Mild Oxidative Damage in the Diabetic Rat Heart Is Attenuated by Glyoxalase-1 Overexpression
Abstract
:1. Introduction
2. Results
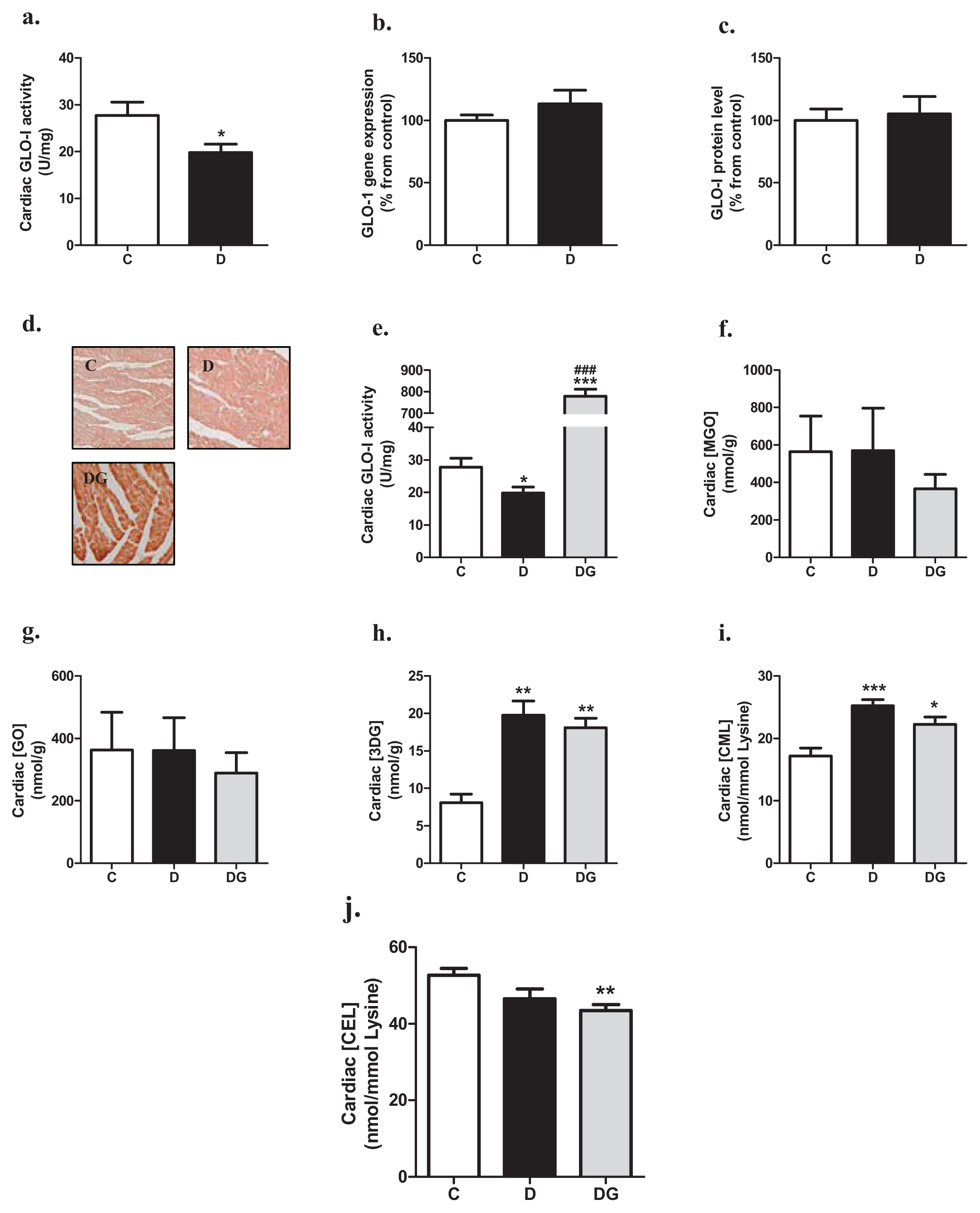
2.1. Glyoxalase, Carbonyl Stress and Glycation in the Diabetic Heart
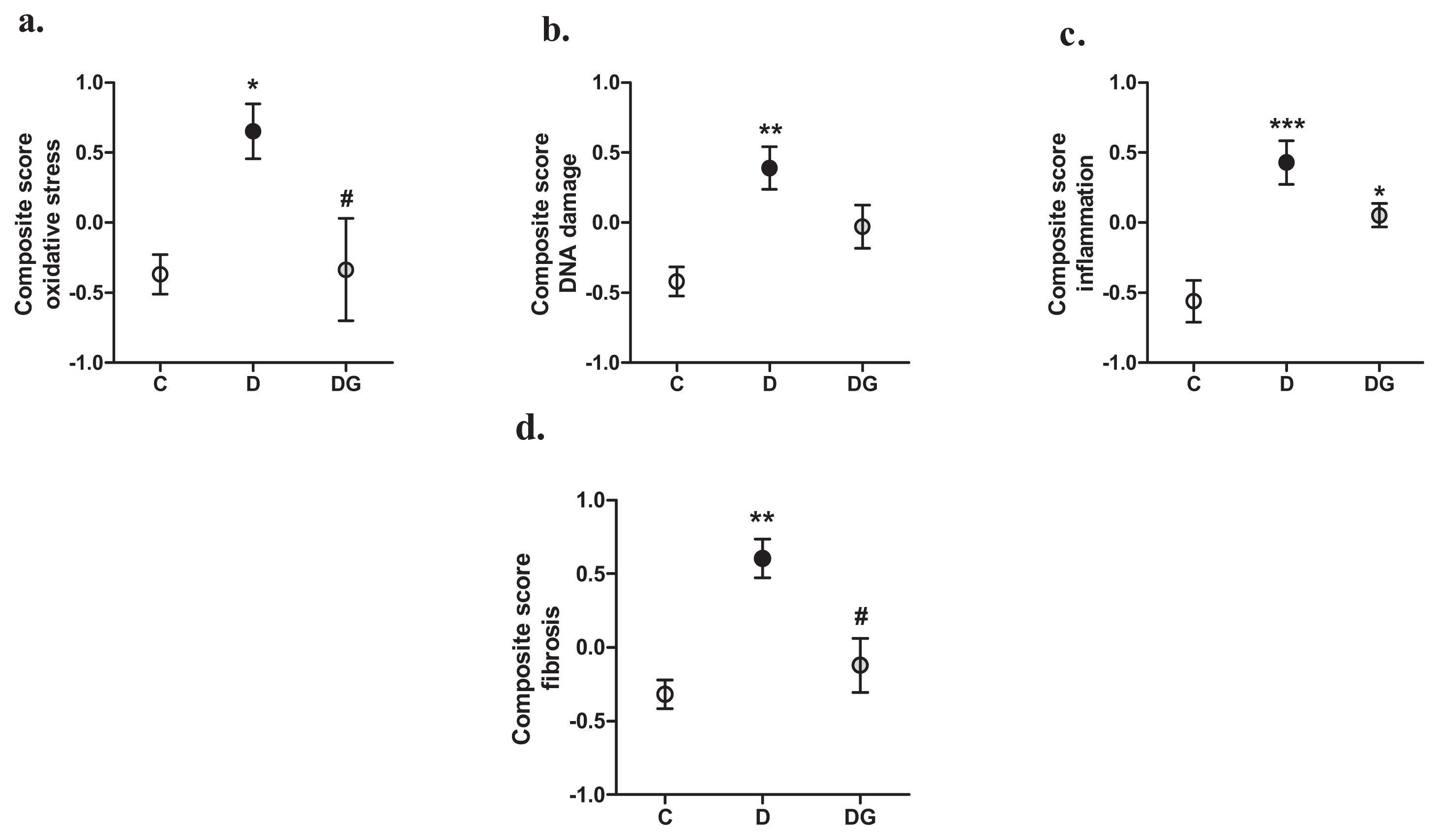
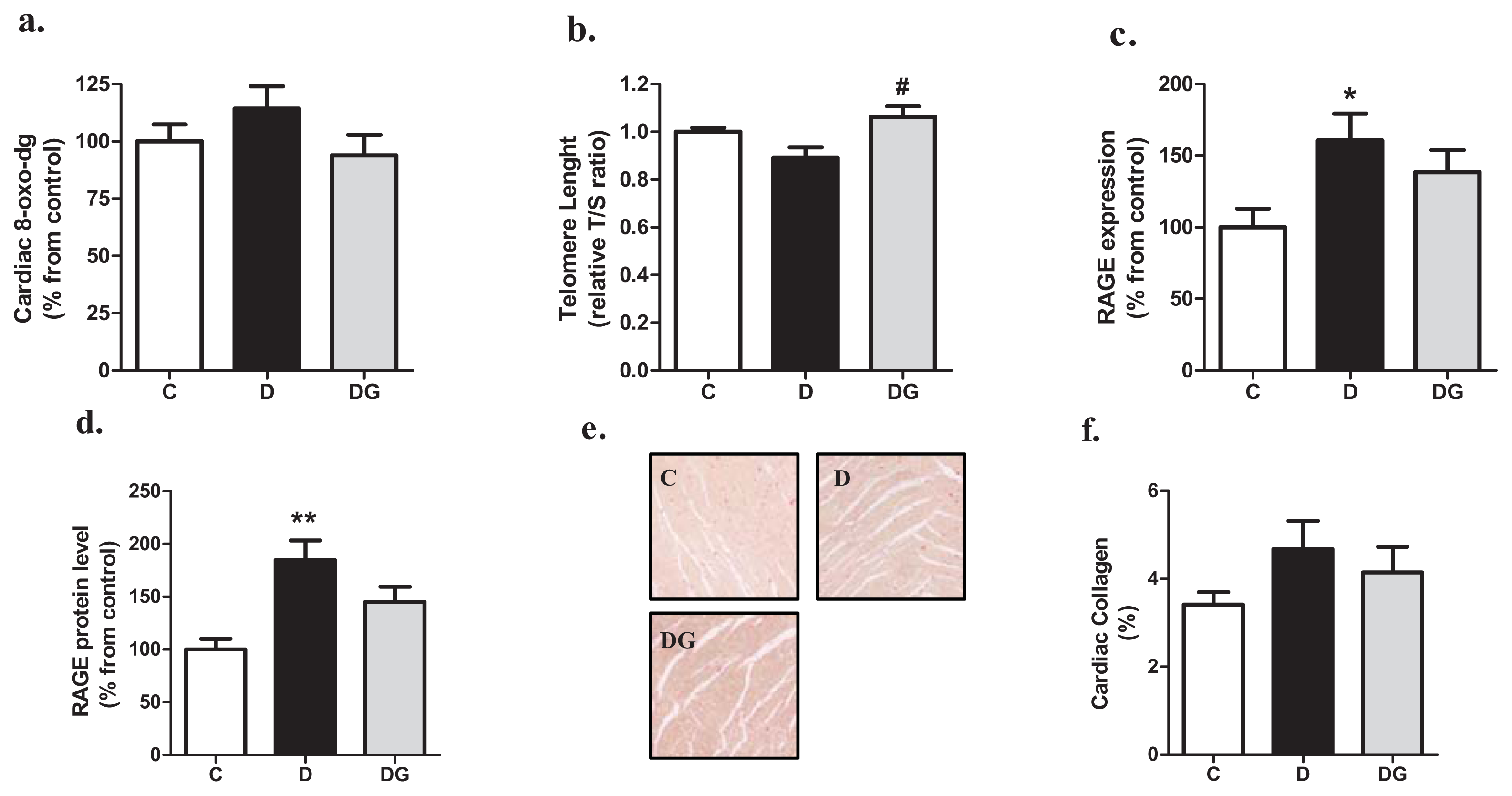
2.2. Oxidative Damage, Inflammation and Fibrosis in the Diabetic Heart
2.3. The Diabetic Heart Displays Signs of Structural Alteration but No Systolic Dysfunction
3. Experimental Section
3.1. Rat Model of Diabetes
3.2. Glyoxalase-I Activity Assay
3.3. Measurement of AGEs and Oxo-Aldehydes in the Heart
3.4. Cardiac Gene Expression Analysis
3.5. DNA Isolation and Telomere Length Measurement
3.6. Western Blotting Analyses
3.7. 8-Oxo-dG Quantification in the Heart
3.8. Heart Tissue Analyses for Cardiomyocyte Area and Collagen
3.9. Measurement of Circulating Cardiac Injury Markers
3.10. Echocardiographic Analyses of Cardiac Dimensions and Function
3.11. Statistical Analysis
4. Discussion and Conclusions
Acknowledgments
Conflict of Interest
References
- The diabetes control and complications trial research group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986.
