Use of a Combined SpO2/PtcCO2 Sensor in the Delivery Room
Abstract
: Arterial oxygen saturation (SaO2) and partial arterial pressure of carbon dioxide (PaCO2) are important respiratory parameters in critically ill neonates. A sensor combining a pulse oximeter with the Stow-Severinghaus electrode, required for the measurement of peripheral oxygen saturation (SpO2) and transcutaneous partial pressure of carbon dioxide (PtcCO2), respectively, has been recently used in neonatal clinical practice (TOSCA500ÒRadiometer). We evaluated TOSCA usability and reliability in the delivery room (DR), throughout three different periods, on term, late-preterm, and preterm neonates. During the first period (period A), 30 healthy term neonates were simultaneously monitored with both TOSCA and a MASIMO pulse oximeter. During the second period (period B), 10 healthy late-preterm neonates were monitored with both TOSCA and a transcutaneous device measuring PtcCO2 (TINAÒ TCM3, Radiometer). During the third period (period C), 15 preterm neonates were monitored with TOSCA and MASIMO after birth, during stabilization, and during transport to the neonatal intensive care unit (NICU). Blood gas analyses were performed to compare transcutaneous and blood gas values. TOSCA resulted easily and safely usable in the DR, allowing reliable noninvasive SaO2 estimation. Since PtcCO2 measurements with TOSCA required at least 10 min to be stable and reliable, this parameter was not useful during the early resuscitation immediately after birth. Moreover, PtcCO2 levels were less precise if compared to the conventional transcutaneous monitoring. However, PtcCO2 measurement by TOSCA was useful as trend-monitoring after stabilization and during transport to NICU.1. Introduction
Arterial oxygen saturation (SaO2) and partial arterial pressure of carbon dioxide (PaCO2) are two of the most important respiratory parameters in the evaluation of critically ill neonates. SaO2 is commonly estimated by pulse oximetry (SpO2), a non invasive technique widely used in neonatal intensive care units. Partial pressure of carbon dioxide (PCO2) can be assessed by sampling blood (PCO2BGA) or by noninvasive techniques such as transcutaneous monitoring (PtcCO2), which reduces the need for frequent blood drawing and painful punctures and provides a continuous estimation of PCO2.
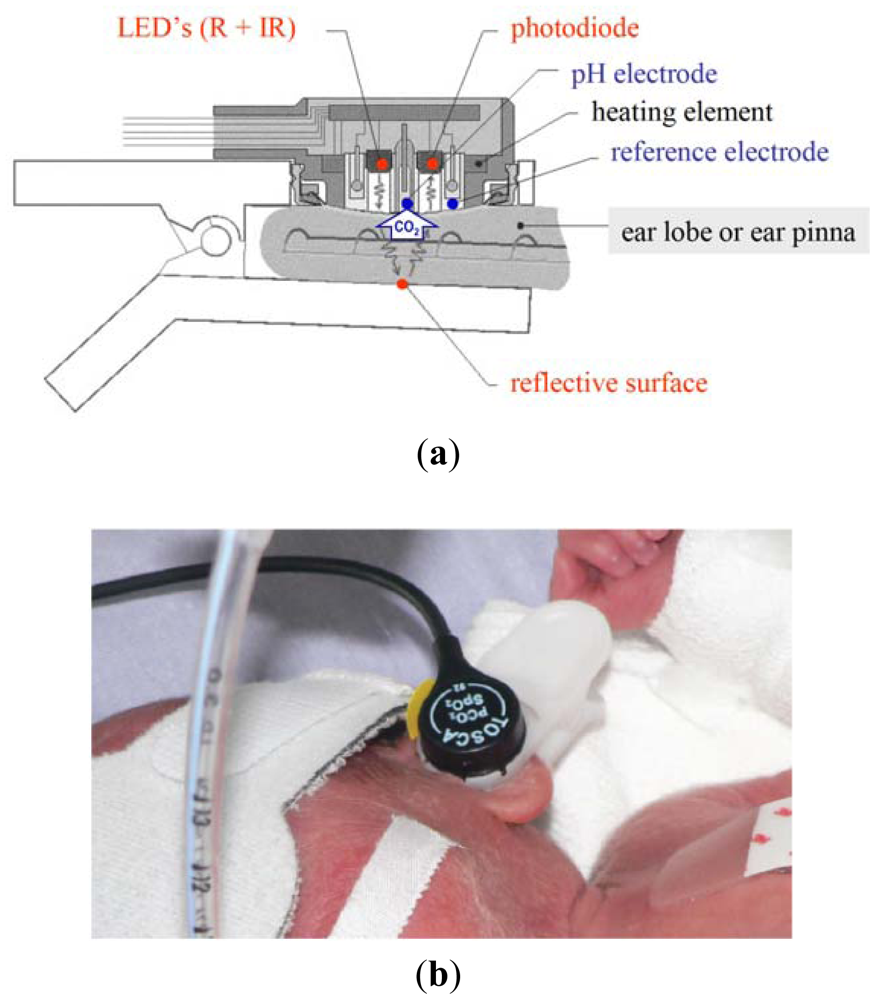
As already described elsewhere [1], the TOSCA monitor combines a pulse oximeter of new technology (Masimo SET) with the Stow-Severinghaus electrode for PtcCO2 measurement in a single sensor, which is also equipped with a heating element to increase the local perfusion Figure 1(a). The sensor is attached to the ear by a clip having an adhesive holder and a reflective element placed onto the inner surface.
