New Evidence for Cross Talk between Melatonin and Mitochondria Mediated by a Circadian-Compatible Interaction with Nitric Oxide
Abstract
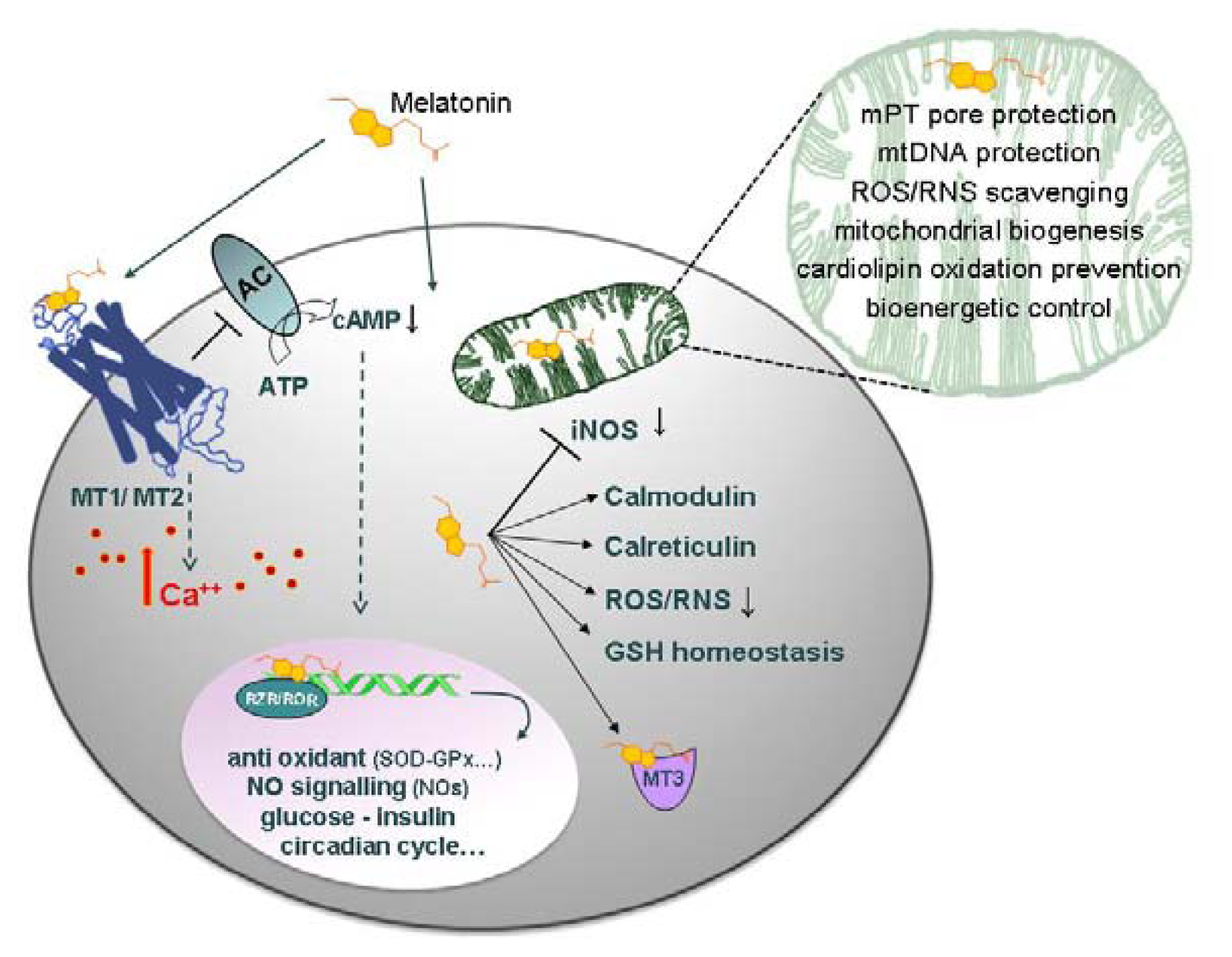
:1. Introduction
2. Results and Discussion
2.1. Experimental Results
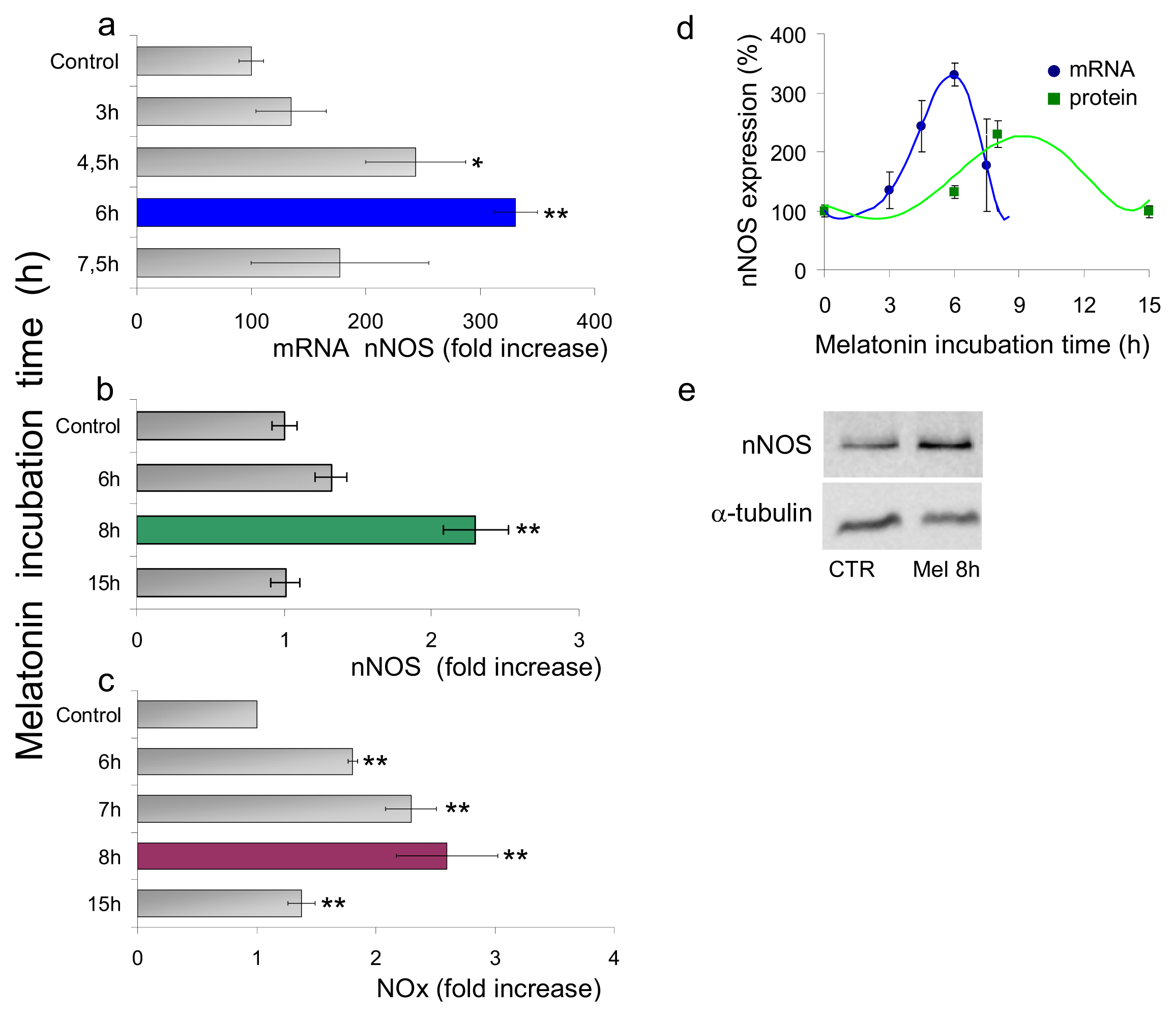
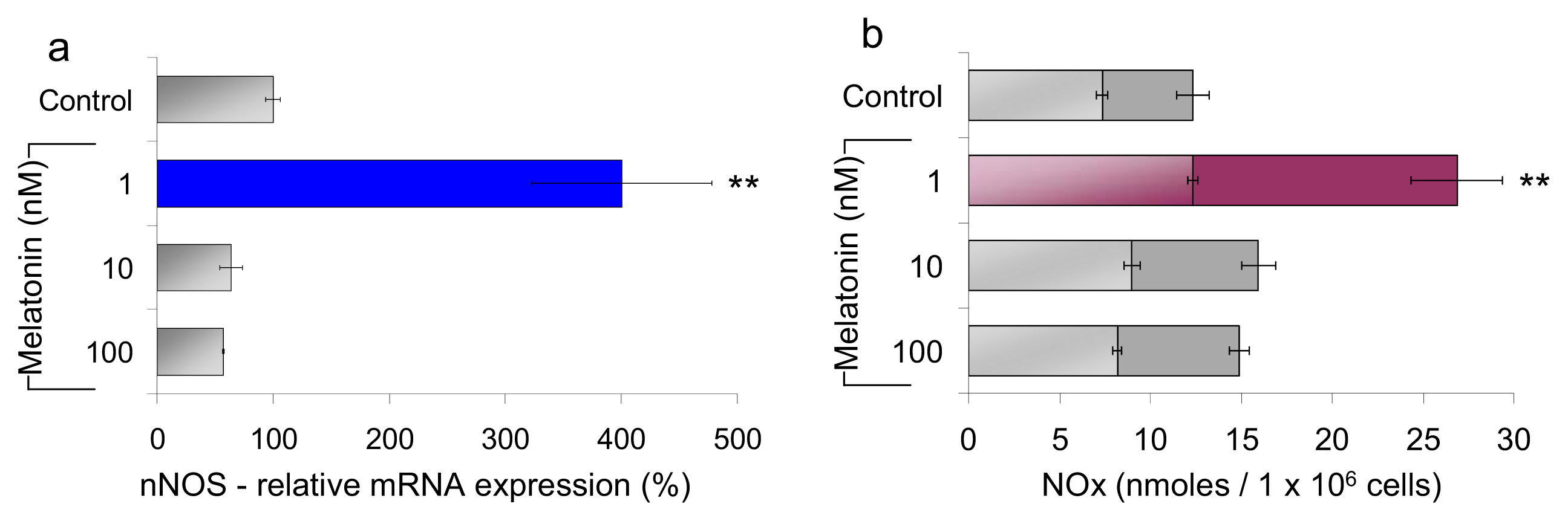
2.1.1. Neuronal NOS (nNOS) Expression and NOx Production
2.1.2. Mitochondrial Respiration and Membrane Potential
2.1.3. ATP and Lactate Production
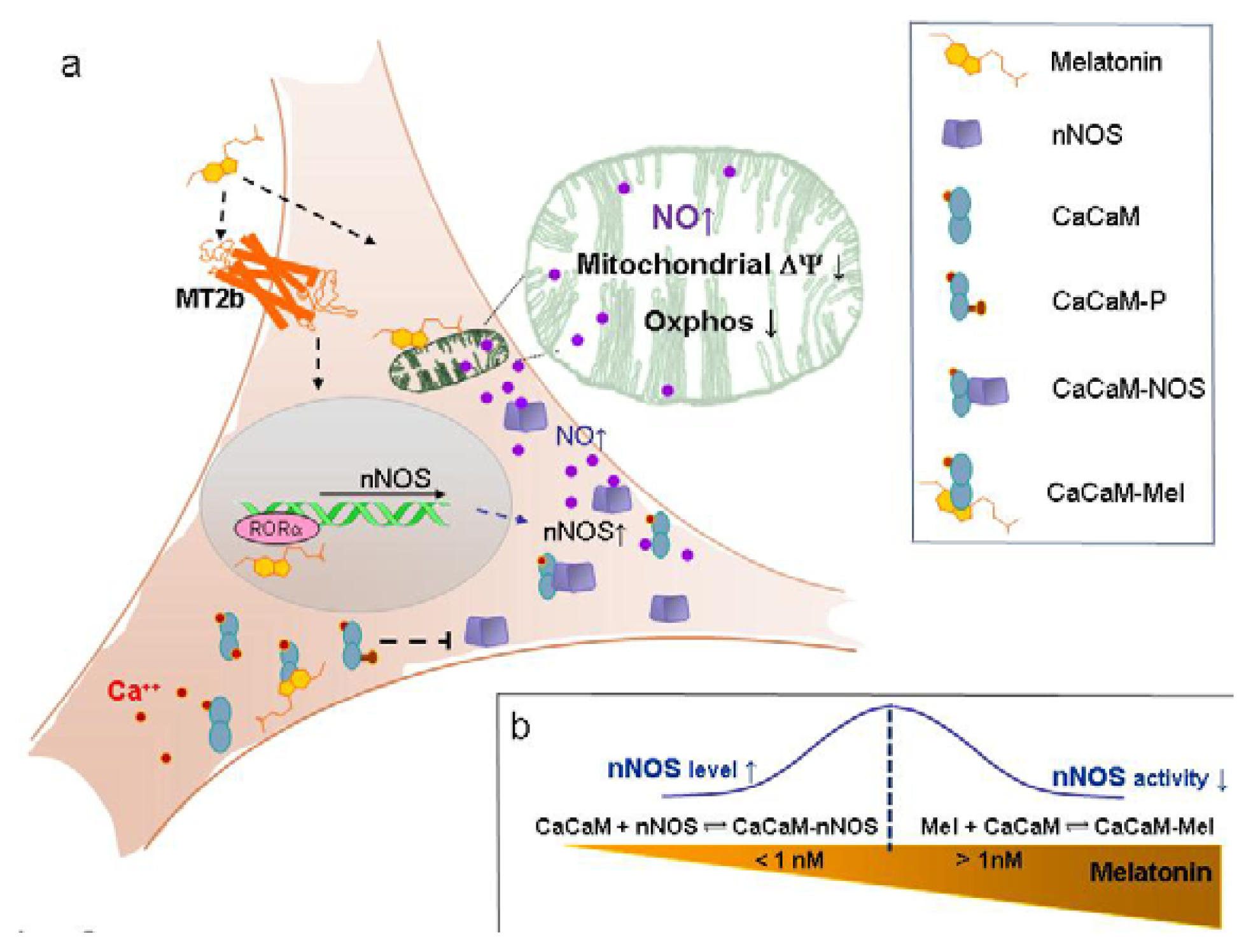
2.2. Discussion
2.2.1. Concentration of Melatonin and Protocol of Administration
2.2.2. Nanomolar Melatonin, nNOS Synthesis and Involvement of Complex IV
3. Experimental Section
3.1. Chemicals
3.2. Cell Culture
3.3. nNOS mRNA Determination
3.4. nNOS Detection by Western Blot
3.5. Nitrite/Nitrate (NOx) Determination
3.6. Cell Respiration
3.7. Mitochondrial Membrane Potential
3.8. ATP Measurements
3.9. Lactate Measurements
3.10. Statistics
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Maharaj, D.S.; Glass, B.D.; Daya, S. Melatonin: New places in therapy. Biosci. Rep 2007, 27, 299–320. [Google Scholar]
- Reiter, R.J.; Paredes, S.D.; Manchester, L.C.; Tan, D.X. Reducing oxidative/nitrosative stress: A newly-discovered genre for melatonin. Crit. Rev. Biochem. Mol. Biol 2009, 44, 175–200. [Google Scholar]
- Conti, A.; Conconi, S.; Hertens, E.; Skwarlo-Sonta, K.; Markowska, M.; Maestroni, J.M. Evidence for melatonin synthesis in mouse and human bone marrow cells. J. Pineal Res 2000, 28, 193–202. [Google Scholar]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab 2008, 19, 17–24. [Google Scholar]
- Champier, J.; Claustrat, B.; Besancon, R.; Eymin, C.; Killer, C.; Jouvet, A.; Chamba, G.; Fevre-Montange, M. Evidence for tryptophan hydroxylase and hydroxy-indol-O-methyl-transferase mRNAs in human blood platelets. Life Sci 1997, 60, 2191–2197. [Google Scholar]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; Garcia-Maurino, S.; Reiter, R.J.; Guerrero, J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J 2004, 18, 537–539. [Google Scholar]
