Oleic Acid Increases Synthesis and Secretion of VEGF in Rat Vascular Smooth Muscle Cells: Role of Oxidative Stress and Impairment in Obesity
Abstract
:1. Introduction
2. Results
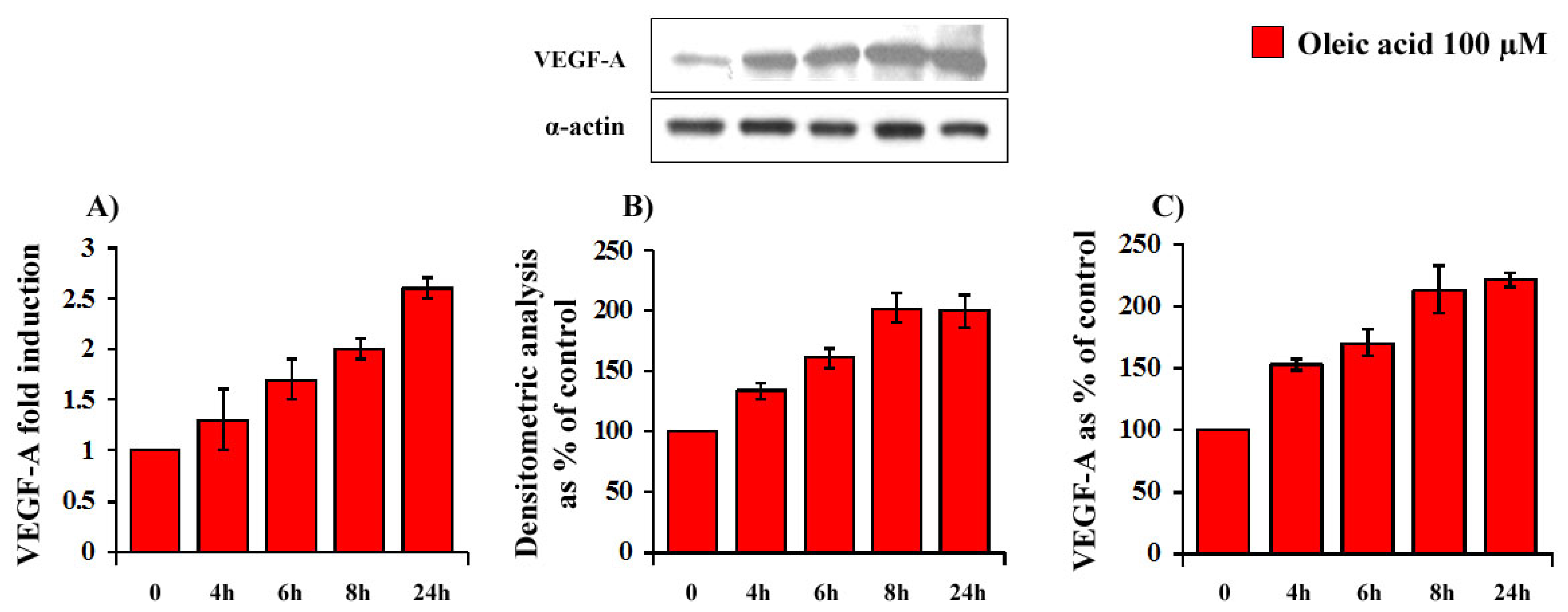
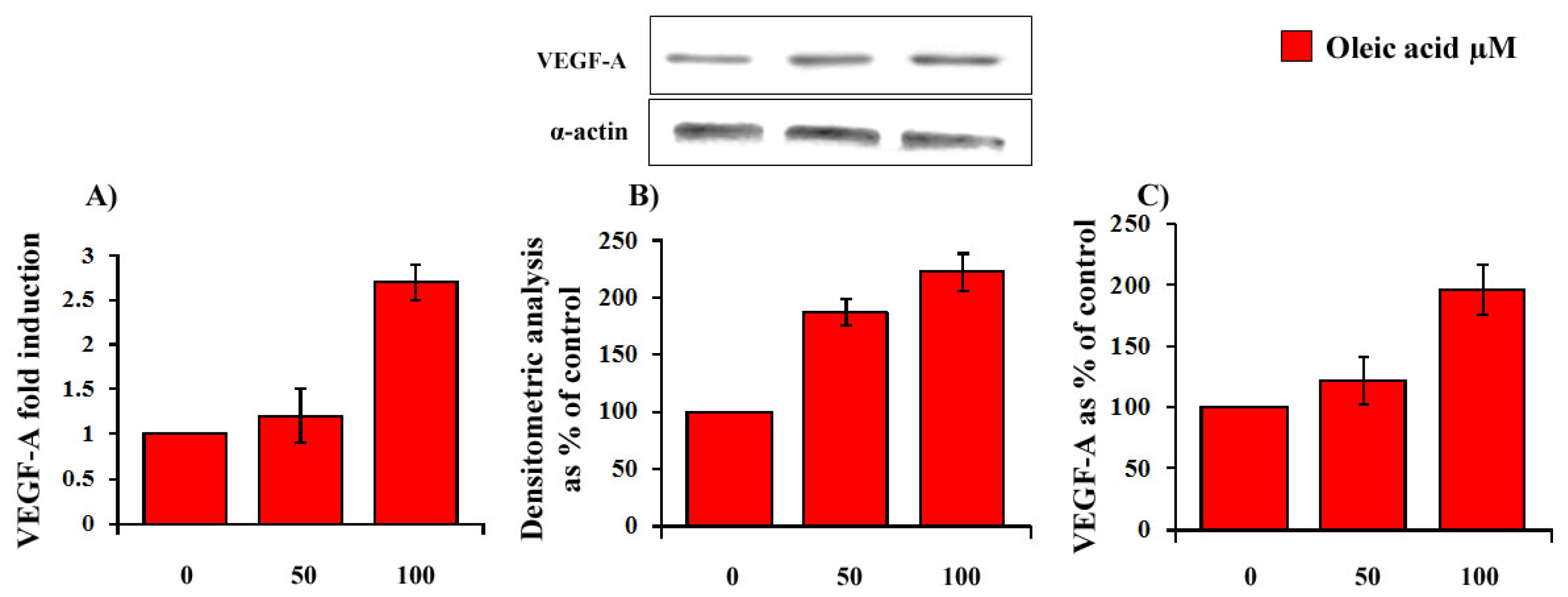
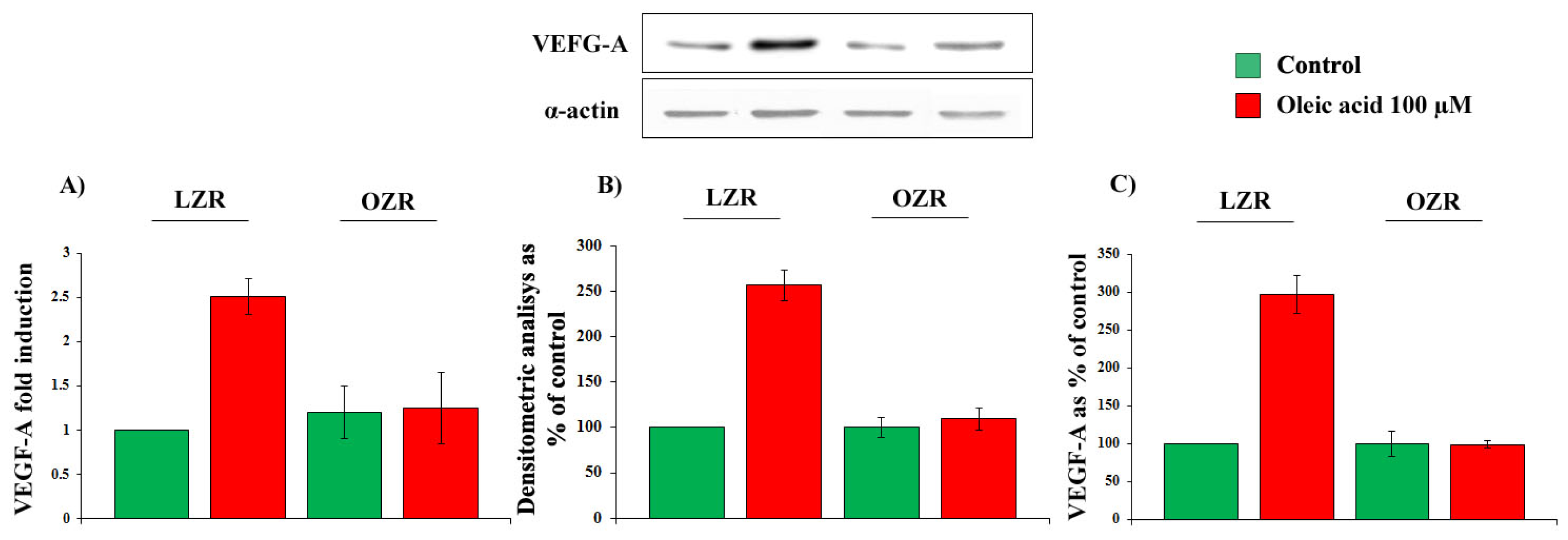
2.1. Time- and Concentration-Dependence of the Oleic Acid Effects on VEGF-A mRNA Transcription and on VEGF-A Protein Synthesis and Secretion in VSMC from LZR and OZR
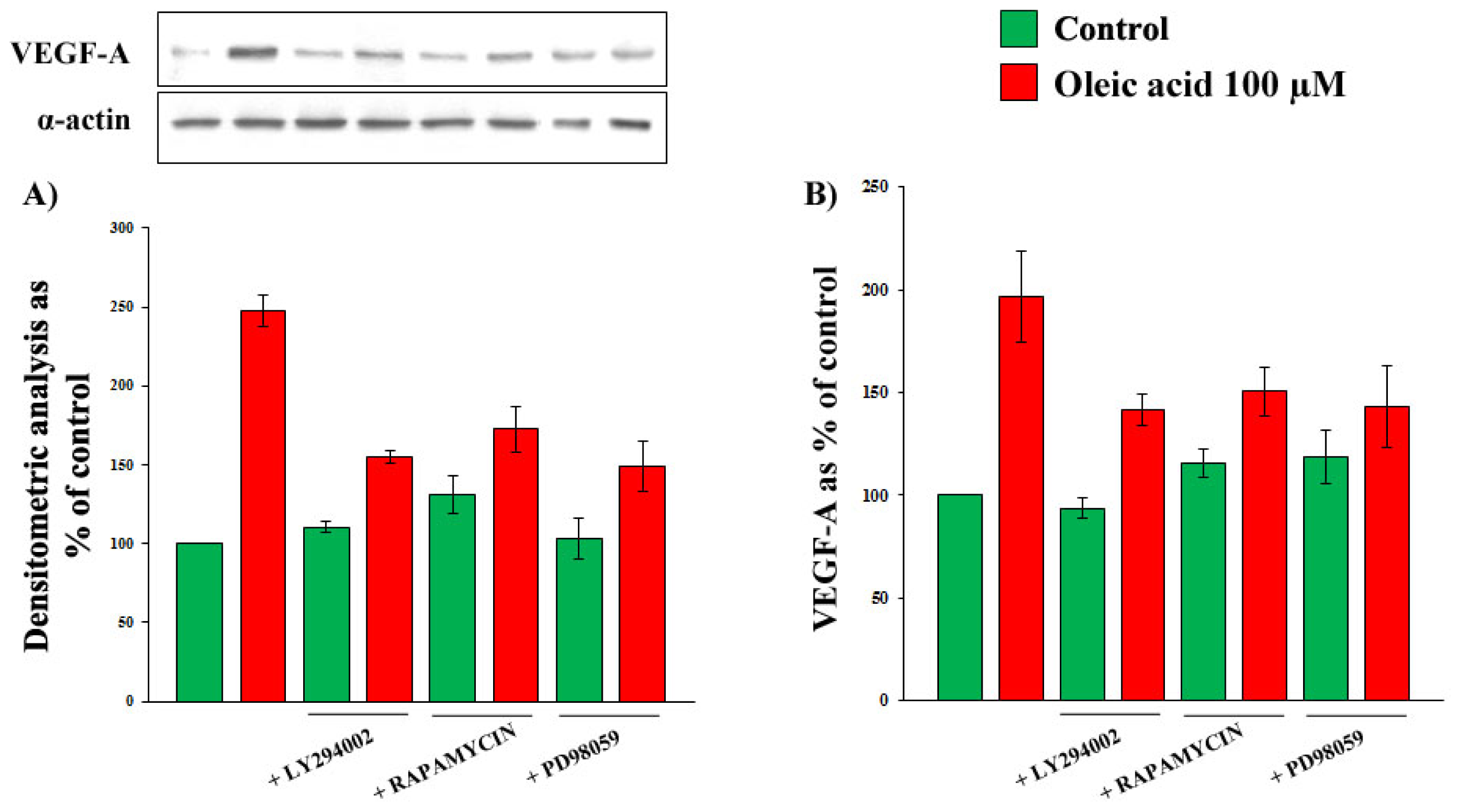
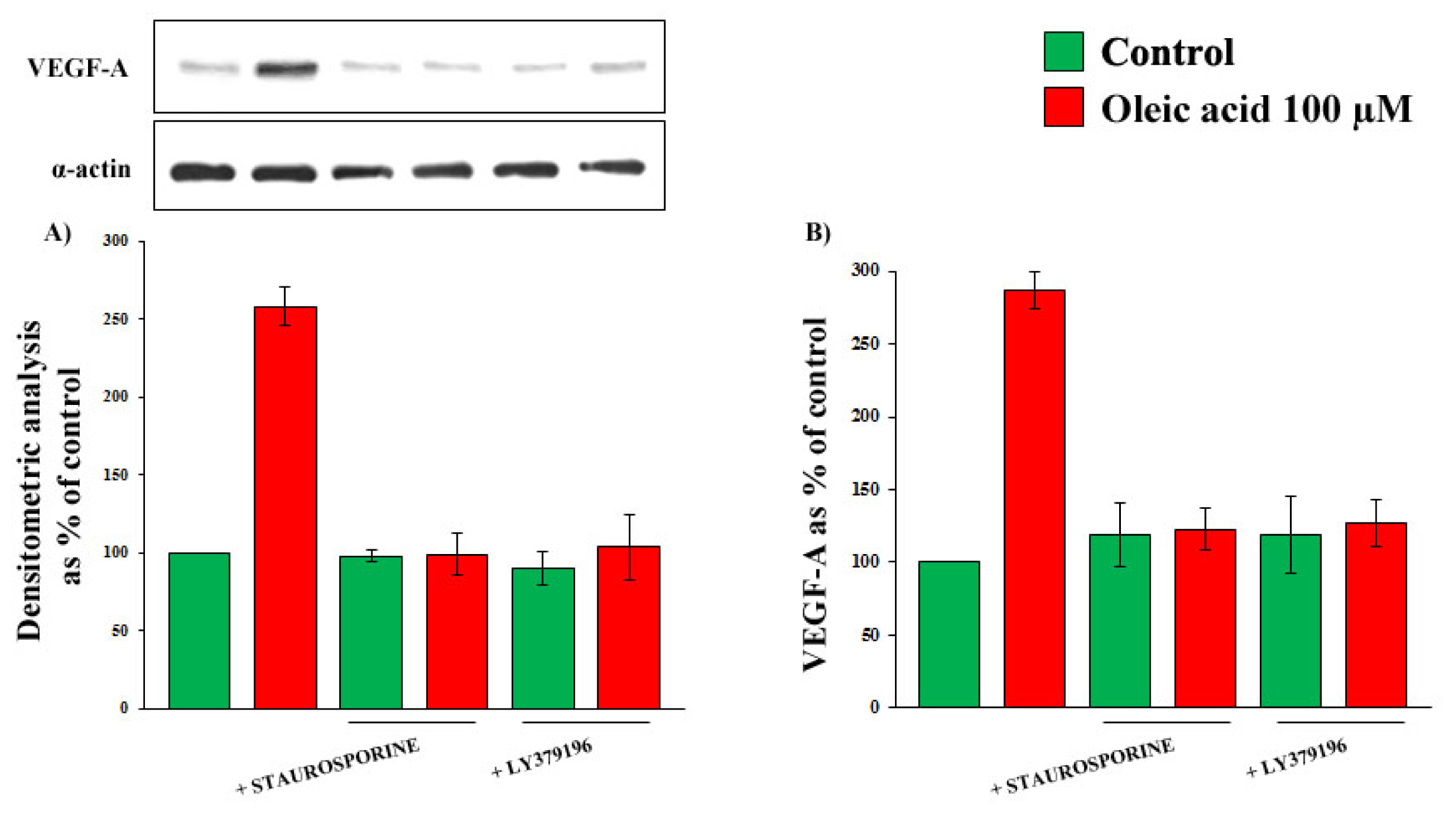
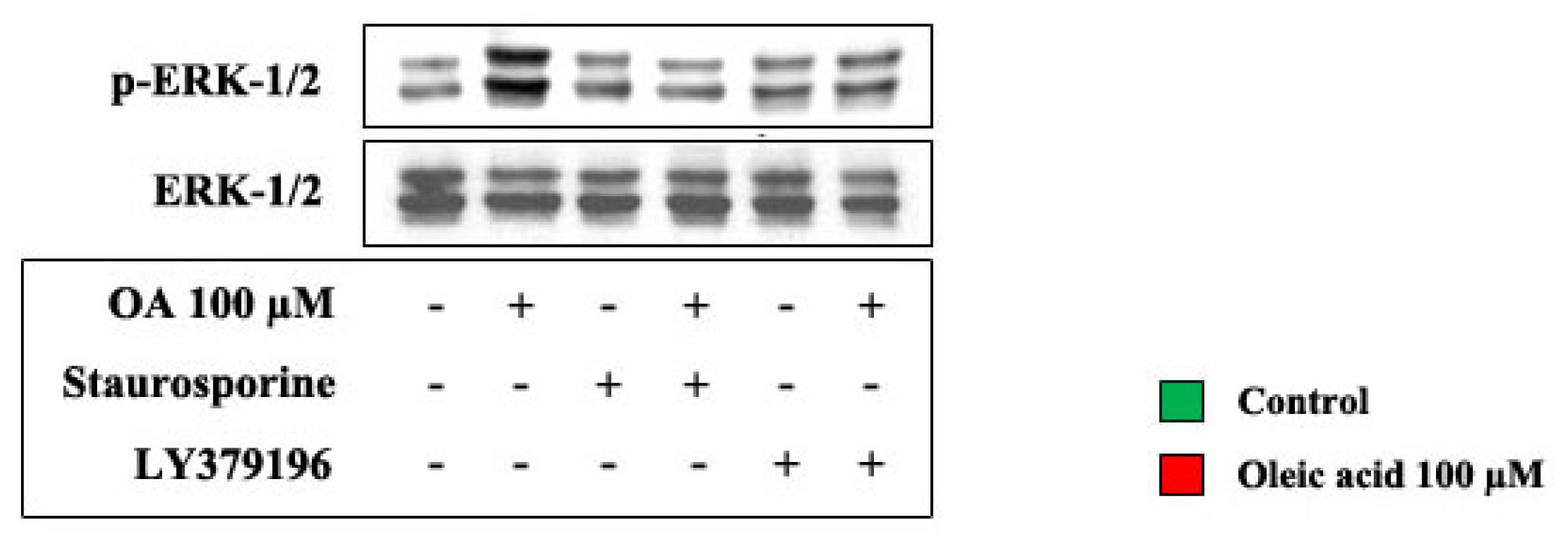
2.2. Role of PI3-K and MAPK Pathways in the Increase of VEGF-A Synthesis and Secretion Induced by Oleic Acid in VSMC from LZR
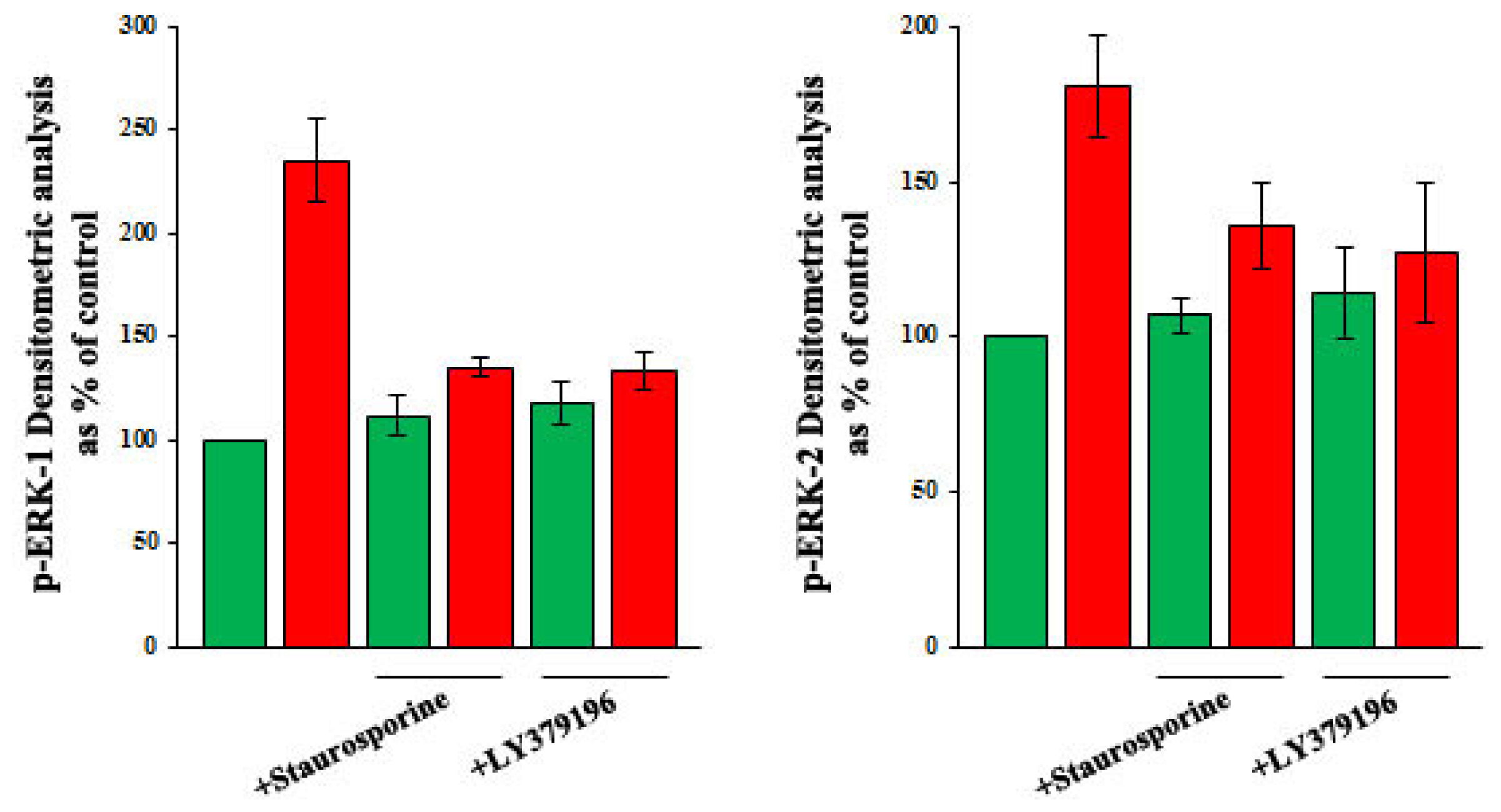
2.3. Role of PKC, and of PKC-Beta in Particular, in the Increase of VEGF-A Synthesis and Secretion Induced by Oleic Acid in VSMC from LZR
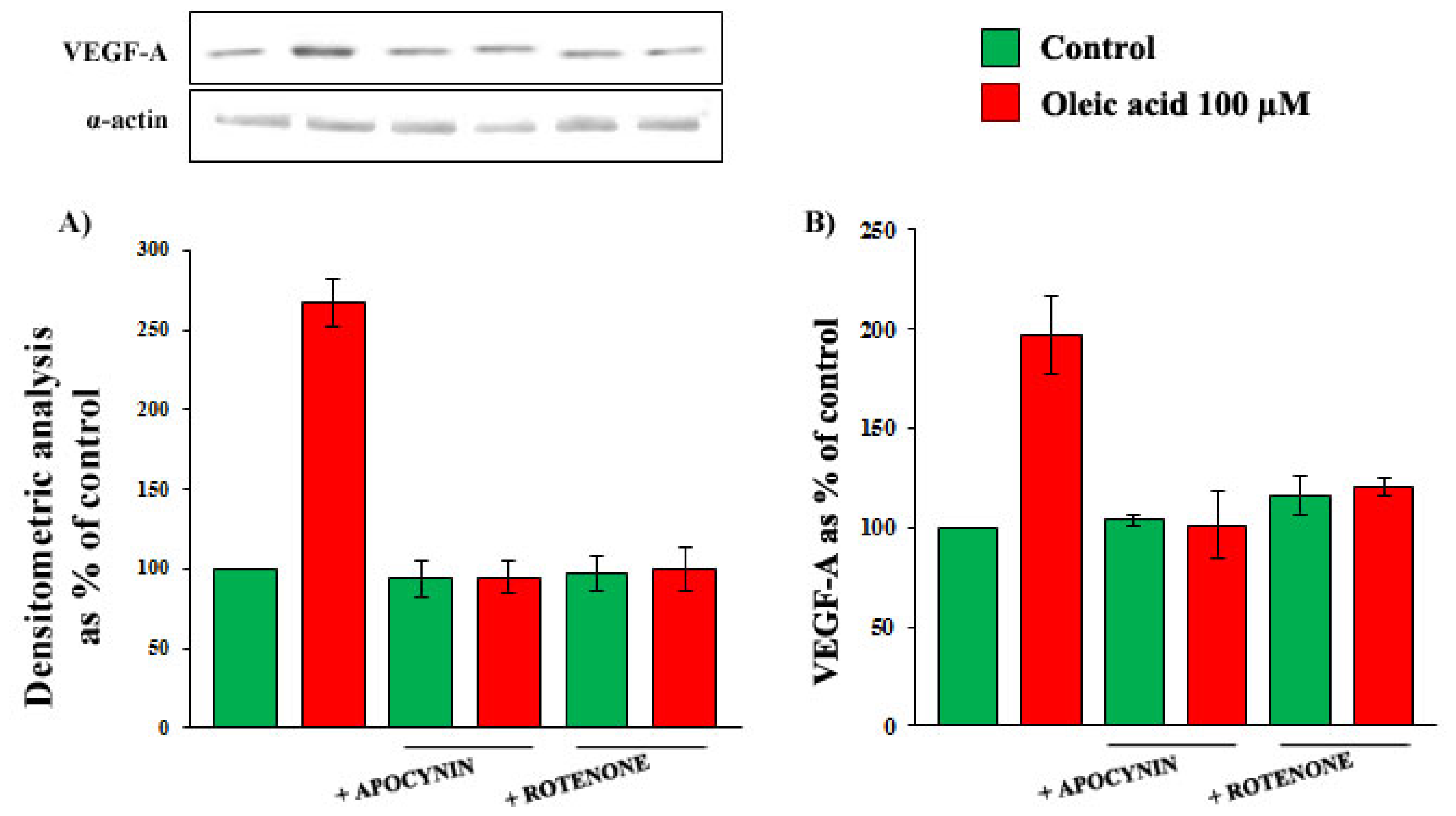
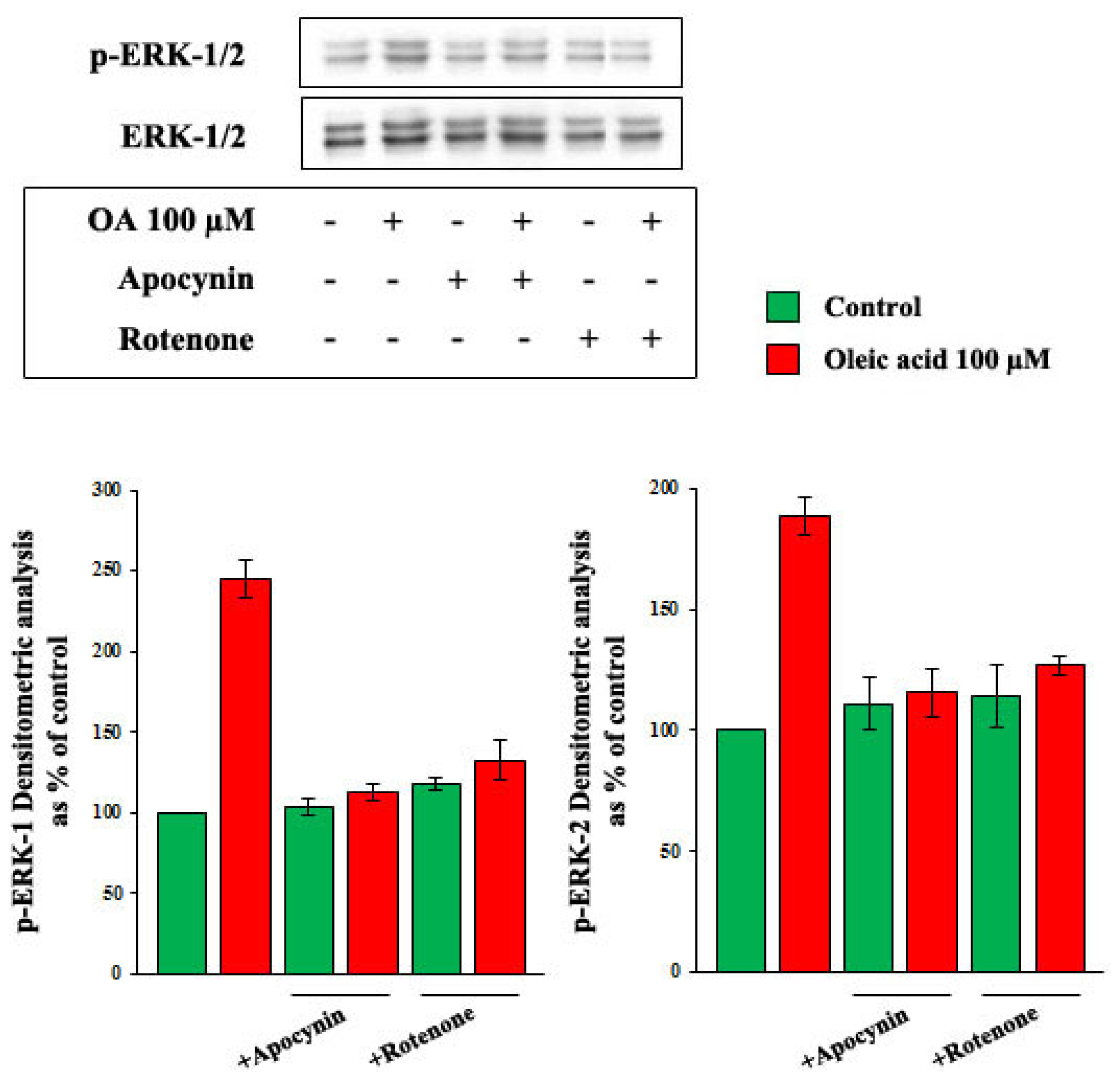
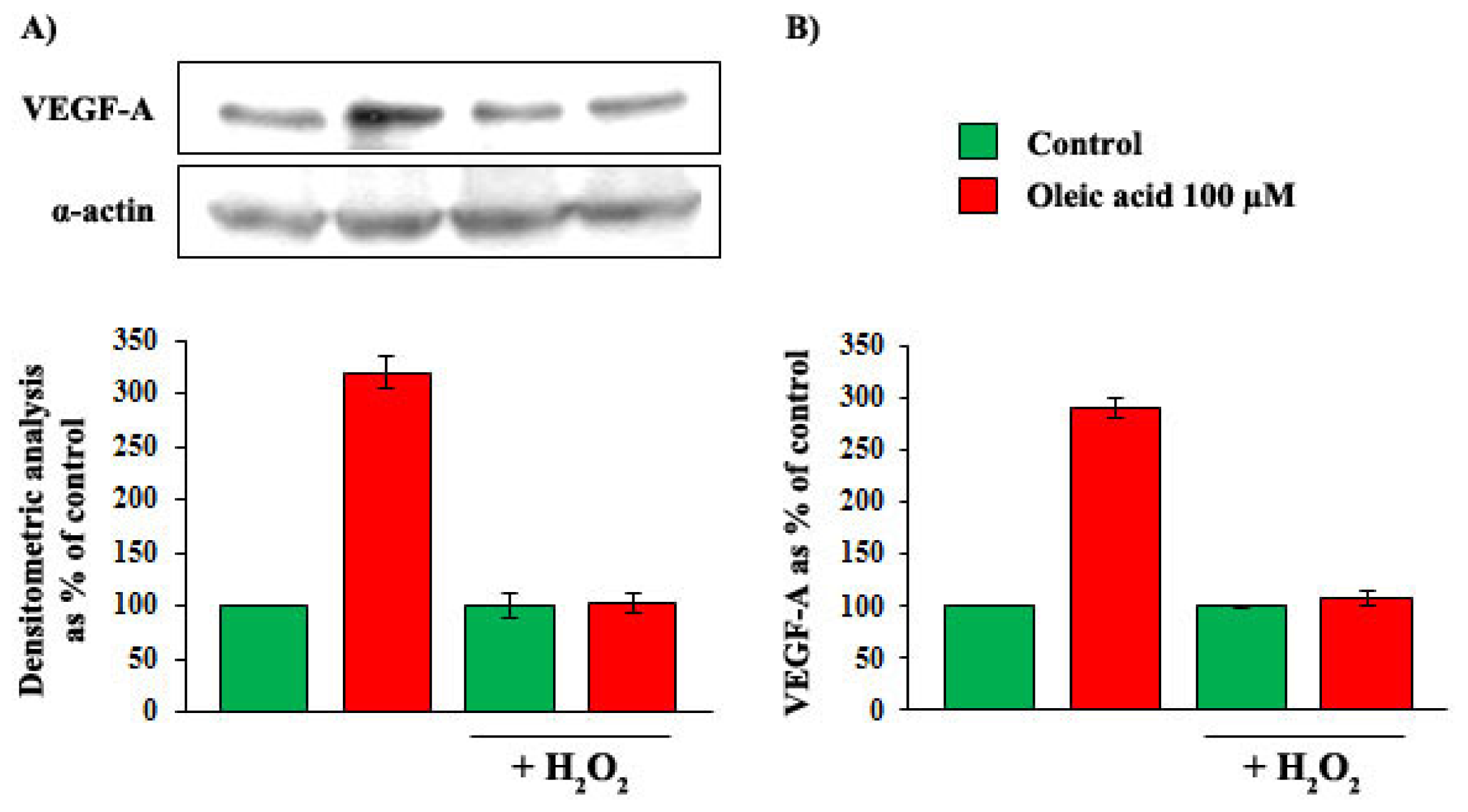
2.4. Role of Reactive Oxygen Species (ROS) in the Increase of VEGF-A Synthesis and Secretion Induced by Oleic Acid in VSMC from LZR
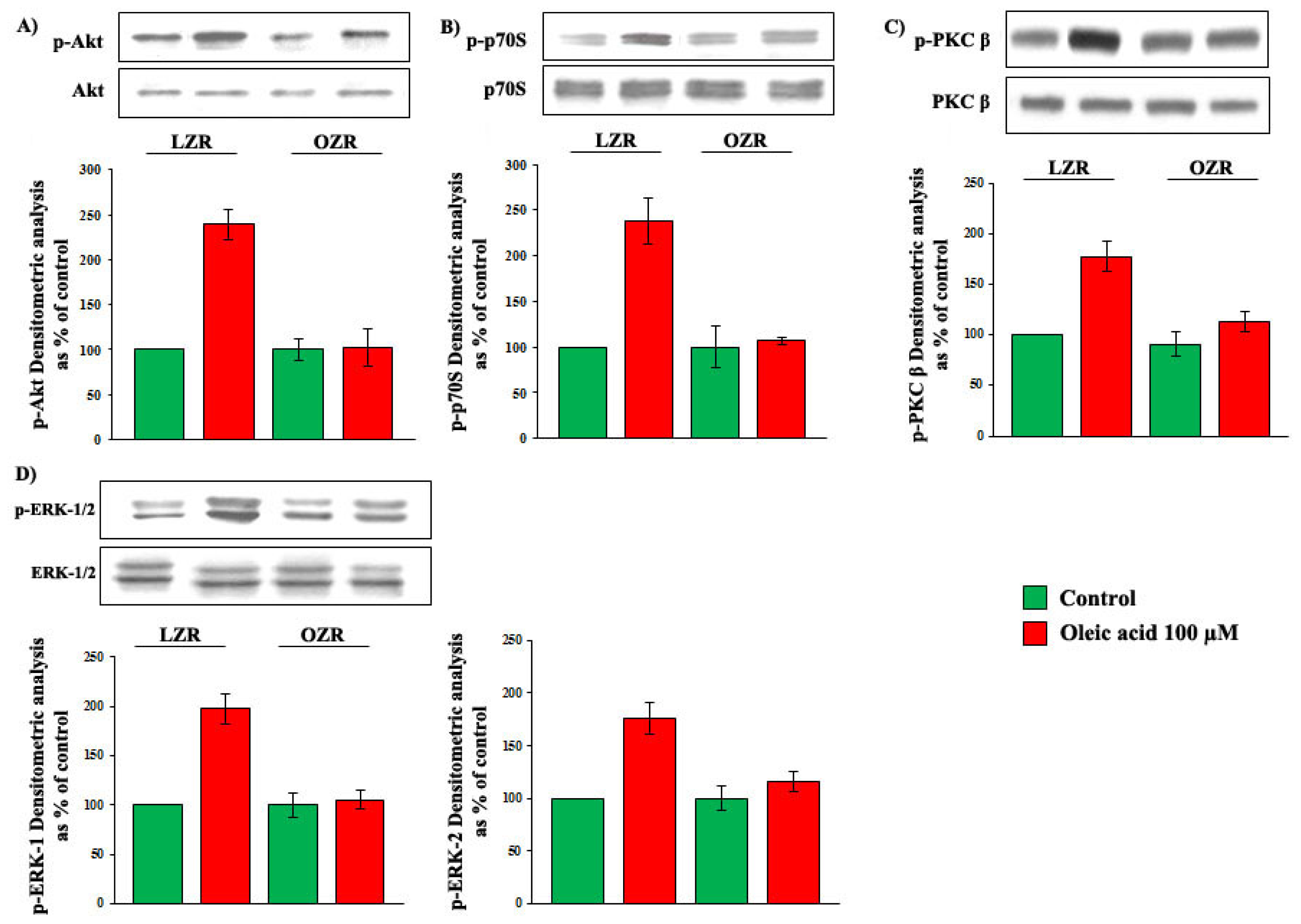
2.5. Oleic Acid Signalling in VSMC from LZR and OZR
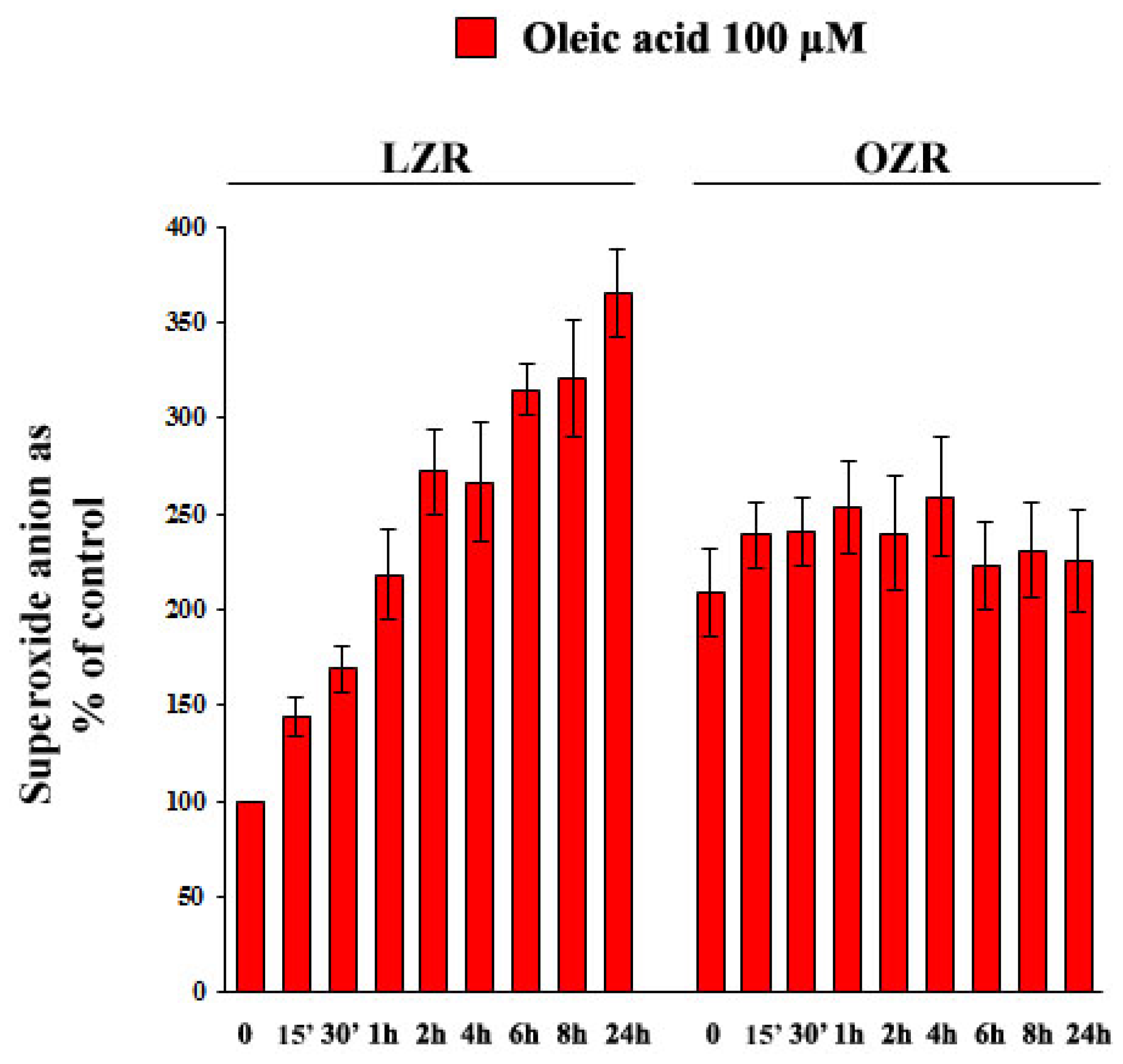
2.6. Oleic Acid-Induced Increase of Superoxide Anion in VSMC from LZR and OZR: Influence of PKC, NADPH Oxidase and Mitochondrial Electron Transport Chain Complex Inhibition
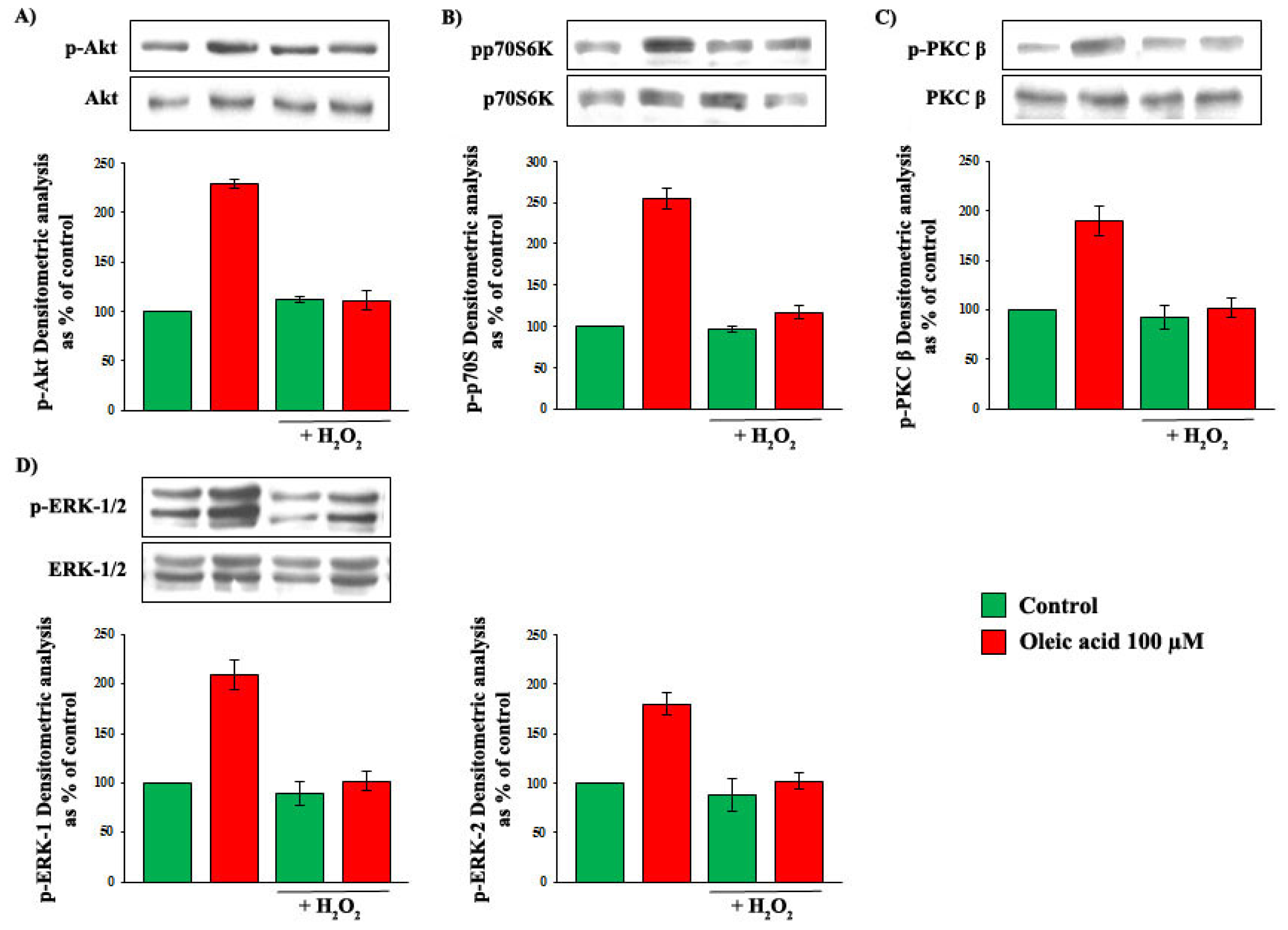
2.7. Influence of Baseline Concentration of ROS on Oleic Acid Signalling in VSMC from LZR
2.8. Influence of Baseline Concentration of ROS on the Increase of VEGF-A Synthesis and Secretion Induced by Oleic Acid in VSMC from LZR
3. Discussion
4. Experimental Section
4.1. Research Design
4.2. Chemicals
