Cell-Based in Vitro Blood–Brain Barrier Model Can Rapidly Evaluate Nanoparticles’ Brain Permeability in Association with Particle Size and Surface Modification
Abstract
:1. Introduction
2. Results and Discussion
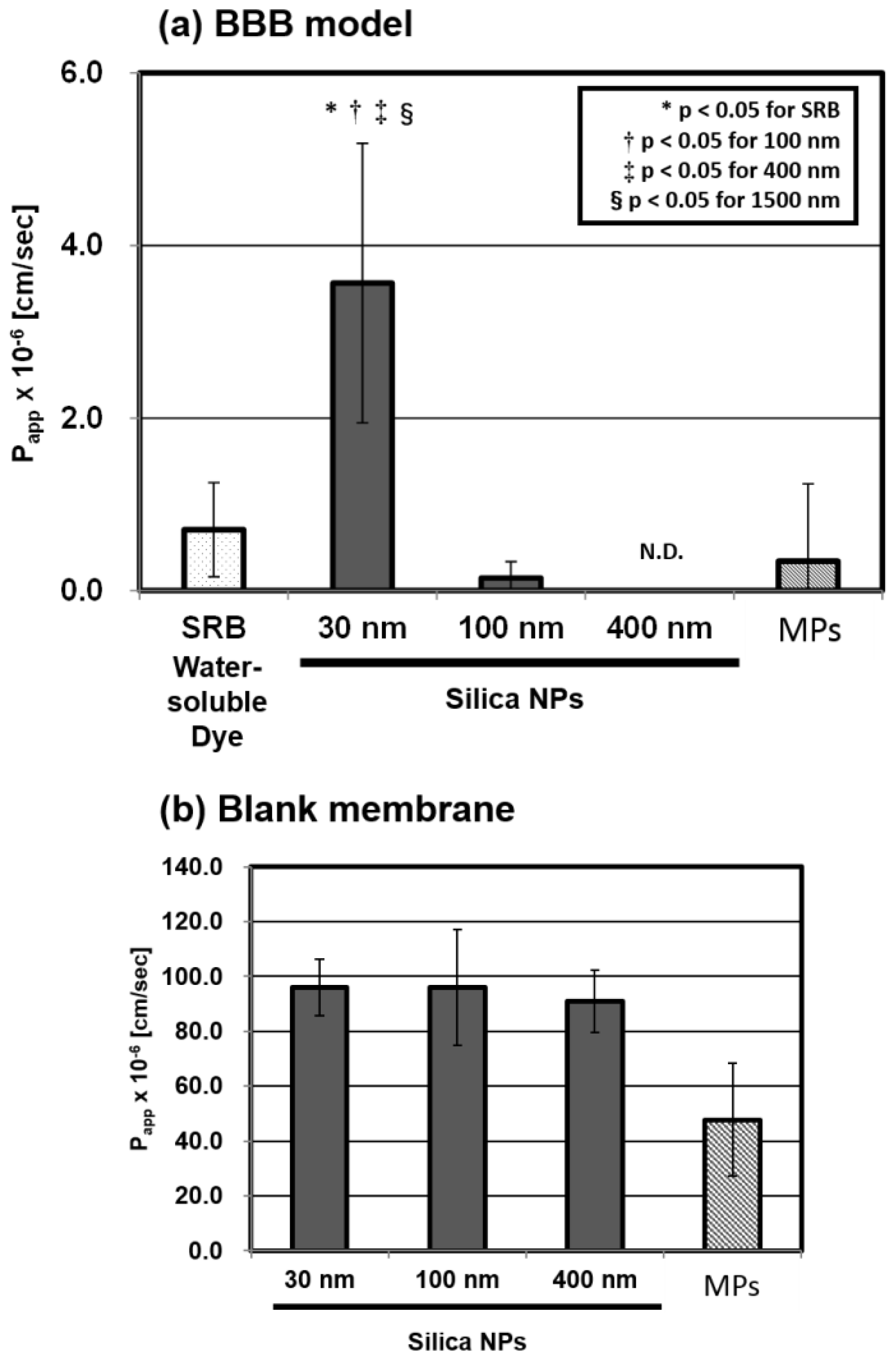
2.1. Size Dependency of BBB Permeability by Silica NPs
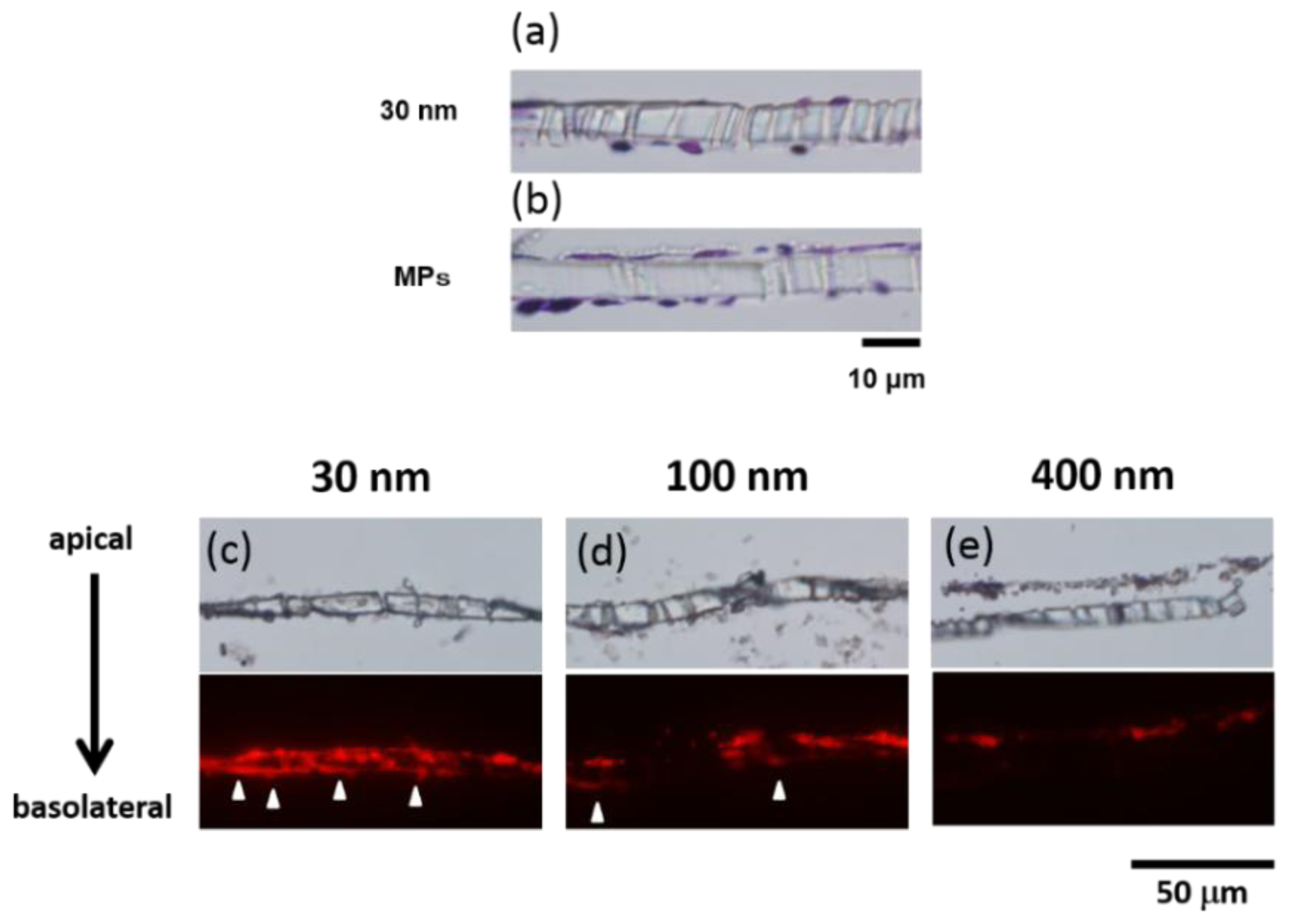
2.2. Microscopic Analysis of the BBB Model
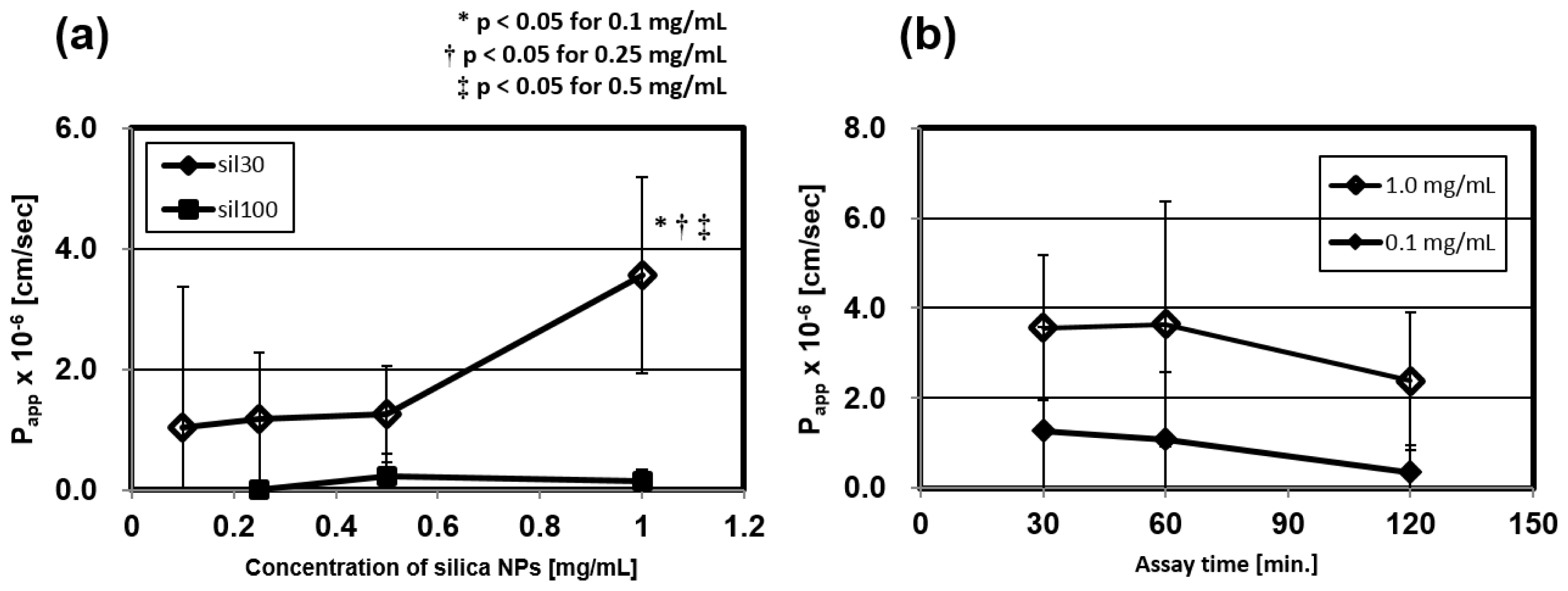
2.3. Detailed Evaluation of BBB Permeability by Silica NPs
2.3.1. Concentration Dependency of the Papp
2.3.2. Time Dependency of the Papp
2.4. Surface-Charge Dependency of BBB Permeability Using the Qdots
3. Experimental Section
3.1. Nanoparticles
3.2. The Rat Blood–Brain Barrier (BBB) in Vitro Model
3.3. Experimental Conditions for the BBB Permeability Assay
3.4. Calculation of Permeability Coefficient (Papp)
3.5. Histology and Fluorescent Microscopy Experiments
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsS.H. and K.F. designed the experiment under the supervision of Y.M. and K.Y.; S.H. performed the experiments and analyzed the data with the advice of K.F. and Y.I.; K.F., Y.I., F.K. and Y.M. discussed the results; K.Y. gave conceptual advice; S.H. wrote the manuscript; all authors commented on the manuscript at all stages.