- Liu, C.; Fukuhara, C.; Wessel, J.H., 3rd; Iuvone, P.M.; Tosini, G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res 2004, 315, 197–201. [Google Scholar]
- Reiter, R.J.; Tan, D.X. What constitutes a physiological concentration of melatonin? J. Pineal Res 2003, 34, 79–80. [Google Scholar]
- Escames, G.; Acuna-Castroviejo, D. Melatonin, synthetic analogs, and the sleep/wake rhythm. Rev. Neurol 2009, 48, 245–254. [Google Scholar]
- Morris, C.J.; Aeschbach, D.; Scheer, F.A. Circadian system, sleep and endocrinology. Mol. Cell. Endocrinol 2012, 349, 91–104. [Google Scholar]
- Dollins, A.B.; Zhdanova, I.V.; Wurtman, R.J.; Lynch, H.J.; Deng, M.H. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc. Natl. Acad. Sci. USA 1994, 91, 1824–1828. [Google Scholar]
- Luchetti, F.; Canonico, B.; Betti, M.; Arcangeletti, M.; Pilolli, F.; Piroddi, M.; Canesi, L.; Papa, S.; Galli, F. Melatonin signaling and cell protection function. FASEB J 2010, 24, 3603–3624. [Google Scholar]
- Ciftci, M.; Bilici, D.; Kufrevioglu, O.I. Effects of melatonin on enzyme activities of glucose-6-phosphate dehydrogenase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol. Res 2001, 44, 7–11. [Google Scholar]
- Martin, V.; Herrera, F.; Carrera-Gonzalez, P.; Garcia-Santos, G.; Antolin, I.; Rodriguez-Blanco, J.; Rodriguez, C. Intracellular signaling pathways involved in the cell growth inhibition of glioma cells by melatonin. Cancer Res 2006, 66, 1081–1088. [Google Scholar]
- Korkmaz, A.; Ma, S.; Topal, T.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Glucose: A vital toxin and potential utility of melatonin in protecting against the diabetic state. Mol. Cell. Endocrinol 2012, 349, 128–137. [Google Scholar]
- Acuna-Castroviejo, D.; Escames, G.; Rodriguez, M.I.; Lopez, L.C. Melatonin role in the mitochondrial function. Front. Biosci 2007, 12, 947–963. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Manchester, L.C. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev. Med. Chem 2013, 13, 373–384. [Google Scholar]
- Poeggeler, B.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J. Pineal Res 1993, 14, 151–168. [Google Scholar]