4.3. Cell Culture and Characterization
4.4. VEGF-A mRNA Expression
4.5. VEGF-A Secretion and Protein Expression
4.6. Activation of Akt, p70S6K, ERK-1/2, JNK-1/2, p38 MAPK and PKC
4.7. Measurement of Superoxide Anion
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar]
- Lakka, H.M.; Lakka, T.A.; Tuomiletho, J.; Salonen, J.T. Abdominal obesity is associated with increased risk of acute coronary events in men. Eur. Heart J 2002, 23, 706–713. [Google Scholar]
- Hu, F.B.; Willett, W.C.; Li, T.; Stampfer, M.J.; Colditz, G.A.; Manson, J.E. Adiposity as compared with physical activity in predicting mortality among women. N. Engl. J. Med 2004, 351, 2694–2703. [Google Scholar]
- Reaven, G.; Abbasi, F.; McLaughlin, T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog. Horm. Res 2004, 59, 207–223. [Google Scholar]
- Anfossi, G.; Russo, I.; Doronzo, G.; Trovati, M. Contribution of insulin resistance to vascular dysfunction. Arch. Physiol. Biochem 2009, 115, 199–217. [Google Scholar]
- Anfossi, G.; Russo, I.; Trovati, M. Platelet dysfunction in central obesity. Nutr. Metab. Cardiovasc. Dis 2009, 19, 440–449. [Google Scholar]
- Reaven, G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler. Thromb. Vasc. Biol 2012, 32, 1754–1759. [Google Scholar]
- Romeo, G.R.; Lee, J.; Shoelson, S.E. Metabolic syndrome, insulin resistance, and roles of inflammation: Mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol 2012, 8, 1771–1776. [Google Scholar]
- Després, J.P. Abdominal obesity and cardiovascular disease: Is inflammation the missing link? Can. J. Cardiol 2012, 28, 642–652. [Google Scholar]
- Taube, A.; Schlich, R.; Sell, H.; Eckardt, K.; Eckel, J. Inflammation and metabolic dysfunction: Links to cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol 2012, 302, H2148–H2165. [Google Scholar]