References
- Ahn, J.H.; Kim, H.S.; Lee, K.J.; Jeon, S.; Kang, S.J.; Sun, Y.; Nuzzo, R.G.; Rogers, J.A. Heterogeneous three-dimensional electronics by use of printed semiconductor nanomaterials. Science 2006, 314, 1754–1757. [Google Scholar]
- Sirbuly, D.J.; Law, M.; Yan, H.; Yang, P. Semiconductor nanowires for subwavelength photonics integration. J. Phys. Chem. B 2005, 109, 15190–15213. [Google Scholar]
- Chan, W.C.W.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotech 2002, 13, 40–46. [Google Scholar]
- Tan, W.; Wang, K.; He, X.; Zhao, X.J.; Drake, T.; Wang, L.; Bagwe, R.P. Bionanotechnology based on silica nanoparticles. Med. Res. Rev 2004, 24, 621–638. [Google Scholar]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng 2005, 100, 1–11. [Google Scholar]
- Alivisatos, A.P.; Gu, W.; Larabell, C. Quantum dots as cellular probes. Ann. Rev. Biomed. Eng 2005, 7, 55–76. [Google Scholar]
- Landsiedel, R.; Ma-Hock, L.; Van Ravenzwaay, B.; Schulz, M.; Wiench, K.; Champ, S.; Schulte, S.; Wohlleben, W.; Oesch, F. Gene toxicity studies on titanium dioxide and zinc oxide nanomaterials used for UV-protection in cosmetic formulations. Nanotoxicology 2010, 4, 364–381. [Google Scholar]
- Chang, J.S.; Chang, K.L.B.; Hwang, D.F.; Kong, Z.L. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ. Sci. Technol 2007, 41, 2064–2068. [Google Scholar]
- Auffan, M.; Pedeutour, M.; Rose, J.; Masion, A.; Ziarelli, F.; Borschneck, D.; Chaneac, C.; Botta, C.; Chaurand, P.; Labille, J.; et al. Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ. Sci. Technol 2010, 44, 2689–2694. [Google Scholar]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol 2008, 3, 145–150. [Google Scholar]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater 2009, 8, 543–557. [Google Scholar]
- Hoshino, A.; Manabe, N.; Fujioka, K.; Hanada, S.; Yasuhara, M.; Kondo, A.; Yamamoto, K. GFP expression by intracellular gene delivery of GFP-coding fragments using nanocrystal quantum dots. Nanotechnology 2008, 19, 495102. [Google Scholar]
- Manabe, N.; Hoshino, A.; Liang, Y.; Goto, T.; Kato, N.; Yamamoto, K. Quantum dot as a drug tracer in vivo. IEEE Trans. NanoBiosci. 2006, 5, 263–267. [Google Scholar]
- Hanada, S.; Fujioka, K.; Futamura, Y.; Manabe, N.; Hoshino, A.; Yamamoto, K. Evaluation of anti-inflammatory drug-conjugated silicon quantum dots: Their cytotoxicity and biological effect. Int. J. Mol. Sci 2013, 14, 1323–1334. [Google Scholar]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol 2004, 22, 969–976. [Google Scholar]
- Morgan, N.Y.; English, S.; Chen, W.; Chernomordik, V.; Russo, A.; Smith, P.D.; Gandjbakhche, A. Real time in vivo non-invasive optical imaging using near-infrared fluorescent quantum dots. Acad. Radiol 2005, 12, 313–323. [Google Scholar]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar]
- Yamamoto, S.; Manabe, N.; Fujioka, K.; Hoshino, A.; Yamamoto, K. Visualizing vitreous using quantum dots as imaging agents. IEEE Trans. NanoBiosci 2007, 6, 94–98. [Google Scholar]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol 2006, 40, 4374–4381. [Google Scholar]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharmacol 2006, 217, 252–259. [Google Scholar]
