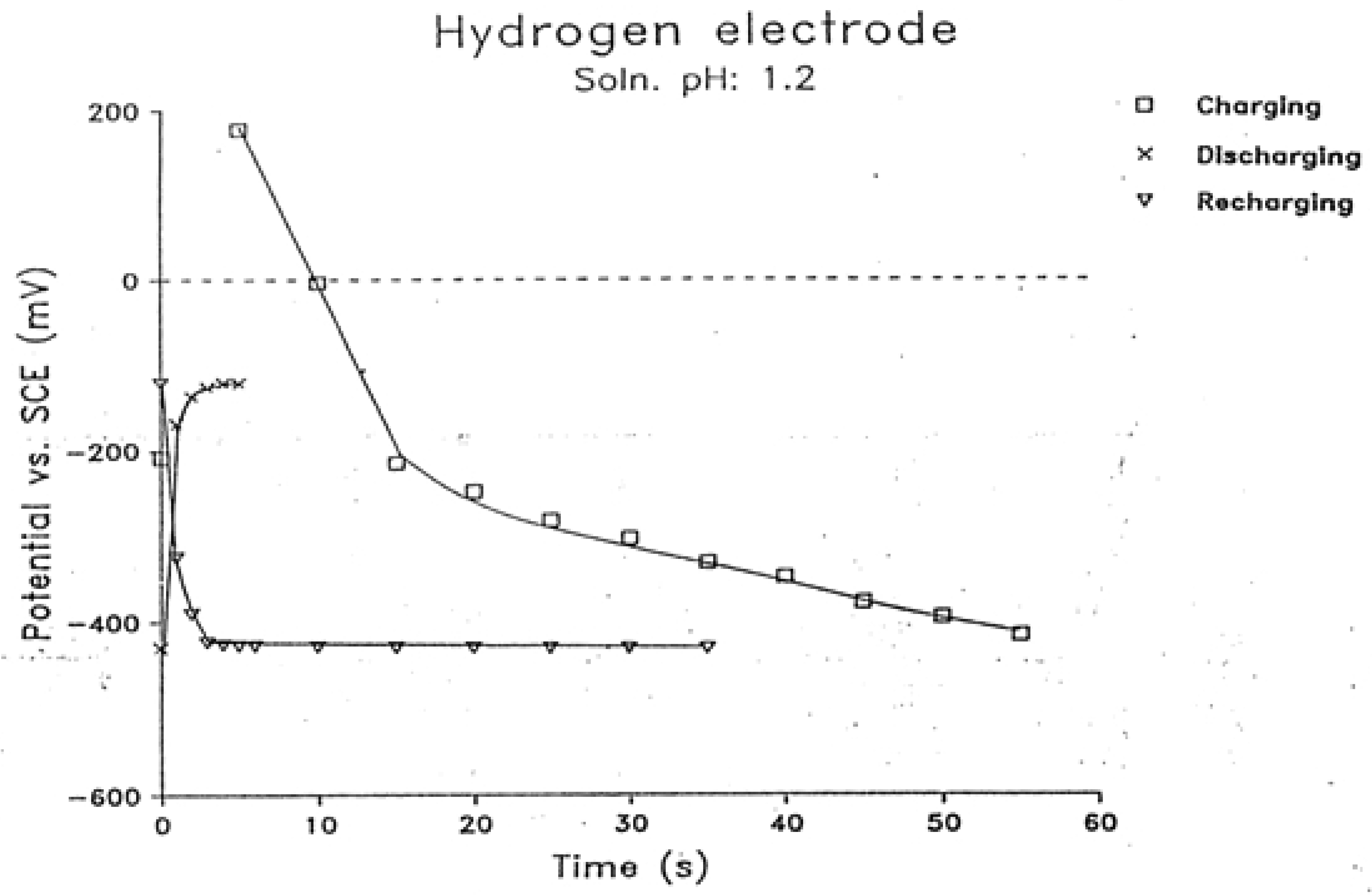
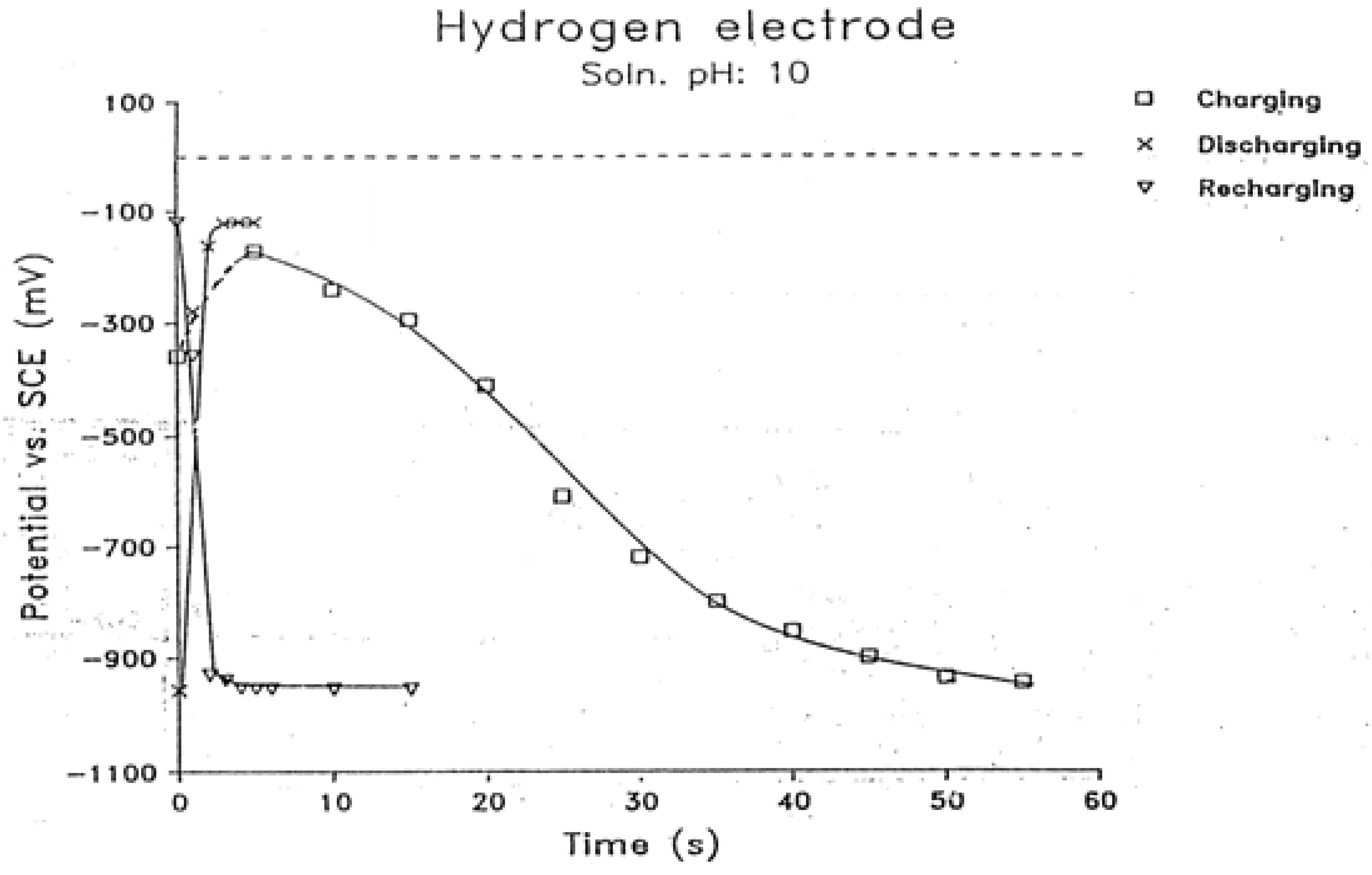
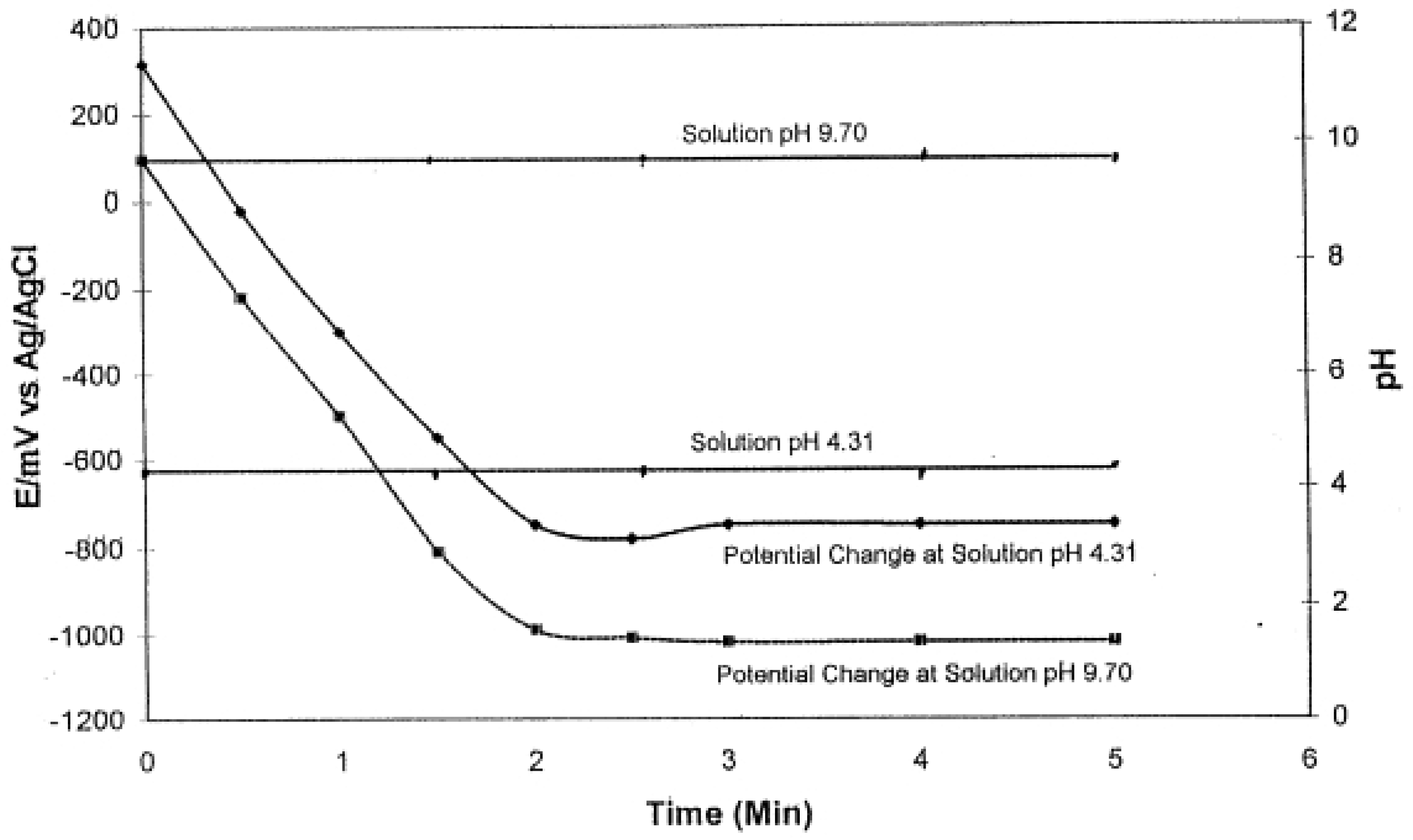
2.3. Charging, Discharging and Recharging in Connection with Overpotential
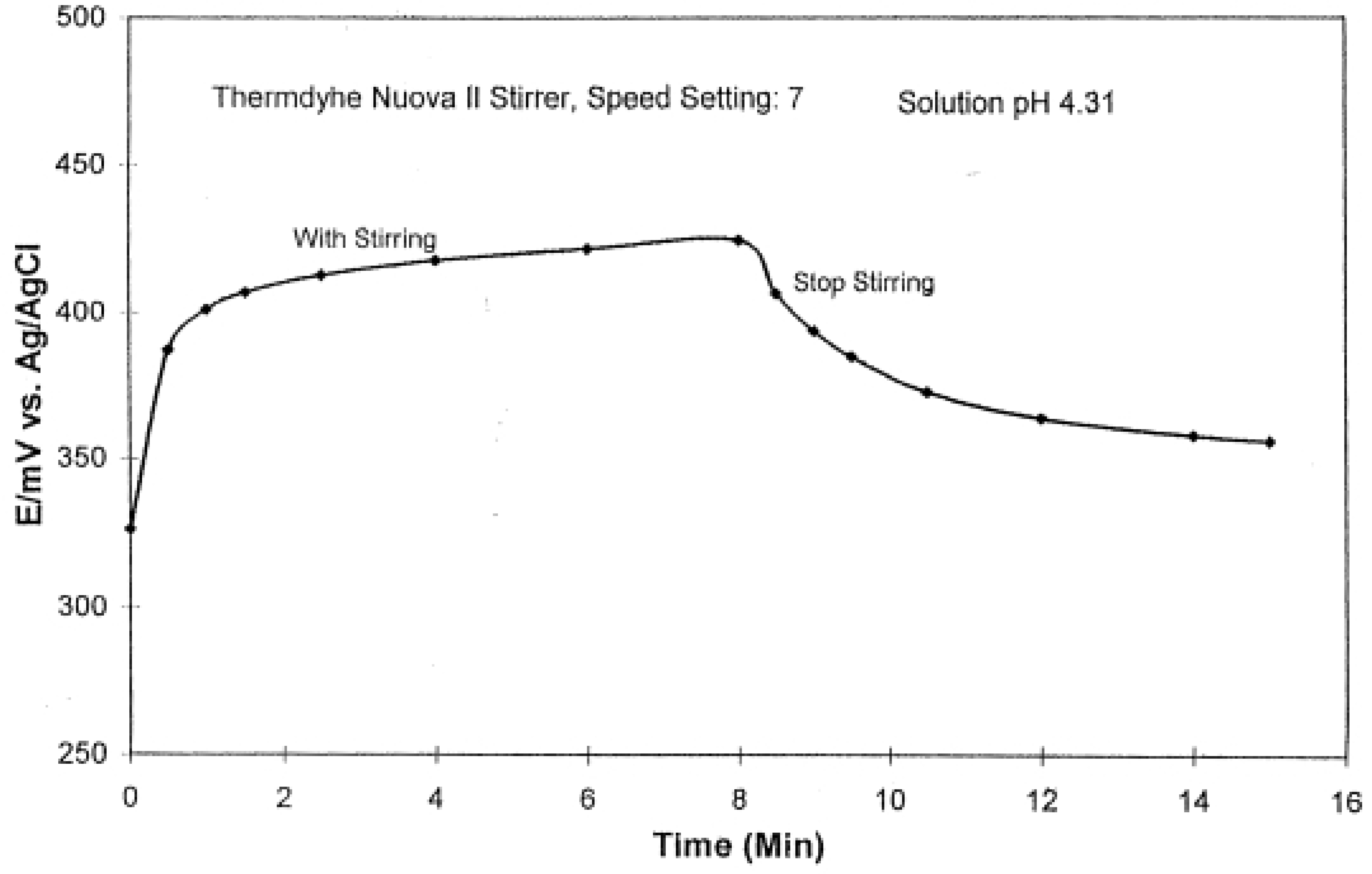
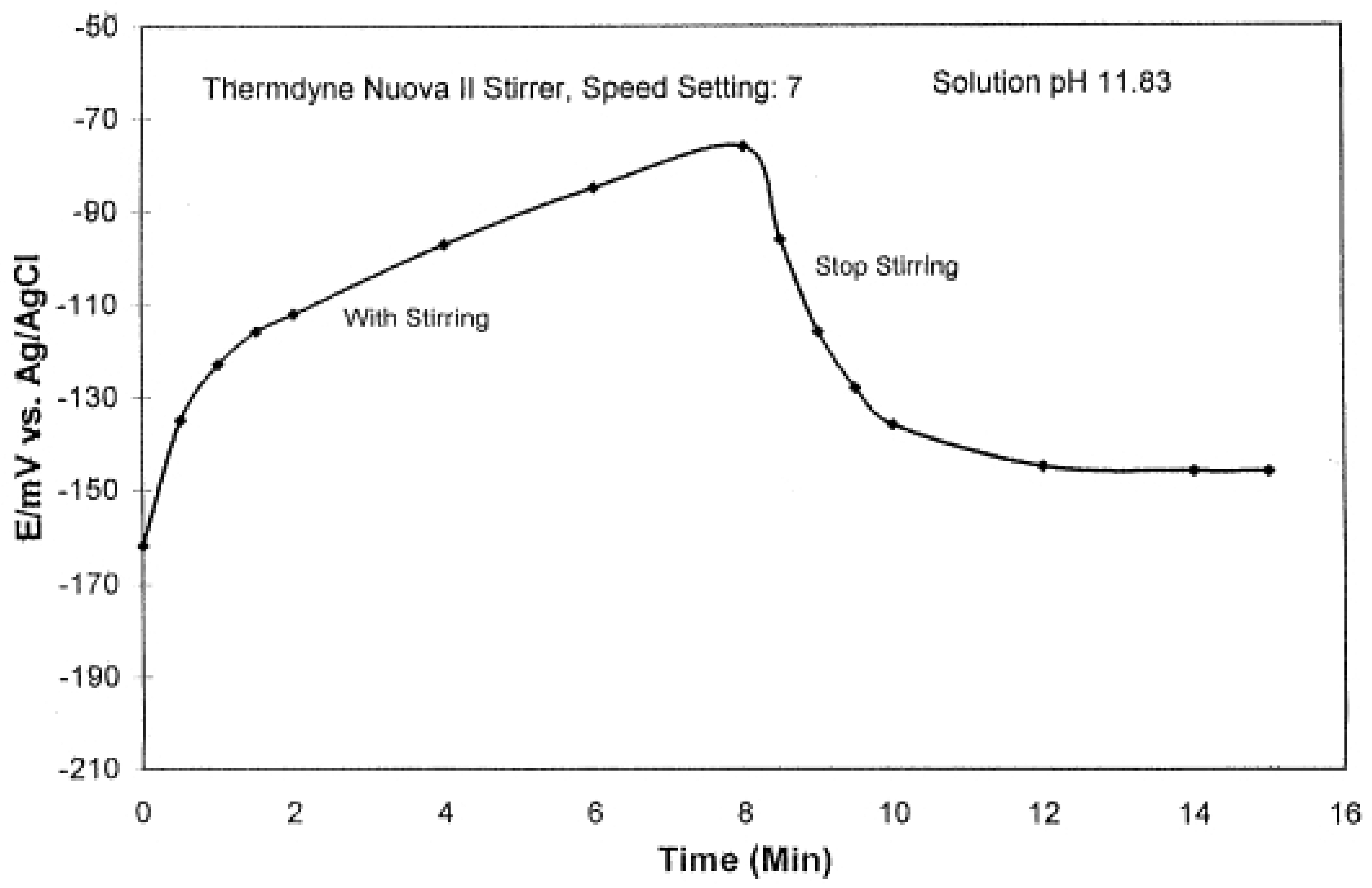
2.4. Stirring Effect
2.5. pH of the Solution Was Independent of Pt Black Electrode Potential
2.6. No Catalyst
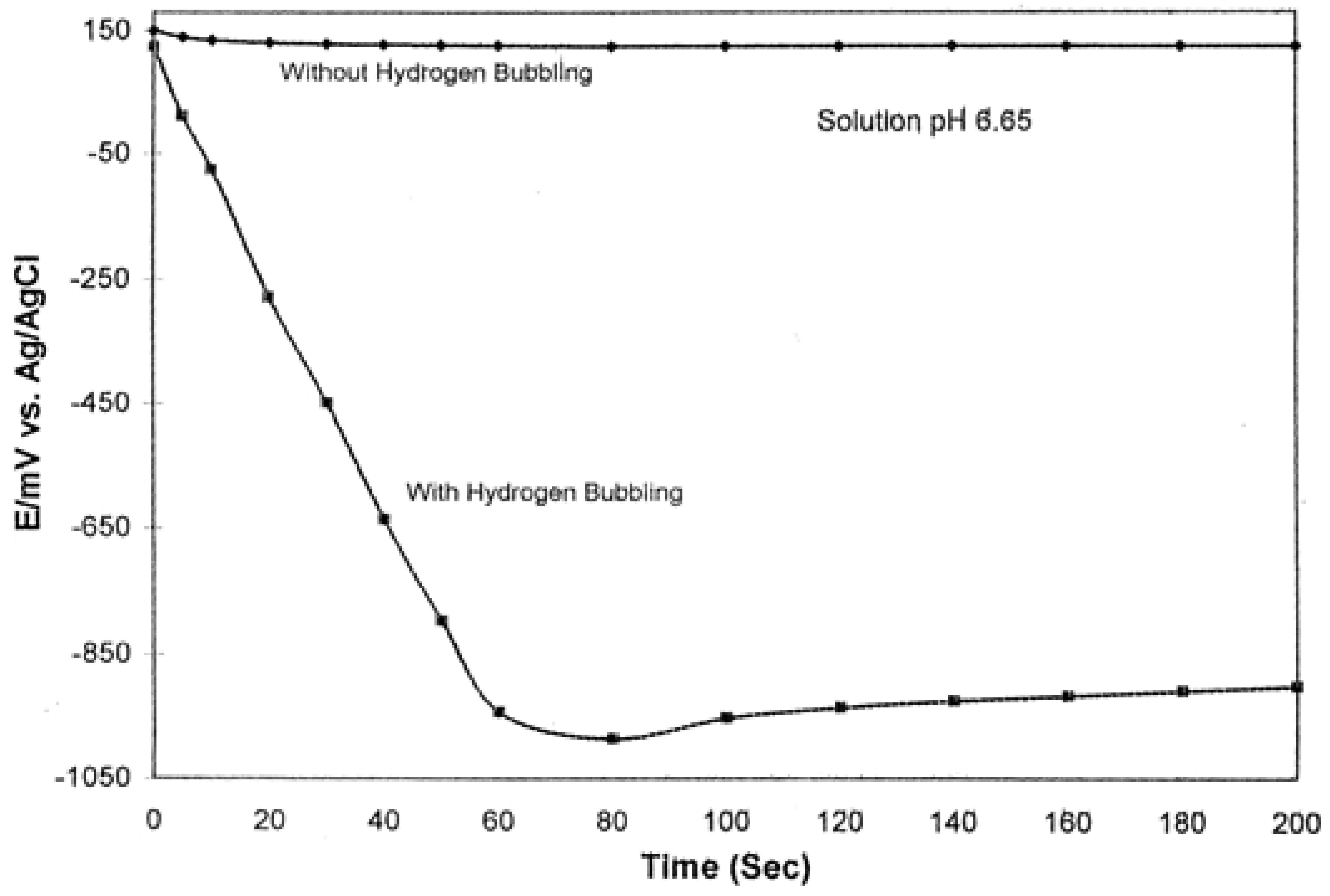