- Yilmaz, M.B.; Biyikoglu, S.F.; Akin, Y.; Guray, U.; Kisacik, H.L.; Korkmaz, S. Obesity is associated with impaired coronary collateral vessel development. Int. J. Obes. Relat. Metab. Disord 2003, 27, 1541–1545. [Google Scholar]
- Goldberg, H.L.; Goldstein, J.; Burer, J.S.; Moses, J.W.; Collins, M.B. Functional importance of coronary collateral vessels. Am. J. Cardiol 1984, 53, 694–699. [Google Scholar]
- De Groot, D.; Pasterkamp, G.; Hoefer, I.E. Cardiovascular risk factors and collateral artery formation. Eur. J. Clin. Investig 2009, 39, 1036–1047. [Google Scholar]
- Seiler, C. The human coronary collateral circulation. Eur. J. Clin. Invest 2010, 40, 465–476. [Google Scholar]
- Shuaib, A.; Butcher, K.; Mohammad, A.A.; Saggur, M.; Liebeskind, D.S. Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. Lancet. Neurol 2011, 10, 909–921. [Google Scholar]
- Johnston, P.C.; Vartanian, S.M.; Runge, S.J.; Hiramoto, J.S.; Eichler, C.M.; Owens, C.D.; Schneider, D.B.; Conte, M.S. Risk factors for clinical failure after stent graft treatment for femoropopliteal occlusive disease. J. Vasc. Surg 2012, 56, 998–1006. [Google Scholar]
- Ruiter, M.S.; van Golde, J.M.; Schaper, N.C.; Stehouwer, C.D.; Huijberts, M.S. Diabetes impairs arteriogenesis in the peripheral circulation: Review of molecular mechanisms. Clin. Sci. (Lond. ) 2010, 119, 225–238. [Google Scholar]
- Phillips, M.S.; Liu, Q.; Hammond, H.A.; Dugan, V.; Hey, P.J.; Caskey, C.J.; Hess, J.F. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet 1966, 13, 18–19. [Google Scholar]
- Chou, E.; Suzuma, I.; Way, K.J.; Opland, D.; Clermont, A.C.; Naruse, K.; Suzuma, K.; Bowling, N.L.; Vlahos, C.J.; Aiello, L.P.; et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: A possible explanation for impaired collateral formation in cardiac tissue. Circulation 2002, 105, 373–379. [Google Scholar]