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol 1972, 30, 595–602. [Google Scholar]
- Aneja, A.; Tang, W.H.; Bansilal, S.; Garcia, M.J.; Farkouh, M.E. Diabetic cardiomyopathy: Insights into pathogenesis, diagnostic challenges, and therapeutic options. Am. J. Med 2008, 121, 748–757. [Google Scholar]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar]
- Ansley, D.M.; Wang, B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol 2013, 229, 232–241. [Google Scholar]
- Aragno, M.; Mastrocola, R.; Alloatti, G.; Vercellinatto, I.; Bardini, P.; Geuna, S.; Catalano, M.G.; Danni, O.; Boccuzzi, G. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology 2008, 149, 380–388. [Google Scholar]
- Brouwers, O.; Niessen, P.M.; Ferreira, I.; Miyata, T.; Scheffer, P.G.; Teerlink, T.; Schrauwen, P.; Brownlee, M.; Stehouwer, C.D.; Schalkwijk, C.G. Overexpression of glyoxalase-i reduces hyperglycemia-induced levels of advanced glycation endproducts and oxidative stress in diabetic rats. J. Biol. Chem 2010, 286, 1374–1380. [Google Scholar]
- Kumagai, T.; Nangaku, M.; Kojima, I.; Nagai, R.; Ingelfinger, J.R.; Miyata, T.; Fujita, T.; Inagi, R. Glyoxalase i overexpression ameliorates renal ischemia-reperfusion injury in rats. Am. J. Physiol. Renal Physiol 2009, 296, F912–F921. [Google Scholar]
- Schlotterer, A.; Kukudov, G.; Bozorgmehr, F.; Hutter, H.; Du, X.; Oikonomou, D.; Ibrahim, Y.; Pfisterer, F.; Rabbani, N.; Thornalley, P.; et al. Diabetes 2009, 58, 2450–2456.
- Thornalley, P.J. Glyoxalase I—structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans 2003, 31, 1343–1348. [Google Scholar]
- Beisswenger, P.J.; Drummond, K.S.; Nelson, R.G.; Howell, S.K.; Szwergold, B.S.; Mauer, M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 2005, 54, 3274–3281. [Google Scholar]
- Fosmark, D.S.; Torjesen, P.A.; Kilhovd, B.K.; Berg, T.J.; Sandvik, L.; Hanssen, K.F.; Agardh, C.D.; Agardh, E. Increased serum levels of the specific advanced glycation end product methylglyoxal-derived hydroimidazolone are associated with retinopathy in patients with type 2 diabetes mellitus. Metabolism 2006, 55, 232–236. [Google Scholar]
- Ogawa, S.; Nakayama, K.; Nakayama, M.; Mori, T.; Matsushima, M.; Okamura, M.; Senda, M.; Nako, K.; Miyata, T.; Ito, S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension 2010, 56, 471–476. [Google Scholar]
- Daoud, S.; Schinzel, R.; Neumann, A.; Loske, C.; Fraccarollo, D.; Diez, C.; Simm, A. Advanced glycation endproducts: Activators of cardiac remodeling in primary fibroblasts from adult rat hearts. Mol. Med 2001, 7, 543–551. [Google Scholar]
- Berner, A.K.; Brouwers, O.; Pringle, R.; Klaassen, I.; Colhoun, L.; McVicar, C.; Brockbank, S.; Curry, J.W.; Miyata, T.; Brownlee, M.; et al. Protection against methylglyoxal-derived ages by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia 2012, 55, 845–854. [Google Scholar]
- McLellan, A.C.; Phillips, S.A.; Thornalley, P.J. The assay of s-d-lactoylglutathione in biological systems. Biochem. Soc. Trans 1993, 211, 37–43. [Google Scholar]