- Nabeshi, H.; Yoshikawa, T.; Matsuyama, K.; Nakazato, Y.; Matsuo, K.; Arimori, A.; Isobe, M.; Tochigi, S.; Kondoh, S.; Hirai, T.; et al. Systemic distribution, nuclear entry and cytotoxicity of amorphous nanosilica following topical application. Biomaterials 2011, 32, 2713–2724. [Google Scholar]
- Wang, F.; Gao, F.; Lan, M.; Yuan, H.; Huang, Y.; Liu, J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol. In Vitro 2009, 23, 808–815. [Google Scholar]
- Hoshino, A.; Fujioka, K.; Oku, T.; Suga, M.; Sasaki, Y.F.; Ohta, T.; Yasuhara, M.; Suzuki, K.; Yamamoto, K. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett 2004, 4, 2163–2169. [Google Scholar]
- Hoshino, A.; Hanada, S.; Yamamoto, K. Toxicity of nanocrystal quantum dots: The relevance of surface modifications. Arch. Toxicol 2011, 85, 707–720. [Google Scholar]
- Praetner, M.; Rehberg, M.; Bihari, P.; Lerchenberger, M.; Uhl, B.; Holzer, M.; Eichhorn, M.E.; Fürst, R.; Perisic, T.; Reichel, C.A.; et al. The contribution of the capillary endothelium to blood clearance and tissue deposition of anionic quantum dots in vivo. Biomaterials 2010, 31, 6692–6700. [Google Scholar]
- Fujioka, K.; Hiruoka, M.; Sato, K.; Manabe, N.; Miyasaka, R.; Hanada, S.; Hoshino, A.; Tilley, R.D.; Manome, Y.; Hirakuri, K.; et al. Luminescent passive-oxidized silicon quantum dots as biological staining labels and their cytotoxicity effects at high concentration. Nanotechnology 2008, 19, 415102. [Google Scholar]
- Shiohara, A.; Hanada, S.; Prabakar, S.; Fujioka, K.; Lim, T.H.; Yamamoto, K.; Northcote, P.T.; Tilley, R.D. Chemical reactions on surface molecules attached to silicon quantum dots. J. Am. Chem. Soc 2010, 132, 248–253. [Google Scholar]
- Byrne, J.D.; Baugh, J.A. The significance of nanoparticles in particle-induced pulmonary fibrosis. McGill J. Med 2008, 11, 43–50. [Google Scholar]
- Alyautdin, R.N.; Petrov, V.E.; Langer, K.; Berthold, A.; Kharkevich, D.A.; Kreuter, J. Delivery of loperamide across the blood–brain barrier with polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Pharm. Res 1997, 14, 325–328. [Google Scholar]
- Calvo, P.; Gouritin, B.; Chacun, H.; Desmaële, D.; D’Angelo, J.; Noel, J.P.; Georgin, D.; Fattal, E.; Andreux, J.P.; Couvreur, P. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm. Res 2001, 18, 1157–1166. [Google Scholar]
- Costantino, L.; Gandolfi, F.; Tosi, G.; Rivasi, F.; Vandelli, M.A.; Forni, F. Peptide-derivatized biodegradable nanoparticles able to cross the blood–brain barrier. J. Control. Release 2005, 108, 84–96. [Google Scholar]
- Fenart, L.; Casanova, A.; Dehouck, B.; Duhem, C.; Slupek, S.; Cecchelli, R.; Betbeder, D. Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood–brain barrier. J. Pharmacol. Exp. Ther 1999, 291, 1017–1022. [Google Scholar]
- Schroeder, U.; Sommerfeld, P.; Ulrich, S.; Sabel, B.A. Nanoparticle technology for delivery of drugs across the blood–brain barrier. J. Pharm. Sci 1998, 87, 1305–1307. [Google Scholar]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and transferrin-receptor-antibody-modified nanoparticles enable drug delivery across the blood–brain barrier (BBB). Eur. J. Pharm. Biopharm 2009, 71, 251–256. [Google Scholar]
- Wohlfart, S.; Gelperina, S.; Kreuter, J. Transport of drugs across the blood–brain barrier by nanoparticles. J. Control. Release 2012, 161, 264–273. [Google Scholar]