Like other similar devices, the TOSCA pulse oximeter determines SaO2 by measuring the difference in absorption of selected light wavelengths by oxyhemoglobin and deoxyhemoglobin. It utilizes two light-emitting diodes (LEDs): one LED emits red light (LED-R; 658 nm wavelength) selectively adsorbed by deoxyhemoglobin, while the other one emits infrared light (LED-IR; 880 nm wavelength) selectively absorbed by oxyhemoglobin. The sensor also contains a photodiode (photodetector), an electronic device which converts light into an electronic signal proportional to the incident light intensity and which sends it to the TOSCA monitor for calculation. Unlike other pulse oximeters, either the LEDs or the photodiode are positioned on the same surface, since the light emitted is reflected by the clip surface opposite to the sensor place. The TOSCA pulse oximeter is equipped with Masimo SET technology and uses a set of algorithms designed to improve SpO2 monitoring during motion and low perfusion.
Transcutaneous measurement of CO2 is based on the observation that this gas has a high tissue solubility and diffusion through the skin, and that the application of local heat dilates blood vessels and enhances skin permeability. The PCO2 component of the TOSCA consists of a Stow-Severinghaus type electrode, a potentiometric sensor combining a silver/silver chloride reference electrode, and a glass pH electrode. Over the sensor surface there is an electrolyte solution, provided within a thin hydrophilic spacer and coupled to the skin via a highly gas permeable hydrophobic membrane. PCO2 determines a pH change of this electrolyte solution by diffusing through the skin to the sensor. The equation below illustrates the reaction occurring between carbon dioxide and water:
Generation of hydrogen ions at the glass electrode is directly proportional to the amount of carbon dioxide which is present. An increase in the hydrogen ion concentration leads to a fall in pH and this change is proportional to the logarithm of the PCO2 change.
The heating element, increasing local perfusion, induces the arterialization of the capillary blood flow. However, due to the elevated temperature of the sensor, the transcutaneous PCO2 results higher than the arterial value mostly due to two factors [2]: first, the elevated temperature increases local blood and tissue PCO2 (“anaerobic factor”); second, the epidermal cells produce CO2, which contributes to the capillary CO2 concentration by a constant amount (“metabolic constant”). As a consequence, in order to provide a monitor readout corresponding as close as possible to PaCO2, the device applies an algorithm correction of the transcutaneous values as already specifically described by Severinghaus [3].