- Poeggeler, B.; Thuermann, S.; Dose, A.; Schoenke, M.; Burkhardt, S.; Hardeland, R. Melatonin’s unique radical scavenging properties—Roles of its functional substituents as revealed by a comparison with its structural analogs. J. Pineal Res 2002, 33, 20–30. [Google Scholar]
- Poeggeler, B.; Saarela, S.; Reiter, R.J.; Tan, D.X.; Chen, L.D.; Manchester, L.C.; Barlow-Walden, L.R. Melatonin—A highly potent endogenous radical scavenger and electron donor: New aspects of the oxidation chemistry of this indole accessed in vitro. Ann. N. Y. Acad. Sci 1994, 738, 419–420. [Google Scholar]
- Mahal, H.S.; Sharma, H.S.; Mukherjee, T. Antioxidant properties of melatonin: A pulse radiolysis study. Free Radic. Biol. Med 1999, 26, 557–565. [Google Scholar]
- Srinivasan, V.; Spence, D.W.; Pandi-Perumal, S.R.; Brown, G.M.; Cardinali, D.P. Melatonin in mitochondrial dysfunction and related disorders. Int. J. Alzheimers Dis. 2011. [Google Scholar] [CrossRef]
- Griefahn, B.; Brode, P.; Remer, T.; Blaszkewicz, M. Excretion of 6-hydroxymelatonin sulfate (6-OHMS) in siblings during childhood and adolescence. Neuroendocrinology 2003, 78, 241–243. [Google Scholar]
- Bergiannaki, J.D.; Soldatos, C.R.; Paparrigopoulos, T.J.; Syrengelas, M.; Stefanis, C.N. Low and high melatonin excretors among healthy individuals. J. Pineal Res 1995, 18, 159–164. [Google Scholar]
- Grof, E.; Grof, P.; Brown, G.M.; Arato, M.; Lane, J. Investigations of melatonin secretion in man. Prog. Neuropsychopharmacol. Biol. Psychiatry 1985, 9, 609–612. [Google Scholar]
- Wetterberg, L.; Iselius, L.; Lindsten, J. Genetic regulation of melatonin excretion in urine. A preliminary report. Clin. Genet 1983, 24, 399–402. [Google Scholar]
- Galecki, P.; Szemraj, J.; Bartosz, G.; Bienkiewicz, M.; Galecka, E.; Florkowski, A.; Lewinski, A.; Karbownik-Lewinska, M. Single-nucleotide polymorphisms and mRNA expression for melatonin synthesis rate-limiting enzyme in recurrent depressive disorder. J. Pineal Res 2010, 48, 311–317. [Google Scholar]