- Janiak, P.; Lainée, P.; Grataloup, Y.; Luyt, C.E.; Bidouard, J.P.; Michel, J.B.; O'Connor, S.E.; Herbert, J.M. Serotonin receptor blockade improves distal perfusion after lower limb ischemia in the fatty Zucker rat. Cardiovasc. Res 2002, 56, 293–302. [Google Scholar]
- Hattan, N.; Chilian, W.M.; Park, F.; Rocic, P. Restoration of coronary collateral growth in the Zucker obese rat: Impact of VEGF and ecSOD. Basic Res. Cardiol 2007, 102, 217–223. [Google Scholar]
- Pung, Y.F.; Rocic, P.; Murphy, M.P.; Smith, R.A.; Hafemeister, J.; Ohanyan, V.; Guarini, G.; Yin, L.; Chilian, W.M. Resolution of mitochondrial oxidative stress rescues coronary collateral growth in zucker obese fatty rats. Arterioscler. Thromb. Vasc. Biol 2012, 32, 325–334. [Google Scholar]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med 2000, 6, 389–395. [Google Scholar]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med 2003, 9, 666–669. [Google Scholar]
- Hattan, N.; Warltier, D.; Gu, W.; Kolz, C.; Chilian, W.M.; Weihrauch, D. Autologous vascular smooth muscle cell-based myocardial gene therapy to induce coronary collateral growth. Am. J. Physiol. Heart Circ. Physiol 2004, 287, H488–H493. [Google Scholar]
- Doronzo, G.; Russo, I.; Mattiello, L.; Anfossi, G.; Bosia, A.; Trovati, M. Insulin activates vascular endothelial growth factor in vascular smooth muscle cells: Influence of nitric oxide and insulin resistance. Eur. J. Clin. Investig 2004, 34, 664–673. [Google Scholar]
- Doronzo, G.; Viretto, M.; Russo, I.; Mattiello, L.; Anfossi, G.; Trovati, M. Effects of high glucose on vascular endothelial growth factor synthesis and secretion in aortic vascular smooth muscle cells from obese and lean Zucker rats. Int. J. Mol. Sci 2012, 13, 9478–9488. [Google Scholar]
