Quantitative Structure-Activity Relationships Predicting the Antioxidant Potency of 17β-Estradiol-Related Polycyclic Phenols to Inhibit Lipid Peroxidation
Abstract
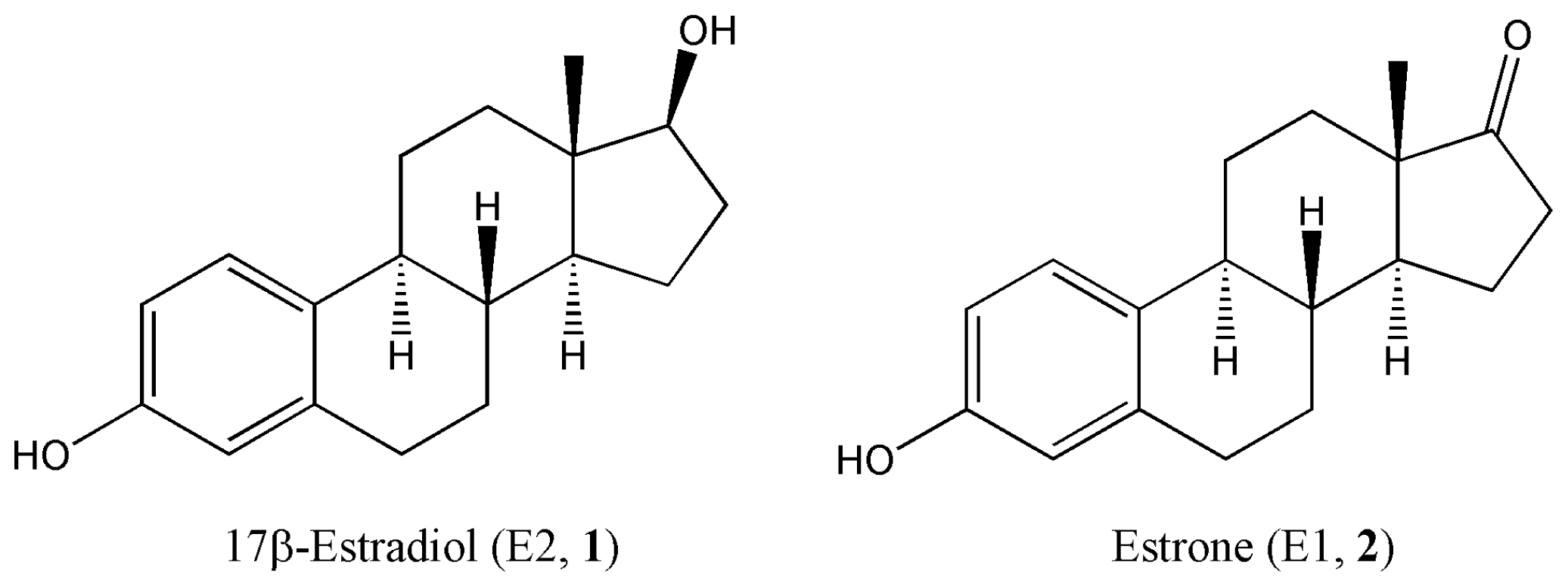
:1. Introduction
2. Results and Discussion
2.1. Construction of QSAR Models
2.2. Validation of QSAR Models (Equations 1–8)
3. Experimental Section
3.1. Materials and Methods
3.2. QSAR
4. Conclusions
Acknowledgments
- Conflict of InterestThe authors declare no conflict of interest.