- Arese, M.; Magnifico, M.C.; Mastronicola, D.; Altieri, F.; Grillo, C.; Blanck, T.J.; Sarti, P. Nanomolar melatonin enhances nNOS expression and controls HaCaT-cells bioenergetics. IUBMB Life 2012, 64, 251–258. [Google Scholar]
- Cleeter, M.W.; Cooper, J.M.; Darley-Usmar, V.M.; Moncada, S.; Schapira, A.H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 1994, 345, 50–54. [Google Scholar]
- Brown, G.C.; Bolanos, J.P.; Heales, S.J.; Clark, J.B. Nitric oxide produced by activated astrocytes rapidly and reversibly inhibits cellular respiration. Neurosci. Lett 1995, 193, 201–204. [Google Scholar]
- Kalinchuk, A.V.; Stenberg, D.; Rosenberg, P.A.; Porkka-Heiskanen, T. Inducible and neuronal nitric oxide synthases (NOS) have complementary roles in recovery sleep induction. Eur. J. Neurosci 2006, 24, 1443–1456. [Google Scholar]
- Tsikas, D.; Gutzki, F.M.; Stichtenoth, D.O. Circulating and excretory nitrite and nitrate as indicators of nitric oxide synthesis in humans: Methods of analysis. Eur. J. Clin. Pharmacol 2006, 62, 51–59. [Google Scholar]
- Chen, L.; Majde, J.A.; Krueger, J.M. Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Res 2003, 973, 214–222. [Google Scholar]
- Leonard, T.O.; Lydic, R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J. Neurosci 1997, 17, 774–785. [Google Scholar]
- Alonso, M.; Collado, P.S.; Gonzalez-Gallego, J. Melatonin inhibits the expression of the inducible isoform of nitric oxide synthase and nuclear factor kappa B activation in rat skeletal muscle. J. Pineal Res 2006, 41, 8–14. [Google Scholar]
- Petrosillo, G.; Moro, N.; Ruggiero, F.M.; Paradies, G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free Radic. Biol. Med 2009, 47, 969–974. [Google Scholar]
- Lopez, A.; Garcia, J.A.; Escames, G.; Venegas, C.; Ortiz, F.; Lopez, L.C.; Acuna-Castroviejo, D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res 2009, 46, 188–198. [Google Scholar]
- Hevia, D.; Sainz, R.M.; Blanco, D.; Quiros, I.; Tan, D.X.; Rodriguez, C.; Mayo, J.C. Melatonin uptake in prostate cancer cells: Intracellular transport versus simple passive diffusion. J. Pineal Res 2008, 45, 247–257. [Google Scholar]