- Teerlink, T.; Barto, R.; Ten Brink, H.J.; Schalkwijk, C.G. Measurement of nepsilon-(carboxymethyl) lysine and nepsilon-(carboxyethyl) lysine in human plasma protein by stable-isotope-dilution tandem mass spectrometry. Clin. Chem 2004, 50, 1222–1228. [Google Scholar]
- Scheijen, J.L.; Schalkwijk, C.G. Quantification of glyoxal, methylglyoxal and 3-deoxyglucosone in blood and plasma by ultra performance liquid chromatography tandem mass spectrometry: Evaluation of blood specimen. Clin. Chem. Lab. Med. 2013. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere measurement by quantitative pcr. Nucleic Acids Res 2002, 30, e47. [Google Scholar]
- Houben, J.M.; Mercken, E.M.; Ketelslegers, H.B.; Bast, A.; Wouters, E.F.; Hageman, G.J.; Schols, A.M. Telomere shortening in chronic obstructive pulmonary disease. Respir. Med 2009, 103, 230–236. [Google Scholar]
- Langie, S.A.; Kowalczyk, P.; Tudek, B.; Zabielski, R.; Dziaman, T.; Olinski, R.; van Schooten, F.J.; Godschalk, R.W. The effect of oxidative stress on nucleotide-excision repair in colon tissue of newborn piglets. Mutat. Res 2010, 695, 75–80. [Google Scholar]
- Qwin morphometric software, version 3. Available online: http://www.leica-microsystems.com/products/microscope-software/materials-sciences/quantitative-imaging/details/product/leica-qwin/ (accessed on 26 July 2013).
- Heijnen, B.F.; Pelkmans, L.P.; Danser, A.J.; Garrelds, I.M.; Mullins, J.J.; De Mey, J.G.; Struijker-Boudier, H.A.; Janssen, B.J. Cardiac remodeling during and after renin-angiotensin system stimulation in cyp1a1-ren2 transgenic rats. J. Renin Angiotensin Aldosterone Syst. 2013. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Obata, T.; Suzaki, M.; Takagi, Y.; Kida, Y.; Ogawa, T.; Tanaka, Y.; Asahina, T.; Ikebuchi, M.; Saeki, Y.; et al. Increase in cardiac muscle fructose content in streptozotocin-induced diabetic rats. Metabolism 1992, 41, 1041–1046. [Google Scholar]
- Lal, S.; Randall, W.C.; Taylor, A.H.; Kappler, F.; Walker, M.; Brown, T.R.; Szwergold, B.S. Fructose-3-phosphate production and polyol pathway metabolism in diabetic rat hearts. Metabolism 1997, 46, 1333–1338. [Google Scholar]
- Frisard, M.; Ravussin, E. Energy metabolism and oxidative stress: Impact on the metabolic syndrome and the aging process. Endocrine 2006, 29, 27–32. [Google Scholar]
- Desai, K.M.; Chang, T.; Wang, H.; Banigesh, A.; Dhar, A.; Liu, J.; Untereiner, A.; Wu, L. Oxidative stress and aging: Is methylglyoxal the hidden enemy? Can. J. Physiol. Pharmacol 2010, 88, 273–284. [Google Scholar]
- Adaikalakoteswari, A.; Balasubramanyam, M.; Ravikumar, R.; Deepa, R.; Mohan, V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis 2007, 195, 83–89. [Google Scholar]
- Collerton, J.; Martin-Ruiz, C.; Kenny, A.; Barrass, K.; von Zglinicki, T.; Kirkwood, T.; Keavney, B. Telomere length is associated with left ventricular function in the oldest old: The newcastle 85+ study. Eur. Heart J 2007, 28, 172–176. [Google Scholar]