References
- Mariani, E.; Polidori, M.C.; Cherubini, A.; Mecocci, P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. J. Chromatogr. B 2005, 827, 65–75. [Google Scholar]
- Niki, E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors 2008, 34, 171–180. [Google Scholar]
- Wagner, B.A.; Buettner, G.R.; Burns, C.P. Free radical-mediated lipid peroxidation in cells: Oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry 1995, 33, 4449–4453. [Google Scholar]
- Butterfield, D.A.; Lange, M.L.B.; Sultana, R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 924–929. [Google Scholar]
- Mancuso, C.; Barone, E.; Guido, P.; Miceli, F.; Di Domenico, F.; Perluigi, M.; Santangelo, R.; Preziosi, P. Inhibition of lipid peroxidation and protein oxidation by endogenous and exogenous antioxidants in rat brain microsomes in vitro. Neurosci. Lett 2012, 19, 101–105. [Google Scholar]
- Mooradian, A.D. Antioxidant properties of steroids. J. Steroid Biochem. Mol. Biol 1993, 45, 509–511. [Google Scholar]
- Prokai-Tatrai, K.; Prokai, L.; Jung, M.E. Phenolic compounds protect cultured hippocampal neurons against ethanol-withdrawal induced oxidative stress. Int. J. Mol. Sci 2009, 10, 1773–1787. [Google Scholar]
- Siriphorn, A.; Chompoopong, S.; Floyd, C.L. 17β-estradiol protects Schwann cells against H2O2-induced cytotoxicity and increases transplanted Schwann cell survival in a cervical hemicontusion spinal cord injury model. J. Neurochem 2010, 115, 864–872. [Google Scholar]
- Wilson, M.E.; Dubal, D.B.; Wise, P.M. Estradiol protects against injury-induced cell death in cortical explant cultures: A role for estrogen receptors. Brain Res 2000, 873, 235–242. [Google Scholar]
- Prokai, L.; Prokai-Tatrai, K.; Perjesi, P.; Zharikova, A.D.; Perez, E.J.; Liu, R.; Simpkins, J.W. Quinol-based cyclic antioxidant mechanism in estrogen neuroprotection. Proc. Nat. Acad. Sci. USA 2003, 96, 8867–8872. [Google Scholar]
- Gerstner, B.; Lee, J.; DeSilva, T.M.; Jensen, F.E.; Volpe, J.J.; Rosenberg, P.A. 17beta-estradiol protects against hypoxic/ischemic white matter damage in the neonatal rat brain. J. Neurosci. Res 2009, 87, 2078–2086. [Google Scholar]
- Kii, N.; Adachi, N.; Liu, K.; Arai, T. Acute effects of 17beta-estradiol on oxidative stress in ischemic rat striatum. J. Neurosurg. Anesthesiol 2005, 17, 27–32. [Google Scholar]
- Strom, J.O.; Theodorsson, A.; Theodorsson, E. Dose-related neuroprotective versus neurodamaging effects of estrogens in rat cerebral ischemia: A systematic analysis. J. Cereb. Blood Flow Metabol 2009, 29, 1359–1372. [Google Scholar]
- Prokai, L.; Oon, S.M.; Prokai-Tatrai, K.; Abboud, K.A.; Simpkins, J.W. Synthesis and biological evaluation of 17beta-alkoxyestra-1,3,5(10)-trienes as potential neuroprotectants against oxidative stress. J. Med. Chem 2001, 4, 110–114. [Google Scholar]
- Prokai, L.; Simpkins, J.W. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. J. Pharmacol. Ther 2007, 114, 1–12. [Google Scholar]
- Moosmann, B.; Behl, C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. USA 1999, 96, 8867–8872. [Google Scholar]
- Prokai-Tatrai, K.; Perjesi, P.; Rivera-Portalatin, N.M.; Prokai, L. Mechanistic investigations on the antioxidant action of a neuroprotective estrogen derivative. Steroids 2009, 73, 280–288. [Google Scholar]
- Hum, P.D.; Macrae, M. Estrogen as a neuroprotectant in stroke. J. Cereb. Blood Flow Metabol 2000, 2, 631–652. [Google Scholar]