- Moncada, S.; Erusalimsky, J.D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol 2002, 3, 214–220. [Google Scholar]
- Sarti, P.; Forte, E.; Giuffre, A.; Mastronicola, D.; Magnifico, M.C.; Arese, M. The chemical interplay between nitric oxide and mitochondrial cytochrome c oxidase: Reactions, effectors and pathophysiology. Int. J. Cell Biol. 2012. [Google Scholar] [CrossRef]
- Sgarbi, G.; Baracca, A.; Lenaz, G.; Valentino, L.M.; Carelli, V.; Solaini, G. Inefficient coupling between proton transport and ATP synthesis may be the pathogenic mechanism for NARP and Leigh syndrome resulting from the T8993G mutation in mtDNA. Biochem. J 2006, 395, 493–500. [Google Scholar]
- Martin, M.; Macias, M.; Escames, G.; Reiter, R.J.; Agapito, M.T.; Ortiz, G.G.; Acuna-Castroviejo, D. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J. Pineal Res 2000, 28, 242–248. [Google Scholar]
- Okatani, Y.; Wakatsuki, A.; Reiter, R.J. Melatonin protects hepatic mitochondrial respiratory chain activity in senescence-accelerated mice. J. Pineal Res 2002, 32, 143–148. [Google Scholar]
- Jimenez-Ortega, V.; Cano, P.; Cardinali, D.P.; Esquifino, A.I. 24-Hour variation in gene expression of redox pathway enzymes in rat hypothalamus: Effect of melatonin treatment. Redox Rep 2009, 14, 132–138. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res 2007, 42, 28–42. [Google Scholar]
- Martin, M.; Macias, M.; Escames, G.; Leon, J.; Acuna-Castroviejo, D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 2000, 14, 1677–1679. [Google Scholar]
- Inarrea, P.; Casanova, A.; Alava, M.A.; Iturralde, M.; Cadenas, E. Melatonin and steroid hormones activate intermembrane Cu,Zn-superoxide dismutase by means of mitochondrial cytochrome P450. Free Radic. Biol. Med 2011, 50, 1575–1581. [Google Scholar]
- Zawilska, J.B.; Skene, D.J.; Arendt, J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep 2009, 61, 383–410. [Google Scholar]
- Martin, M.; Macias, M.; Leon, J.; Escames, G.; Khaldy, H.; Acuna-Castroviejo, D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell Biol 2002, 34, 348–357. [Google Scholar]