- Waki, H.; Tontonoz, P. Endocrine functions of adipose tissue. Annu. Rev. Pathol 2007, 2, 31–56. [Google Scholar]
- Anfossi, G.; Russo, I.; Doronzo, G.; Pomero, A.; Trovati, M. Adipocytokines in atherothrombosis: Focus on platelets and vascular smooth muscle cells. Mediat. Inflamm 2010, 2010, 174341. [Google Scholar]
- Trayhurn, P.; Drevon, C.A.; Eckel, J. Secreted proteins from adipose tissue and skeletal muscle-adipokines, myokines and adipose/muscle cross-talk. Arch. Physiol. Biochem 2011, 117, 47–56. [Google Scholar]
- Northcott, J.M.; Yeganeh, A.; Taylor, C.G.; Zahradka, P.; Wigle, J.T. Adipokines and the cardiovascular system: Mechanisms mediating heath and disease. Can. J. Physiol. Pharmacol 2012, 90, 1029–1059. [Google Scholar]
- Van Dijk, S.J.; Feskens, E.J.M.; Bos, M.B.; Hoelen, D.W.; Heijligenberg, R.; Bromhaar, M.G.; de Groot, L.C.; de Vries, J.H.; Müller, M.; Afman, L.A. A saturated fatty acid-rich diet induces an obesity-linked pronflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am. J. Clin. Nutr 2009, 90, 1656–1664. [Google Scholar]
- Bermudez, B.; Lopez, S.; Ortega, A.; Varela, L.M.; Pacheco, Y.M.; Abia, R.; Muriana, F.J. Oleic acid in olive oil: From a metabolic framework toward a clinical perspective. Curr. Pharm. Des 2011, 17, 831–843. [Google Scholar]
- Lu, G.; Morinelli, T.A.; Meier, K.E.; Rosenzweig, S.A.; Egan, B.M. Oleic acid induced mitogenic signaling in vascular smooth muscle cells: A role for protein kinase C. Circ. Res 1996, 79, 611–618. [Google Scholar]
- Lu, G.; Greene, E.L.; Nagai, T.; Egan, B.M. Reactive oxygen species are critical in the oleic acid-mediated mitogenic signaling pathway in vascular smooth muscle cells. Hypertension 1998, 32, 1003–1010. [Google Scholar]