- Yao, D.; Brownlee, M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (rage) and rage ligands. Diabetes 2010, 59, 249–255. [Google Scholar]
- Dai, Q.M.; Lu, J.; Liu, N.F. Fluvastatin attenuates myocardial interstitial fibrosis and cardiac dysfunction in diabetic rats by inhibiting over-expression of connective tissue growth factor. Chin. Med. J. (Engl. ) 2011, 124, 89–94. [Google Scholar]
- Miric, G.; Dallemagne, C.; Endre, Z.; Margolin, S.; Taylor, S.M.; Brown, L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br. J. Pharmacol 2001, 133, 687–694. [Google Scholar]
- Van Linthout, S.; Seeland, U.; Riad, A.; Eckhardt, O.; Hohl, M.; Dhayat, N.; Richter, U.; Fischer, J.W.; Bohm, M.; Pauschinger, M.; et al. Reduced mmp-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res. Cardiol 2008, 103, 319–327. [Google Scholar]
- Westermann, D.; Rutschow, S.; Jager, S.; Linderer, A.; Anker, S.; Riad, A.; Unger, T.; Schultheiss, H.P.; Pauschinger, M.; Tschope, C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: The role of angiotensin type 1 receptor antagonism. Diabetes 2007, 56, 641–646. [Google Scholar]
- Daniels, A.; Linz, D.; van Bilsen, M.; Rutten, H.; Sadowski, T.; Ruf, S.; Juretschke, H.P.; Neumann-Haefelin, C.; Munts, C.; van der Vusse, G.J.; et al. Long-term severe diabetes only leads to mild cardiac diastolic dysfunction in zucker diabetic fatty rats. Eur. J. Heart Fail 2012, 14, 193–201. [Google Scholar]
- Bidani, A.K.; Picken, M.; Hacioglu, R.; Williamson, G.; Griffin, K.A. Spontaneously reduced blood pressure load in the rat streptozotocin-induced diabetes model: Potential pathogenetic relevance. Am. J. Physiol. Renal Physiol 2007, 292, F647–F654. [Google Scholar]
- Akula, A.; Kota, M.K.; Gopisetty, S.G.; Chitrapu, R.V.; Kalagara, M.; Kalagara, S.; Veeravalli, K.K.; Gomedhikam, J.P. Biochemical, histological and echocardiographic changes during experimental cardiomyopathy in stz-induced diabetic rats. Pharmacol. Res 2003, 48, 429–435. [Google Scholar]
- Mihm, M.J.; Seifert, J.L.; Coyle, C.M.; Bauer, J.A. Diabetes related cardiomyopathy time dependent echocardiographic evaluation in an experimental rat model. Life Sci 2001, 69, 527–542. [Google Scholar]
- Irlbeck, M.; Zimmer, H.G. Functional responses of the left and right heart of diabetic rats to alpha- and beta-adrenergic receptor stimulation. Diabetes Res. Clin. Pract 1996, 31, S79–S86. [Google Scholar]
- Schenk, J.; Hebden, R.A.; Dai, S.; McNeill, J.H. Integrated cardiovascular function in the conscious streptozotocin-diabetic deoxycorticosterone-acetate-hypertensive rats. Pharmacology 1994, 48, 211–215. [Google Scholar]
- Hoit, B.D.; Castro, C.; Bultron, G.; Knight, S.; Matlib, M.A. Noninvasive evaluation of cardiac dysfunction by echocardiography in streptozotocin-induced diabetic rats. J. Card. Fail 1999, 5, 324–333. [Google Scholar]