- Brun, E.; Carrière, M.; Mabondzo, A. In vitro evidence of dysregulation of blood–brain barrier function after acute and repeated/long-term exposure to TiO2 nanoparticles. Biomaterials 2012, 33, 886–896. [Google Scholar]
- Ragnaill, M.N.; Brown, M.; Ye, D.; Bramini, M.; Callanan, S.; Lynch, I.; Dawson, K.A. Internal benchmarking of a human blood–brain barrier cell model for screening of nanoparticle uptake and transcytosis. Eur. J. Pharm. Biopharm 2011, 77, 360–367. [Google Scholar]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol 2004, 16, 437–445. [Google Scholar]
- Wang, J.; Liu, Y.; Jiao, F.; Lao, F.; Li, W.; Gu, Y.; Li, Y.; Ge, C.; Zhou, G.; Li, B.; et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO2 nanoparticles. Toxicology 2008, 254, 82–90. [Google Scholar]
- Kato, S.; Itoh, K.; Yaoi, T.; Tozawa, T.; Yoshikawa, Y.; Yasui, H.; Kanamura, N.; Hoshino, A.; Manabe, N.; Yamamoto, K.; et al. Organ distribution of quantum dots after intraperitoneal administration, with special reference to area-specific distribution in the brain. Nanotechnology 2010, 21, 335103. [Google Scholar]
- Yamashita, K.; Yoshioka, Y.; Higashisaka, K.; Mimura, K.; Morishita, Y.; Nozaki, M.; Yoshida, T.; Ogura, T.; Nabeshi, H.; Nagano, K.; et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol 2011, 6, 321–328. [Google Scholar]
- Moore, M.N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ. Int 2006, 32, 967–976. [Google Scholar]
- Dosunmu, R.; Wu, J.; Basha, M.R.; Zawia, N.H. Environmental and dietary risk factors in Alzheimer’s disease. Expert Rev. Neurother 2007, 7, 887–900. [Google Scholar]
- Hoet, P.H.M.; Bruske-Hohlfeld, I.; Salata, O.V. Nanoparticles—Known and unknown health risks. J. Nanobiotechnol 2004, 2, 12. [Google Scholar]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, A.; Tanaka, K.; Niwa, M. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int 2009, 54, 253–263. [Google Scholar]
- Nakagawa, S.; Deli, M.A.; Nakao, S.; Honda, M.; Hayashi, K.; Nakaoke, R.; Kataoka, Y.; Niwa, M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell. Mol. Neurobiol 2007, 27, 687–694. [Google Scholar]
- Weissleder, R.; Kelly, K.; Sun, E.Y.; Shtatland, T.; Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotech 2005, 23, 1418–1423. [Google Scholar]
- Lemarchand, C.; Gref, R.; Passirani, C.; Garcion, E.; Petri, B.; Müller, R.; Couvreur, P. Influence of polysaccharide coating on the interactions of nanoparticles with biological systems. Biomaterials 2006, 27, 108–118. [Google Scholar]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 2006, 6, 662–668. [Google Scholar]
- Lu, F.; Wu, S.H.; Hung, Y.; Mou, C.Y. Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar]
- Doran, A.; Obach, R.S.; Smith, B.J.; Hosea, N.A.; Becker, S.; Callegari, E.; Chen, C.; Chen, X.; Choo, E.; Cianfrogna, J.; et al. The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: Evaluation using the MDR1A/1B knockout mouse model. Drug Metabol. Dispos 2005, 33, 165–174. [Google Scholar]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size. Colloids Surf. B 2008, 66, 274–280. [Google Scholar]
- Tricklar, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W.; et al. Silver nanoparticle induced blood–brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol. Sci 2010, 118, 160–170. [Google Scholar]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar]