Since the monitor automatically performs calibration, the sensor is steadily ready to use. The small-sized sensor is easily attached to the ear lobe with a low pressure clip, and has been tested in adults [4–6], children [7], and neonates with birth weight (BW) >1,500 g and gestational age (GA) >28 weeks [8,9]. TOSCA monitor resulted reliable and applicable also in very low birth weight infants when the sensor was placed at the ear pinna, rather than at the earlobe, because of its small size. Figure 1(b) [10].
Since the use of pulse oximetry immediately after birth is indicated to titrate oxygen supplementation, as recommended by the Guidelines for Neonatal Resuscitation [11], the present study was designed to test the usability and reliability of TOSCA in the delivery room (DR) among term, late-preterm, and preterm neonates.
2. Experimental Section
2.1. Materials and Methods
This prospective observational study was carried out between January and October 2010. It was approved by the ethical committee of our institution and oral informed parental consent was obtained. The study was conducted throughout three different periods among neonates recruited immediately after birth.
During the first period (period A), usability and reliability of TOSCA (TOSCA500® Radiometer, Copenhagen, Denmark) were evaluated in 30 term healthy neonates. A simultaneous SpO2 monitoring with TOSCA and pulse oximeter MASIMO Radical-7 SET (MASIMO® Corporation, Irvine, CA, USA) was performed. SpO2 values reported by TOSCA (SpO2TOSCA) were compared to those reported by MASIMO (SpO2MASIMO) throughout 10 min of monitoring. During this period, also PtcCO2 levels were recorded with TOSCA (PtcCO2TOSCA) and a blood gas analysis (BGA) by capillary sampling was performed at the end of monitoring.
During the second period (period B), PtcCO2TOSCA levels were recorded in 10 late-preterm neonates and compared to PtcCO2 values (PtcCO2TINA) recorded by a transcutaneous device (TINA® TCM3, Radiometer, Copenhagen, Denmark). TINA was equipped with a sensor applied on the abdomen and set at 44 °C. After 10 minutes of monitoring, a BGA was performed from umbilical line.
In the third period (period C), 15 preterm neonates were monitored. SpO2TOSCA and SpO2MASIMO values were recorded immediately after birth and during stabilization and transport to the neonatal intensive care unit (NICU) (total time: 40 min). Also PtcCO2TOSCA values were registered during the same period and a BGA was performed after 10 min of monitoring from umbilical line.
TOSCA was always used in QUICKSTART mode (temperature set at 44 °C during the first 20 min, then at 42 °C). The sensor probe cleaned with alcohol and dried before each application was applied to the adhesive clip at the ear pinna, using one drop of contact solution. Since Lacerenza et al., showed that the placement site at the ear pinna facilitated the application of the sensor, it was electively applied at the right ear pinna rather than at the ear lobe [10]. The MASIMO sensor was placed around the right hand and applied before connection to the oximeter [12,13].
Blood samples were analyzed for PCO2BGA with a blood gas analyzer (Stat Profile® Critical Care Xpress, Nova Biomedical Corporation, Waltham, MA, USA), routinely calibrated according to the manufacturer's instructions. BGA was performed at 10 min of monitoring since this time is indicated as an adequate time for TOSCA equilibration by the manifacturers themselves [14,15].
Patient characteristics (GA, BW, gender) and resuscitative measures required at birth were recorded.
The conduct of the study did not interfere with routine clinical practice: while a neonatologist was assisting the neonate, a nurse placed the pulse oximeter sensor, and a dedicated researcher applied both TOSCA and TINA sensors and collected data. Transcutaneous monitoring began as the sensors were adequately placed. Data were collected every min during the first 5 min, then every 5 min for the remaining time. At the end of the monitoring phase, sensors were removed and the underlying skin was examined to verify the presence of burns.