- Garcia, J.J.; Reiter, R.J.; Guerrero, J.M.; Escames, G.; Yu, B.P.; Oh, C.S.; Munoz-Hoyos, A. Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett 1997, 408, 297–300. [Google Scholar]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Qi, W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: A review of the evidence. Cell Biochem. Biophys 2001, 34, 237–256. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept 2000, 9, 137–159. [Google Scholar]
- Acuna-Castroviejo, D.; Martin, M.; Macias, M.; Escames, G.; Leon, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res 2001, 30, 65–74. [Google Scholar]
- Leon, J.; Acuna-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.X.; Reiter, R.J. Melatonin and mitochondrial function. Life Sci 2004, 75, 765–790. [Google Scholar]
- Leon, J.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.X.; Reiter, R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res 2005, 38, 1–9. [Google Scholar]
- Reyes-Toso, C.F.; Rebagliati, I.R.; Ricci, C.R.; Linares, L.M.; Albornoz, L.E.; Cardinali, D.P.; Zaninovich, A. Effect of melatonin treatment on oxygen consumption by rat liver mitochondria. Amino Acids 2006, 31, 299–302. [Google Scholar]
- Soto-Vega, E.; Meza, I.; Ramirez-Rodriguez, G.; Benitez-King, G. Melatonin stimulates calmodulin phosphorylation by protein kinase C. J. Pineal Res 2004, 37, 98–106. [Google Scholar]
- Leon, J.; Escames, G.; Rodriguez, M.I.; Lopez, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrion, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem 2006, 98, 2023–2033. [Google Scholar]
- Palacios-Callender, M.; Quintero, M.; Hollis, V.S.; Springett, R.J.; Moncada, S. Endogenous NO regulates superoxide production at low oxygen concentrations by modifying the redox state of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 7630–7635. [Google Scholar]
- Mastronicola, D.; Genova, M.L.; Arese, M.; Barone, M.C.; Giuffre, A.; Bianchi, C.; Brunori, M.; Lenaz, G.; Sarti, P. Control of respiration by nitric oxide in Keilin-Hartree particles, mitochondria and SH-SY5Y neuroblastoma cells. Cell. Mol. Life Sci 2003, 60, 1752–1759. [Google Scholar]