- Napierska, D.; Thomassen, L.C.; Rabolli, V.; Lison, D.; Gonzalez, L.; Kirsch-Volders, M.; Martens, J.A.; Hoet, P.H. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small 2009, 5, 846–853. [Google Scholar]
- Slemmer, J.E.; Shacka, J.J.; Sweeney, M.I.; Weber, J.T. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr. Med. Chem 2008, 15, 404–414. [Google Scholar]
- Zhang, L.W.; Monteiro-Riviere, N.A. Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol. Sci 2009, 110, 138–155. [Google Scholar]
- Lesniak, A.; Fenaroli, F.; Monopoli, M.P.; Aberg, C.; Dawson, K.A.; Salvati, A. Effect of the presence or absence of a protein corona on silica nanoparticle uptake and impact on cells. ACS Nano 2012, 6, 5845–5857. [Google Scholar]
- Ge, C.; Du, J.; Zhao, L.; Wang, L.; Liu, Y.; Li, D.; Yang, Y.; Zhou, R.; Zhao, Y.; Chai, Z.; et al. Binding of blood proteins to carbon nanotubes reduces cytotoxicity. Proc. Natl. Acad. Sci. USA 2011, 108, 16968–16973. [Google Scholar]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein corona-mediated mitigation of cytotoxicity of graphene oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar]


| Silica | 30 nm | 100 nm | 400 nm | MPs/1500 nm | |
|---|---|---|---|---|---|
| Particle collection ratio (%) | BBB | 61.0 ± 9.3 | 77.2 ± 6.5 | 68.9 ± 9.0 | 32.3 ± 12.3 |
| Blank | 100 ± 1.7 | 98.1 ± 4.7 | 96.3 ± 6.5 | 75.8 ± 8.0 |
| Qdot | Carboxyl | PEGylated | Amino | |
|---|---|---|---|---|
| Particle collection ratio (%) | without serum | 73.8 ± 4.3 | 82.0 ± 6.0 | 97.0 ± 3.2 |
| with serum | 101.7 ± 0.3 | 98.6 ± 4.1 | 98.2 ± 0.1 |
| Particle | Peak Diameter (nm) | Z-Average Diameter (nm) | PdI (−) | Zeta Potential (mV) |
|---|---|---|---|---|
| Silica | ||||
| 30 nm | 32.0 ± 1.1 | 29.5 ± 0.5 | 0.162 ± 0.013 | −15.6 ± 1.2 |
| 100 nm | 137.0 ± 1.5 | 129.7 ± 1.4 | 0.042 ± 0.005 | −19.7 ± 1.05 |
| 400 nm | 481.4 ± 22.1 | 460.3 ± 10.1 | 0.164 ± 0.030 | −21.8 ± 2.4 |
| MP/1500 nm | 367.4 ± 157.1 | 0.869 ± 0.080 | −29.3 ± 2.1 | |
| Qdots | ||||
| Carboxyl | 49.85 ± 7.8 | 0.49 ± 0.028 | −32.0 ± 1.3 | |
| PEGylated | 32.9 ± 9.07 | 0.31 ± 0.028 | −3.6 ± 2.1 | |
| Amino | 47.9 ± 3.0 | 0.44 ± 0.005 | −7.3 ± 3.8 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hanada, S.; Fujioka, K.; Inoue, Y.; Kanaya, F.; Manome, Y.; Yamamoto, K. Cell-Based in Vitro Blood–Brain Barrier Model Can Rapidly Evaluate Nanoparticles’ Brain Permeability in Association with Particle Size and Surface Modification. Int. J. Mol. Sci. 2014, 15, 1812-1825. https://doi.org/10.3390/ijms15021812
Hanada S, Fujioka K, Inoue Y, Kanaya F, Manome Y, Yamamoto K. Cell-Based in Vitro Blood–Brain Barrier Model Can Rapidly Evaluate Nanoparticles’ Brain Permeability in Association with Particle Size and Surface Modification. International Journal of Molecular Sciences. 2014; 15(2):1812-1825. https://doi.org/10.3390/ijms15021812
Chicago/Turabian StyleHanada, Sanshiro, Kouki Fujioka, Yuriko Inoue, Fumihide Kanaya, Yoshinobu Manome, and Kenji Yamamoto. 2014. "Cell-Based in Vitro Blood–Brain Barrier Model Can Rapidly Evaluate Nanoparticles’ Brain Permeability in Association with Particle Size and Surface Modification" International Journal of Molecular Sciences 15, no. 2: 1812-1825. https://doi.org/10.3390/ijms15021812