2.2. Statistical Analysis
Statistical analysis was performed using the Stata Statistical Software: Release 10 (StataCorp LP, College Station, TX, USA). Results are presented as mean ± standard deviation (SD) for continuous variables or as median (interquartile range) and as number (percentage) for categorical variables. Unpaired Student's t test was used for parametric data, Wilcoxon rank-sum test (Mann-Whitney U test) for nonparametric data, and Fisher's exact test for categorical variables. Bland-Altman analysis [16] was used to evaluate the agreement at 10 min between PtcCO2TOSCA and PCO2TINA, PtcCO2TOSCA and PCO2BGA, PCO2BGA and PtcCO2TINA. Precision was defined as 2 SD of the mean difference (bias). A p < 0.05 was considered statistically significant.
3. Results
Of the 30 neonates born during period A (GA: 37.9 ± 1 weeks; BW: 3138 ± 459 g, 13 males and 17 females), none needed respiratory support at birth. No differences between SpO2TOSCA and SpO2MASIMO were detected at all time points (Figure 2(top)). For both monitors, data were available 60 s after sensor placement. The clipping of the TOSCA sensor was easy. Sensor repositioning was necessary in 16/30 registrations (53%) with TOSCA and in 5/30 registrations (16.6%) with MASIMO (p = 0.003). After sensor repositioning, time required to obtain a stable reading was shorter for TOSCA than for MASIMO (about 10 vs. 15 s). PtcCO2TOSCA progressively increased during the whole monitoring (Figure 2(bottom)).
PCO2BGA at 10 min was 61.5 ± 10.8 mmHg, significantly higher than PtcCO2TOSCA (48.6 ± 11.7 mmHg; p < 0.005). At 10 min, the bias (precision) between PtcCO2TOSCA and PCO2BGA values was 12.9 (26.4). No signs of skin erythema or burn at the ear pinna were found at the end of monitoring.
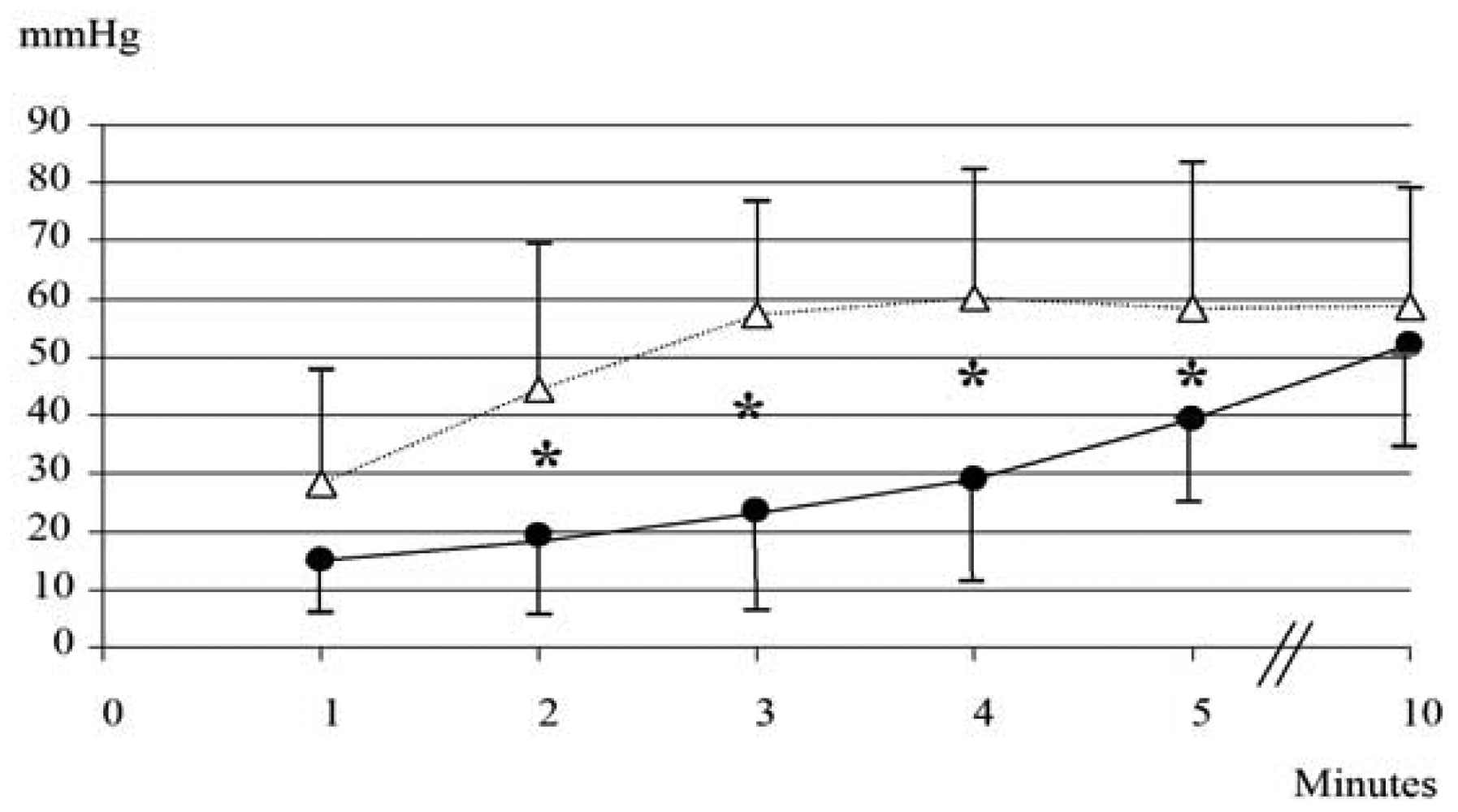