- Sarti, P.; Giuffrè, A.; Barone, M.C.; Forte, E.; Mastronicola, D.; Brunori, M. Nitric oxide and cytochrome oxidase: Reaction mechanisms from the enzyme to the cell. Free Radic. Biol. Med 2003, 34, 509–520. [Google Scholar]
- Cooper, C.E.; Mason, M.G.; Nicholls, P. A dynamic model of nitric oxide inhibition of mitochondrial cytochrome c oxidase. Biochim. Biophys. Acta 2008, 1777, 867–876. [Google Scholar]
- Sarti, P.; Forte, E.; Mastronicola, D.; Giuffre, A.; Arese, M. Cytochrome c oxidase and nitric oxide in action: Molecular mechanisms and pathophysiological implications. Biochim. Biophys. Acta 2012, 1817, 610–619. [Google Scholar]
- Almeida, A.; Almeida, J.; Bolanos, J.P.; Moncada, S. Different responses of astrocytes and neurons to nitric oxide: The role of glycolytically generated ATP in astrocyte protection. Proc. Natl. Acad. Sci. USA 2001, 98, 15294–15299. [Google Scholar]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar]
- Brown, G.C.; Cooper, C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett 1994, 356, 295–298. [Google Scholar]
- Cooper, C.E.; Brown, G.C. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: Chemical mechanism and physiological significance. J. Bioenerg. Biomembr 2008, 40, 533–539. [Google Scholar]
- Sarti, P.; Giuffrè, A.; Forte, E.; Mastronicola, D.; Barone, M.C.; Brunori, M. Nitric oxide and cytochrome c oxidase: Mechanisms of inhibition and NO degradation. Biochem. Biophys. Res. Commun 2000, 274, 183–187. [Google Scholar]
- Brunori, M.; Forte, E.; Arese, M.; Mastronicola, D.; Giuffre, A.; Sarti, P. Nitric oxide and the respiratory enzyme. Biochim. Biophys. Acta 2006, 1757, 1144–1154. [Google Scholar]
- Mason, M.G.; Nicholls, P.; Wilson, M.T.; Cooper, C.E. Nitric oxide inhibition of respiration involves both competitive (heme) and noncompetitive (copper) binding to cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2006, 103, 708–713. [Google Scholar]