2.7. Interference
3. Experimental Section
4. Conclusions
Acknowledgments
References
- Ives, D. J.G.; Janz, G. J. Reference Electrodes.; Academic Press: New York, 1961. [Google Scholar]
- Vetter, K. J. Electrochemical Kinetics.; Academic Press: New York, 1967. [Google Scholar]
- Bates, B. G. Determination of pH. Theory and Practice, 2nd ed.; Wiley: New York, 1964. [Google Scholar]
- Beans, H.T.; Hammett, P. Experimental Studies on the H2 Electrode. J. Am. Chem. Soc. 1925, 47, 1215–1226. [Google Scholar]
- Bockris, J. O'M.; Reddy, A. K. N. Modern Electrochemistry; Vol. 1, Plenum: New York, 1977; p. 16. [Google Scholar]
- Cheng, K.L. Microchem. J. 1990, 42, 5–24.Also Internet J. of Chem. 2001, 4, 1.
- Cheng, K. L. pH Glass Electrode and Its Mechanism. In Electrochemistry, Past and Present; Stock, J.T., Orno, M. V., Eds.; ACS Symposium Series: Washington, D.C., 1989; 390, pp. 286–302. [Google Scholar]
- Huang, C. I.; Huang, H.J.; Cheng, K. L. Applications of Membrane-Mimetic Chemistry; Yen, T. F., et al., Eds.; Plenum: NY, 1994; pp. 209–225. [Google Scholar]
- Cheng, K.L. Microchem. J. 1998, 59, 323–325.
- Cheng, K.L. Microchem. J. 1998, 59, 457–461.
- Cheng, K.L. J. Coll. Interface. Sci. 2001, 239, 385–390.
- Murray, R.W. IUPAC revised, (Editorial). Anal. Chem. 1998, 70, 623 A.Also Anal. Chem. 1997, 69, 521 A.