None of the 10 neonates (GA: 36.1 ± 1.4 weeks; BW: 2743 ± 714 g; five males and five females) born during period B needed respiratory support. As shown in Figure 3, during the first 5 min PtcCO2TOSCA values resulted significantly lower than PtcCO2TINA values, while at 10 min similar values were recorded (PtcCO2TOSCA 52 ± 17.6 mmHg vs. PtcCO2TINA 58.6 ± 20.2 mmHg; p = ns).
PCO2BGA at 10 min was 59.4 ± 17.3 mmHg; the bias (precision) between PtcCO2TOSCA and PCO2BGA values was 7.42 (39.8), between PCO2BGA and PtcCO2TINA 0.82 (31.9), and between PtcCO2TOSCA and PtcCO2TINA 6.6 (43.4). Sensor repositioning was required 3 times (30%) for TOSCA.
Fifteen preterm neonates (GA: 31.6 ± 2.0 weeks; BW: 1622 ± 569 grams; nine males and six females) born during period C were monitored for 40 min with TOSCA and MASIMO. Two neonates required resuscitation and were transferred to NICU mechanically ventilated; six neonates were assisted with nasal continuous positive airway pressure. Sensor repositioning was required six times (40%) for TOSCA and never for MASIMO. SpO2TOSCA and SpO2MASIMO values were similar during the whole study period (Figure 4(top)). PtcCO2TOSCA values progressively increased until 10 min, then became stable (Figure 4(bottom)).
PCO2BGA at 10 min was 63.1 ± 20.3 mmHg and the bias (precision) between PtcCO2TOSCA and PCO2BGA was 11.1 (43.4).
4. Discussion
The Guidelines on Neonatal Resuscitation recommend the use of pulse oximetry to monitor neonates' oxygenation status in the DR [11], since skin color is only partially reliable [17]. Moreover, awareness about neonatal oxygenation status is helpful to titrate oxygen supplementation, if requested.
In case of frequent motion artifacts or low perfusion, often occurring in DR, pulse oximeters with MASIMO Signal Extraction Technology are indicated [18].