- Römer, W.; Oettel, M.; Menzenbach, B.; Droescher, P.; Schwarz, S. Novel estrogens and their radical scavenging effects, iron-chelating, and total antioxidative activities: 17-alpha-substituted analogs of delta 9(11)-dehydro-17 beta-estradiol. Steroids 1997, 62, 688–694. [Google Scholar]
- Yune, T.E.; Kim, S.J.; Lee, S.M.; Lee, Y.K.; Oh, Y.J.; Kim, Y.C.; Markelonis, G.J.; Oh, T.H. Systemic administration of 17b-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J. Neurotrauma 2004, 21, 293–306. [Google Scholar]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-pass metabolism via UDP-glucuronosyltransferase: A barrier to oral bioavailability of phenolics. J. Pharm. Sci 2011, 100, 3655–3681. [Google Scholar]
- DePaolo, L.; Negro-Vilar, A. Estrogenic feminization of the LH response to orchidectomy in the rat: Evidence for a hypothalamic site of action. Neuroendocrinol 1982, 34, 104–111. [Google Scholar]
- Hsueh, A.J.W.; Peck, E.J.; Clark, J.H. Progesterone antagonism of the oestrogen receptor and oestrogen-induced uterine growth. Nature 1975, 254, 337–339. [Google Scholar]
- Ruiz-Larrea, M.B.; Martin, C.; Martinez, R.; Navarro, R.; Lacort, M.; Miller, N.J. Antioxidant activities of estrogens against aqueous and lipophilic radicals; differences between phenol and catechol estrogens. Chem. Phys. Lipids 2000, 105, 179–188. [Google Scholar]
- Perez, E.; Cai, Z.Y.; Covey, D.F.; Simpkins, J.F. Neuroprotective effects of estratriene analogs: Structure-activity relationships and molecular optimization. Drug Dev. Res 2005, 66, 78–92. [Google Scholar]
- Cummins, C.H. Synthesis and radiochemistry of 2,4-disubstituted 17-iodovinylestradiols. Steroids 1994, 59, 590–596. [Google Scholar]
- Miller, C.P.; Jirkovsky, I.; Hayhurst, D.A.; Adelmant, S.J. In vitro antioxidant effects of estrogens with a hindered 3-OH function on the copper-induced oxidation of low density lipoprotein. Steroids 1996, 61, 305–308. [Google Scholar]
- Tong, W.; Perkins, R.; Strelitz, R.; Collantes, E.R.; Keenan, S.; Welsh, W.J.; Branham, W.S.; Sheehan, D.M. Quantitative structure-activity relationships (QSARs) for estrogen binding to the estrogen receptor: predictions across species. Environ. Health Perspect 1997, 105, 1116–1124. [Google Scholar]
- Hu, J.-Y.; Aizawa, T. Quantitative structure-activity relationships for estrogen receptor binding affinity of phenolic chemicals. Water Res 2003, 37, 1213–1222. [Google Scholar]
- Hong, H.; Tong, W.; Fang, H.; Shi, L.; Xie, Q.; Wu, J.; Perkins, R.; Walker, J.D.; Branham, W.; Sheehan, D.M. Prediction of estrogen receptor binding for 58,000 chemicals using an integrated system of a tree-based model with structural alerts. Environ. Health Perspect 2002, 110, 29–36. [Google Scholar]
- Behl, C.; Skutella, T.; Lezoualc’h, F.; Post, A.; Widmann, M.; Newton, C.J.; Holsboer, F. Neuroprotection against oxidative stress by estrogens: Structure-activity relationship. Mol. Pharmacol 1997, 51, 535–541. [Google Scholar]
- Badeau, M.; Adlercreutz, H.; Kaihocaara, P.; Tikkanen, M.J. Estrogen A-ring structure and antioxidative effect on lipoproteins. J. Steroid Biochem. Mol. Biol 2005, 96, 271–278. [Google Scholar]
- Callaway, J.K.; Beart, P.M.; Jarrott, B. A reliable procedure for comparison of antioxidants in rat brain homogenates. J. Pharmacol. Toxicol. Meth 1998, 39, 155–162. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malondialdehyde and 4-hydroxynonenal. Methods Enzymol 1990, 186, 407–421. [Google Scholar]