- Yun, M.R.; Lee, G.J.; Park, H.S.; Heo, H.J.; Park, J.Y.; Bae, S.S.; Hong, K.W.; Sung, S.M.; Kim, C.D. Oleic acid enhances vascular smooth muscle cell proliferation via phosphatidylinositol 3-kinase/Akt signaling pathway. Pharmacol. Res 2006, 54, 97–102. [Google Scholar]
- Jiang, X.; Zhang, Y.; Hou, D.; Yun, M.R.; Lee, J.Y.; Park, H.S.; Heo, H.J.; Park, J.Y.; Bae, S.S.; Hong, K.W. 17-beta-Estradiol inhibits oleic acid-induced rat VSMC Proliferation and migration by restoring PGC-1 expression. Mol. Cell. Endocrinol 2010, 315, 74–80. [Google Scholar]
- Lu, G.; Meier, K.E.; Jaffa, A.A.; Rosenzweig, S.A.; Egan, B.M. Oleic acid and angiotensin II induce synergistic mitogenic response in vascular smooth muscle cells. Hypertension 1998, 31, 978–985. [Google Scholar]
- Greene, E.L.; Lu, G.; Zhang, D.; Egan, B.M. Signaling events mediating the additive effect of oleic acid and angiotensin II on vascular smooth muscle cell migration. Hypertension 2001, 37, 308–312. [Google Scholar]
- Kwok, C.F.; Shih, K.C.; Hwu, C.M.; Ho, L.T. Linoleic acid and oleic acid increase the endothelin-1 binding and action in cultured rat aortic smooth muscle cells. Metabolism 2000, 49, 1386–1389. [Google Scholar]
- Askari, B.; Carroll, M.A.; Capparelli, M.; Kramer, F.; Gerrity, R.G.; Bornfeldt, K.E. Oleate and linoleate enhance the growth-promoting effects of insulin-like growth factor-1 through a phospholipase D-dependent pathway in arterial smooth muscle cells. J. Biol. Chem 2002, 277, 36338–36344. [Google Scholar]
- Lamers, D.; Schlich, R.; Greulich, S.; Sasson, S.; Sell, H.; Eckel, J. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signalling in human vascular smooth muscle cells. J. Cell Mol. Med 2011, 15, 1177–1188. [Google Scholar]