TOSCA monitor records SpO2 with MASIMO technology and simultaneously detects PtcCO2, allowing a combined assessment of both parameters. The present study investigated for the first time the possible use of TOSCA monitor in neonates immediately after birth, a period in which the use of excessive pressure or volume (overstretching) and oxidative aggression (reactive oxygen and nitrogen species) may cause acute damage to the lung and other organs, in particular in preterm babies. The simultaneous measurement of SpO2 and PtcCO2 could be helpful both to verify the physiologic changes occurring during transition from the intra- to the extrauterine life and to determine the appropriate grade of respiratory support when resuscitation is required.
Concerning usability, our results showed that TOSCA sensor can be easily applied, even in the DR immediately after birth, to the ear pinna of both term and preterm neonates. As previously demonstrated [10], sensor application to the ear pinna rather than to the ear lobe did not alter data displaying. The size of the clipped sensor is well suited for the thin, flat, and soft ear pinna of the newborn, while it may result too large compared to the small ear lobe. Nevertheless, dislocations occurred more often using TOSCA than MASIMO. In this study, repositioning of the TOSCA sensor was more frequently required among term than among late-preterm or preterm neonates, presumably because of their greater mobility. However, the viability of TOSCA monitoring was not impaired thanks both to the shorter time required for sensor clipping and to its faster reading, due to the high sensor's stability [1,2].
No burns underneath the TOSCA sensor were identified in any of the infants, including premature neonates monitored for longer period (40 min) in period C. This was presumably due to a lower electrode temperature after 20 min of monitoring in the QUICKSTART mode. In fact, this modality allows a long term (up to 12 h) application of the sensor without adverse events, although measurements of PtCO2 may result less reliable [9]. This could be due to the lower sensor temperature which is not able to provide an adequate grade of arterialization in order to compensate the greater CO2 local production induced by temperature itself. However, this occurrence has only limited implications in our study, since we performed a “long time evaluation” of the PtcCO2TOSCA exclusively in Period C, throughout a monitoring period which is much shorter than the one described by other authors [9].
Concerning the reliability of SpO2TOSCA, the recorded values were comparable to those detected by SpO2MASIMO. As formerly demonstrated [10], the different placement site of both sensors, always preductal, did not affect SpO2 detection.
Regarding PtcCO2TOSCA measurement in DR, the estimation accuracy of TOSCA resulted generally acceptable compared to blood samples collected at 10 min of use. Although this single BGA, representing a punctual evaluation, may be a limit in the estimation of PtcCO2 values, a higher number of blood collection was avoided to prevent painful punctures. However, the agreement between PtcCO2TOSCA and PCO2BGA was more accentuated when blood samples were taken by the umbilical line (period B and C) compared to the capillary sampling (period A). A possible explanation of these results may be that capillary PCO2BGA is comparable to arterial PCO2BGA only after the first hours of life [19]. Nevertheless, after 10 min of registration, the TOSCA monitor reveals less accurate PtcCO2 values than the TINA one compared to the umbilical PCO2BGA (period B), confirming that the measurement of PtCO2 with TOSCA was less precise than the conventional monitoring [10].
The results achieved throughout period B demonstrated that during the first minutes of registration the PtcCO2TOSCA values were lower than those recorded by TINA and became comparable only after 5 min. This is in agreement with previously reported data achieved among preterm neonates admitted to NICU, and was probably dependent on the placement site of the TOSCA sensor [8]. The thin dermal layer and the poor capillary bed of the ear pinna may induce a lower grade of arterialization than the one obtained in other sites [10].
A similar slowly increasing trend of the PtcCO2TOSCA was also detected among preterm infants monitored after birth and during transport to the NICU (period C), and was followed by value stabilization after 10 min of monitoring.