- Rodrigues, B.; Cam, M.C.; Kong, J.; Goyal, R.K.; McNeill, J.H. Strain differences in susceptibility to streptozotocin-induced diabetes: Effects on hypertriglyceridemia and cardiomyopathy. Cardiovasc. Res 1997, 34, 199–205. [Google Scholar]
- Daniels, A.; van Bilsen, M.; Janssen, B.J.; Brouns, A.E.; Cleutjens, J.P.; Roemen, T.H.; Schaart, G.; van der Velden, J.; van der Vusse, G.J.; van Nieuwenhoven, F.A. Impaired cardiac functional reserve in type 2 diabetic db/db mice is associated with metabolic, but not structural, remodelling. Acta Physiol. (Oxf. ) 2010, 200, 11–22. [Google Scholar]
| Genes | Control | Diabetes | Diabetes + GLO-I |
|---|---|---|---|
| Oxidative stress | (C) | (D) | (DG) |
| SOD-3 | 100 ± 9 | 122 ± 9 | 91 ± 14 |
| CAT | 100 ± 10 | 226 ± 46 * | 190 ± 43 |
| GPX-1 | 100 ± 6 | 149 ± 12 * | 91 ± 12 # |
| NOX-4 | 100 ± 9 | 130 ± 9 | 97 ± 17 |
| DNA damage | - | - | - |
| p300 | 100 ± 6 | 116 ± 6 | 94 ± 16 |
| APEX-1 | 100 ± 1 | 121 ± 9 | 104 ± 8 |
| OGG-1 | 100 ± 6 | 121 ± 4 * | 129 ± 13 |
| p53 | 100 ± 25 | 133 ± 29 | 133 ± 33 |
| Inflammation | - | - | - |
| PAI-1 | 100 ± 13 | 278 ± 28 *** | 359 ± 46 *** |
| TNFα | 100 ± 12 | 126 ± 17 | 114 ± 17 |
| IL-1β | 100 ± 11 | 216 ± 26 * | 162 ± 28 |
| IL-6 | 100 ± 23 | 108 ± 19 | 93 ± 18 |
| Fibrosis | - | - | - |
| CTGF | 100 ± 13 | 180 ± 18 *** | 197 ± 23 |
| Col1α1 | 100 ± 13 | 65 ± 7 * | 109 ± 14 # |
| MMP-2 | 100 ± 6 | 78 ± 6 * | 115 ± 10 # |
| MMP-9 | 100 ± 23 | 150 ± 35 | 128 ± 22 |
| Characteristic | Control | Diabetes | Diabetes + GLO-I |
|---|---|---|---|
| Cardiac Hypertrophy | (C) | (D) | (DG) |
| Cardiac weight (g) | 1.32 ± 0.08 | 1.080 ± 0.05 * | 0.993 ± 0.04 ** |
| Heart/bodyweight (%) | 0.276 ± 0.01 | 0.360 ± 0.01 *** | 0.333 ± 0.01 ** |
| ANP (% from C) | 100 ± 15 | 499 ± 139 ** | 385 ± 48 *** |
| BNP (% from C) | 100 ± 16 | 148 ± 23 * | 157 ± 13 ** |
| Cardiomyocyte CSA (μm3) | 277 ± 12 | 284 ± 21 | 260 ± 21 |
| Ventricular Dimensions | - | - | - |
| Thickness wall diastole (cm) | 0.17 ± 0.01 | 0.14 ± 0.01 ** | 0.15 ± 0.01 * |
| Volume wall diastole (cm3) | 0.49 ± 0.03 | 0.40 ± 0.03 * | 0.45 ± 0.02 |
| Internal diameter cavity diastole (cm) | 0.84 ± 0.06 | 0.84 ± 0.05 | 0.77 ± 0.04 ** |
| Thickness wall systole (cm) | 0.19 ± 0.01 | 0.17 ± 0.01 ** | 0.17 ± 0.01 * |
| Volume wall systole (cm3) | 0.43 ± 0.03 | 0.39 ± 0.02 | 0.40 ± 0.02 |
| Internal diameter cavity systole (cm) | 0.68 ± 0.06 | 0.70 ± 0.04 | 0.64 ± 0.04 |
| Characteristic | Control | Diabetes | Diabetes + GLO-I |
|---|---|---|---|
| Cardiac volume | (C) | (D) | (DG) |
| End diastolic volume (mL) | 0.534 ± 0.04 | 0.536 ± 0.04 | 0.492 ± 0.02 |
| End systolic volume (mL) | 0.211 ± 0.02 | 0.218 ± 0.01 | 0.205 ± 0.01 |
| Stroke volume (mL) | 0.322 ± 0.03 | 0.318 ± 0.03 | 0.287 ± 0.02 |
| Cardiac function | - | - | - |
| Fractional shortening (%) | 16.8 ± 1.0 | 16.0 ± 1.2 | 16.6 ± 0.8 |
| Ejection fraction (%) | 59.4 ± 3.1 | 58.9 ± 1.3 | 58.0 ± 2.2 |
| Cardiac output (mL/min) | 110.7 ± 9.7 | 81.2 ± 7.7 | 77.8 ± 6.5 * |
| Genes | Forward primer | Reverse primer |
|---|---|---|