- Lamers, D.; Schlich, R.; Horrighs, A.; Cramer, A.; Sell, H.; Eckel, J. Differential impact of oleate, palmitate, and adipokines on expression of NF-κB target genes in human vascular smooth muscle cells. Mol. Cell. Endocrinol 2012, 362, 194–201. [Google Scholar]
- St-Denis, C.; Cloutier, I.; Tanguay, J.F. Key fatty acid combinations define vascular smooth cell proliferation and viability. Lipids 2012, 47, 1073–1084. [Google Scholar]
- Yuan, H.; Zhang, X.; Huang, X.; Lu, Y.; Tang, W.; Man, Y.; Wang, S.; Xi, J.; Li, J. NADPH oxidase 2-derived reactive oxygen species mediate FFAs-induced dysfunction and apoptosis of β-cells via JNK, p38 MAPK and p53 pathways. PLoS One 2010, 5, e15726. [Google Scholar]
- Zhu, L.H.; Wang, L.; Wang, D.; Jiang, H.; Tang, Q.Z.; Yan, L.; Bian, Z.Y.; Wang, X.A.; Li, H. Puerarin attenuates high-glucose and diabetes-induced vascular smooth muscle cell proliferation by blocking PKCβ2/Rac1-dependent signaling. Free Radic. Biol. Med 2010, 48, 471–482. [Google Scholar]
- Russo, I.; Del Mese, P.; Doronzo, G.; Mattiello, L.; Viretto, M.; Bosia, A.; Anfossi, G.; Trovati, M. Resistance to the nitric oxide/cyclic guanosine 5′-monophosphate/protein kinase G pathway in vascular smooth muscle cells from the obese Zucker rat, a classical animal model of insulin resistance: Role of oxidative stress. Endocrinology 2008, 49, 1480–1489. [Google Scholar]
- Doronzo, G.; Viretto, M.; Russo, I.; Mattiello, L.; Di Martino, L.; Cavalot, F.; Anfossi, G.; Trovati, M. Nitric oxide activates PI3-K and MAPK signalling pathways in human and rat vascular smooth muscle cells: Influence of insulin resistance and oxidative stress. Atherosclerosis 2011, 216, 44–53. [Google Scholar]
- Rocic, P.; Kolz, C.; Reed, R.; Potter, B.; Chilian, W.M. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am. J. Physiol. Heart Circ. Physiol 2007, 292, H2729–H2736. [Google Scholar]