5. Conclusions
We can assert that TOSCA was easily and safely usable in DR both in term and preterm neonates, allowing a reliable and noninvasive estimation of SpO2. Considering that at least 10 min were required to obtain reliable and stable values of PtcCO2TOSCA, TOSCA reliability results limited during the first minutes of life, when resuscitation procedures may be necessary. However, even if the optimal level of PCO2 during neonatal resuscitation is not known yet [20], PtcCO2 measurement with TOSCA could be useful as trend monitoring after neonatal stabilization and during transport to the NICU to prevent lung injury, since its beginning may occur soon after birth among neonates requiring positive-pressure ventilation. We believe that our study may represent a stimulating starting point for further investigations focusing on the development of respiratory function monitoring devices to be used in DR [21]. In the near future, an efficient technology may enable the individualization of the specific ventilatory and oxygenation requirements, thus minimizing physical and oxygen-derived damage [22].
References
- Eberhard, P.; Gisiger, P.A.; Gardaz, J.P.; Spahn, D.R. Combining transcutaneous blood gas measurement and pulse oximetry. Anesth. Analg. 2002, 94, S76–S80. [Google Scholar]
- Eberhard, P. The design, use, and results of transcutaneous carbon dioxide analysis: Current and future directions. Anesth. Analg. 2007, 105, S48–S52. [Google Scholar]
- Severinghaus, J.W. Transcutaneous blood gas analysis. Respir. Care 1982, 27, 152–159. [Google Scholar]
- Senn, O.; Clarenbach, C.F.; Kaplan, V.; Maggiorini, M.; Bloch, K.E. Monitoring carbon dioxide tension and arterial oxygen saturation by a single earlobe sensor in patients with critical illness or sleep apnea. Chest 2005, 128, 1291–1296. [Google Scholar]
- Bendjelid, K.; Schutz, N.; Stotz, M.; Gerard, I.; Suter, P.M.; Romand, J.A. Transcutaneous PCO2 monitoring in critically ill adults: Clinical evaluation of a new sensor. Crit. Care Med. 2005, 33, 2203–2206. [Google Scholar]
- Parker, S.M.; Gibson, G.J. Evaluation of a transcutaneous carbon dioxide monitor (“TOSCA”) in adult patients in routine respiratory practice. Resp. Med. 2007, 101, 261–264. [Google Scholar]
- Dullenkopf, A.; Di Bernardo, S.; Berger, F.; Fasnacht, M.; Gerber, A.C.; Weiss, M. Evaluation of a new combined SpO2/PtcCO2 sensor in anaesthetized paediatric patients. Paediatr. Anaesth. 2003, 13, 777–784. [Google Scholar]
- Bernet-Buettiker, V.; Ugarte, M.J.; Frey, B.; Hug, M.I.; Baenziger, O.; Weiss, M. Evaluation of a new combined transcutaneous measurement of PCO2/pulse oximetry oxygen saturation ear sensor in newborn patients. Pediatrics 2005, 115, e64–e68. [Google Scholar]
- Bernet, V.; Döll, C.; Cannizzaro, V.; Ersch, J.; Frey, B.; Weiss, M. Longtime performance and reliability of two different PtcCO2 and SpO2 sensors in neonates. Paediatr. Anaesth. 2008, 18, 872–877. [Google Scholar]
- Lacerenza, S.; De Carolis, M.P.; Fusco, F.P.; La Torre, G.; Chiaradia, G.; Romagnoli, C. An evaluation of a new combined SpO2/PtcCO2 sensor in very low birth weight infants. Anesth. Analg. 2008, 107, 125–129. [Google Scholar]
- Kattwinkel, J.; Perlman, J.M.; Aziz, K.; Colby, C.; Fairchild, K.; Gallagher, J.; Hazinski, M.F.; Halamek, L.P.; Kumar, P.; Little, G.; et al. American heart association. Neonatal resuscitation: 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics 2010, 126, e1400–e1413. [Google Scholar]