| Oxidative stress | - | - |
| SOD-3 | CTTCCCAGCCGAGCAGAA | TCAGGTCCCCGAACTCATG |
| CAT | TCCTGAGAGAGTGGTACATGC | AAGGTGTGAGCCATAGCC |
| GPX-1 | AATTCCCTCAAGTACGTCCG | TCAGGTTCGATGTCGATGGT |
| NOX-4 | TGGGTGTCAAACAAGAGATTGC | GGCTCAGGAGGTTCTTCATGTAG |
| DNA Damage | - | - |
| p300 | GGGACTAACCAATGGTGGTG | ATTGGGAGAAGTCAAGCCTG |
| APEX-1 | GAATGTGGATGGGCTTCGA | AAGATGTCTGGTGCTTCTTCCTTT |
| OGG-1 | CGGCTGGCAGCCTAAGAC | CCGGAAAAAGTTTCCCAGTTC |
| p53 | GACAGCTTTGAGGTTCGTGTTTG | TGCTCTTCTTTTTTGCGGAAA |
| Inflammation | - | - |
| PAI-1 | TCCGCCATCACCAACATTTT | GTCAGTCATGCCCAGCTTCTC |
| TNFα | TGATCGGTCCCAACAAGGA | GGGCCATGGAACTGATGAGA |
| IL-1β | GCACCTTCTTTTCCTTCATCTTTG | CACACTAGCAGGTCGTCATCATC |
| IL-6 | GAAACCCTAGTTCATATCTTCAAACAAG | CCATTAGGAGAGCATTGGAAGTTG |
| Fibrosis | - | - |
| CTGF | CACAGAGTGGAGCGCCTGTTC | GATGCACTTTTTGCCCTTCTTAATG |
| Col1α1 | CGAAGGCAACAGTCGATTCA | GGTCTTGGTGGTTTTGTATTCGAT |
| MMP-2 | TTTGCTCGGGCCTTAAAAGTAT | CCATCAAACGGGTATCCATCTC |
| MMP-9 | GACCTGAAAACCTCCAACCTC | CTGCTTCTCTCCCATCATCTG |
| Hypertrophy | - | - |
| ANP | ATCACCAAGGGCTTCTTCCT | TGTTGGACACCGCACTGTAT |
| BNP | AGACAGCTCTCAAAGGACCA | CTATCTTCTGCCCAAAGCAG |
| Telomere length | - | - |
| PGK1 | CGGAGACACCFCCACTTG | AAGGCAGGAAAATACTAAACAT |
| Telomere 1 | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT | |
| Telomere 2 | GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT | |
| Reference gene | - | - |
| β-actin | GACAGGATGCAGAAGGAGATTACTG | CCACCGATCCACACAGAGTACTT |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brouwers, O.; Vos-Houben, J.M.J.d.; Niessen, P.M.G.; Miyata, T.; Nieuwenhoven, F.V.; Janssen, B.J.A.; Hageman, G.; Stehouwer, C.D.A.; Schalkwijk, C.G. Mild Oxidative Damage in the Diabetic Rat Heart Is Attenuated by Glyoxalase-1 Overexpression. Int. J. Mol. Sci. 2013, 14, 15724-15739. https://doi.org/10.3390/ijms140815724
Brouwers O, Vos-Houben JMJd, Niessen PMG, Miyata T, Nieuwenhoven FV, Janssen BJA, Hageman G, Stehouwer CDA, Schalkwijk CG. Mild Oxidative Damage in the Diabetic Rat Heart Is Attenuated by Glyoxalase-1 Overexpression. International Journal of Molecular Sciences. 2013; 14(8):15724-15739. https://doi.org/10.3390/ijms140815724
Chicago/Turabian StyleBrouwers, Olaf, Joyce M. J. de Vos-Houben, Petra M. G. Niessen, Toshio Miyata, Frans Van Nieuwenhoven, Ben J. A. Janssen, Geja Hageman, Coen D. A. Stehouwer, and Casper G. Schalkwijk. 2013. "Mild Oxidative Damage in the Diabetic Rat Heart Is Attenuated by Glyoxalase-1 Overexpression" International Journal of Molecular Sciences 14, no. 8: 15724-15739. https://doi.org/10.3390/ijms140815724
APA StyleBrouwers, O., Vos-Houben, J. M. J. d., Niessen, P. M. G., Miyata, T., Nieuwenhoven, F. V., Janssen, B. J. A., Hageman, G., Stehouwer, C. D. A., & Schalkwijk, C. G. (2013). Mild Oxidative Damage in the Diabetic Rat Heart Is Attenuated by Glyoxalase-1 Overexpression. International Journal of Molecular Sciences, 14(8), 15724-15739. https://doi.org/10.3390/ijms140815724