- Zhou, J.; Zu, Y.; Bard, A.J. Scanning electrochemical microscopy part 39. J. Electroanal. Chem. 2000, 491, 22–29. [Google Scholar]
- Sun, Y.; Wei, G.; Cheng, K. L. An unusually high sensitivity of platinum black pH electrode. Paper presented at the 80thACS Colloid and Surface Science Symposium, the University of Colorado, June 20, 2006.


| pH of solution | |||||||
|---|---|---|---|---|---|---|---|
| Before adding Pt black | 1.24 | 2.21 | 4.31 | 6.50 | 9.43 | 11.79 | 1.44* |
| After adding Pt Black | 1.26 | 2.22 | 5.26 | 6.25 | 7.15 | 11.60 | 1.44 |
© by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Cheng, K.L.; Ashraf, N.; Wei, G. A New Overpotential — Capacitance Mechanism for H2 Electrode. Sensors 2006, 6, 1187-1198. https://doi.org/10.3390/s6101187
Cheng KL, Ashraf N, Wei G. A New Overpotential — Capacitance Mechanism for H2 Electrode. Sensors. 2006; 6(10):1187-1198. https://doi.org/10.3390/s6101187
Chicago/Turabian StyleCheng, K. L., Naila Ashraf, and Glenn Wei. 2006. "A New Overpotential — Capacitance Mechanism for H2 Electrode" Sensors 6, no. 10: 1187-1198. https://doi.org/10.3390/s6101187
APA StyleCheng, K. L., Ashraf, N., & Wei, G. (2006). A New Overpotential — Capacitance Mechanism for H2 Electrode. Sensors, 6(10), 1187-1198. https://doi.org/10.3390/s6101187