- O'Donnell, C.P.; Kamlin, C.O.; Davis, P.G.; Morley, C.J. Feasibility of and delay in obtaining pulse oximetry during neonatal resuscitation. J. Pediatr. 2005, 147, 698–699. [Google Scholar]
- O'Donnell, C.P.; Kamlin, C.O.; Davis, P.G.; Morley, C.J. Obtaining pulse oximetry data in neonates: A randomised crossover study of sensor application techniques. Arch. Dis. Child Fetal Neonatal Ed. 2005, 90, 84–85. [Google Scholar]
- Kelly, A.M.; Klim, S. Agreement between arterial and transcutaneous PCO2 in patients undergoing non-invasive ventilation. Respir. Med. 2011, 105, 226–229. [Google Scholar]
- De Oliveira, G.S., Jr.; Ahmad, S.; Fitzgerald, P.C.; McCarthy, R.J. Detection of hypoventilation during deep sedation in patients undergoing ambulatory gynaecological hysteroscopy: A comparison between transcutaneous and nasal end-tidal carbon dioxide measurements. Br. J. Anaesth. 2010, 104, 774–778. [Google Scholar]
- Bland, M.J.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurements. Lancet 1986, 1, 307–310. [Google Scholar]
- O'Donnell, C.P.; Kamlin, C.O.; Davis, P.G.; Carlin, J.B.; Morley, C.J. Clinical assessment of infant colour at delivery. Arch. Dis. Child-Fetal Neonatal. 2007, 92, 465–467. [Google Scholar]
- Hay, W.W., Jr.; Rodden, D.J.; Collins, S.M.; Melara, D.L.; Hale, K.A.; Fashaw, L.M. Reliability of conventional and new pulse oximetry in neonatal patients. J. Perinatol. 2002, 22, 360–366. [Google Scholar]
- Koch, G.; Wendel, H. Comparison of pH, carbon dioxide tension, standard bicarbonate and oxygen tension in arterial and capillary blood in the newborn period. Acta Paediatr. Scand. 1967, 56, 10–16. [Google Scholar]
- Saugstad, O.D. New guidelines for newborn resuscitation. Acta Paediatr. 2007, 96, 333–337. [Google Scholar]
- Perlman, J.; Kattwinkel, J.; Wyllie, J.; Guinsburg, R.; Velaphi, S. Nalini singhal for the neonatal ILCOR task force group. Neonatal resuscitation: In pursuit of evidence gaps in knowledge. Resuscitation 2012, 83, 545–550. [Google Scholar]
- Vento, M.; Cheung, P.Y.; Aguar, M. The first golden minutes of the extremely-low-gestational-age neonate: A gentle approach. Neonatology 2009, 95, 286–298. [Google Scholar]


© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rubortone, S.A.; De Carolis, M.P.; Lacerenza, S.; Bersani, I.; Occhipinti, F.; Romagnoli, C. Use of a Combined SpO2/PtcCO2 Sensor in the Delivery Room. Sensors 2012, 12, 10980-10989. https://doi.org/10.3390/s120810980
Rubortone SA, De Carolis MP, Lacerenza S, Bersani I, Occhipinti F, Romagnoli C. Use of a Combined SpO2/PtcCO2 Sensor in the Delivery Room. Sensors. 2012; 12(8):10980-10989. https://doi.org/10.3390/s120810980
Chicago/Turabian StyleRubortone, Serena Antonia, Maria Pia De Carolis, Serafina Lacerenza, Iliana Bersani, Federica Occhipinti, and Costantino Romagnoli. 2012. "Use of a Combined SpO2/PtcCO2 Sensor in the Delivery Room" Sensors 12, no. 8: 10980-10989. https://doi.org/10.3390/s120810980
APA StyleRubortone, S. A., De Carolis, M. P., Lacerenza, S., Bersani, I., Occhipinti, F., & Romagnoli, C. (2012). Use of a Combined SpO2/PtcCO2 Sensor in the Delivery Room. Sensors, 12(8), 10980-10989. https://doi.org/10.3390/s120810980