- Brookes, P.S.; Kraus, D.W.; Shiva, S.; Doeller, J.E.; Barone, M.C.; Patel, R.P.; Lancaster, J.R., Jr; Darley-Usmar, V. Control of mitochondrial respiration by NO*, effects of low oxygen and respiratory state. J. Biol. Chem. 2003, 278, 31603–31609. [Google Scholar]
- Trimmer, B.A.; Aprille, J.R.; Dudzinski, D.M.; Lagace, C.J.; Lewis, S.M.; Michel, T.; Qazi, S.; Zayas, R.M. Nitric oxide and the control of firefly flashing. Science 2001, 292, 2486–2488. [Google Scholar]
- Zoche, M.; Bienert, M.; Beyermann, M.; Koch, K.W. Distinct molecular recognition of calmodulin-binding sites in the neuronal and macrophage nitric oxide synthases: A surface plasmon resonance study. Biochemistry 1996, 35, 8742–8747. [Google Scholar]
- Romero, M.P.; Garcia-Perganeda, A.; Guerrero, J.M.; Osuna, C. Membrane-bound calmodulin in Xenopus laevis oocytes as a novel binding site for melatonin. FASEB J 1998, 12, 1401–1408. [Google Scholar]
- Reers, M.; Smith, T.W.; Chen, L.B. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 1991, 30, 4480–4486. [Google Scholar]
- Reed, P.W. Ionophores. Methods Enzymol 1979, 55, 435–454. [Google Scholar]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J 2006, 273, 2813–2838. [Google Scholar]


© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sarti, P.; Magnifico, M.C.; Altieri, F.; Mastronicola, D.; Arese, M. New Evidence for Cross Talk between Melatonin and Mitochondria Mediated by a Circadian-Compatible Interaction with Nitric Oxide. Int. J. Mol. Sci. 2013, 14, 11259-11276. https://doi.org/10.3390/ijms140611259
Sarti P, Magnifico MC, Altieri F, Mastronicola D, Arese M. New Evidence for Cross Talk between Melatonin and Mitochondria Mediated by a Circadian-Compatible Interaction with Nitric Oxide. International Journal of Molecular Sciences. 2013; 14(6):11259-11276. https://doi.org/10.3390/ijms140611259
Chicago/Turabian StyleSarti, Paolo, Maria Chiara Magnifico, Fabio Altieri, Daniela Mastronicola, and Marzia Arese. 2013. "New Evidence for Cross Talk between Melatonin and Mitochondria Mediated by a Circadian-Compatible Interaction with Nitric Oxide" International Journal of Molecular Sciences 14, no. 6: 11259-11276. https://doi.org/10.3390/ijms140611259