- Holland, W.L.; Knotts, T.A.; Chavez, J.A.; Wang, L.P.; Hoen, K.L.; Summers, S.A. Lipid mediators of insulin resistance. Nutr. Rev 2007, 65, S39–S46. [Google Scholar]
- Toyama, T.; Kudo, N.; Hibino, Y.; Mitsumoto, A.; Nishikawa, M.; Kawashima, Y. Effects of pioglitazone on stearoyl-CoA desaturase in obese Zucker fa/fa rats. J. Pharmacol. Sci 2007, 104, 137–145. [Google Scholar]
- Toyota, E.; Waltier, D.C.; Brock, T.; Ritman, E.; Kolz, C.; O'Malley, P.; Rocic, P.; Focardi, M.; Chilian, W.M. Vascular Endothelial Growth Factor is required for coronary collateral growth in the rat. Circulation 2005, 112, 2108–2113. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔ CT method. Methods 2001, 25, 402–408. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Doronzo, G.; Viretto, M.; Barale, C.; Russo, I.; Mattiello, L.; Anfossi, G.; Trovati, M. Oleic Acid Increases Synthesis and Secretion of VEGF in Rat Vascular Smooth Muscle Cells: Role of Oxidative Stress and Impairment in Obesity. Int. J. Mol. Sci. 2013, 14, 18861-18880. https://doi.org/10.3390/ijms140918861
Doronzo G, Viretto M, Barale C, Russo I, Mattiello L, Anfossi G, Trovati M. Oleic Acid Increases Synthesis and Secretion of VEGF in Rat Vascular Smooth Muscle Cells: Role of Oxidative Stress and Impairment in Obesity. International Journal of Molecular Sciences. 2013; 14(9):18861-18880. https://doi.org/10.3390/ijms140918861
Chicago/Turabian StyleDoronzo, Gabriella, Michela Viretto, Cristina Barale, Isabella Russo, Luigi Mattiello, Giovanni Anfossi, and Mariella Trovati. 2013. "Oleic Acid Increases Synthesis and Secretion of VEGF in Rat Vascular Smooth Muscle Cells: Role of Oxidative Stress and Impairment in Obesity" International Journal of Molecular Sciences 14, no. 9: 18861-18880. https://doi.org/10.3390/ijms140918861