- Mihaljevic, B.A.; Katusin-Razem, B.; Razem, D. The reevaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanistic aspects of the response. Free Radic. Biol. Med 1996, 21, 53–63. [Google Scholar]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med 1990, 9, 515–540. [Google Scholar]
- Rivera-Portalatin, N.M. Mechanism of the Antioxidant Action of Estrogens. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2006. [Google Scholar]
- Perez, E.J. Estratriene Neuroprotection through Antioxidant, Non-Estrogen Receptor Mediated Mechanisms. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2004; p. 102. [Google Scholar]
- Mitra, I.; Saha, A.; Roy, K. In silico development, validation and comparison of predictive QSAR models for lipid peroxidation inhibitory activity of cinnamic acid and caffeic acid derivatives using multiple chemometric and cheminformatics tools. J. Mol. Model 2012, 18, 3951–3967. [Google Scholar]
- Cheng, Z.Y.; Ren, J.; Li, Y.Z.; Chang, W.B.; Chen, Z.D. Establishment of a quantitative structure-activity relationship model for evaluating and predicting the protective potentials of phenolic antioxidants on lipid peroxidation. J. Pharm. Sci 2003, 92, 475–484. [Google Scholar]
- Bakalbassis, E.G.; Chatzopoulou, A.; Malissas, V.S.; Tsimidou, M.; Vafiadis, A. Ab initio and density functional theory studies for the explanation of the antioxidant activity of certain phenolic acids. Lipids 2001, 36, 181–190. [Google Scholar]
- Mukai, K.; Uemoto, Y.; Fukuhara, M.; Nagaoka, S.; Ishizu, K. ENDOR study of the cation radicals of vitamin-E derivatives-relation between antioxidant activity and molecular-structure. Bull. Chem. Soc. Jpn 1992, 65, 2016–2020. [Google Scholar]
- Bordwell, F.G.; Zhang, X.-M.; Satish, A.V.; Cheng, J.-P. Assessment of the importance of changes in ground-state energies on the bond dissociation enthalpies of the O–H bonds in phenols and the S–H bonds in thiophenols. J. Am. Chem. Soc. 1994, 116, 6605–6610. [Google Scholar]
- Prokai, L.; Prokai-Tatrai, K.; Perjesi, P.; Simpkins, J.W. Mechanistic insights into the direct antioxidant effects of estrogens. Drug Dev. Res 2006, 66, 118–125. [Google Scholar]
- Pedulli, G.F.; Lucarini, A.; Pedrielli, P. Bond Dissociation Energies of Phenolic and Amine Antioxidants. In Free Radicals in Biology and Environment; Minisci, F., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherland, 1997; pp. 169–180. [Google Scholar]
- Kier, L.B. Indexes of molecular shape from chemical graphs. Med. Res. Rev 1987, 7, 417–440. [Google Scholar]
- Katritzky, A.R.; Gordeeva, E.V. Traditional topological indices vs. electronic, geometric, combined molecular descriptors in QSAR/QSPR research. J. Chem. Inf. Comput. Sci 1993, 33, 835–857. [Google Scholar]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans 1993, 2, 799–805. [Google Scholar]
- Lorand, T.; Kocsis, B.; Sohar, P.; Nagy, G.; Jozsef, P.; Kispal, G.; Laszlo, R.; Prokai, L. Synthesis and antibacterial effect of fused Mannich ketones. Eur. J. Med. Chem 2002, 37, 803–812. [Google Scholar]
- Kocsis, B.; Kustos, I.; Kilar, F.; Nyul, A.; Jakus, P.B.; Kerekes, S.; Villarreal, V.; Prokai, L.; Lorand, T. Antifungal unsaturated cyclic Mannich ketones and aminoalcohols: Study of mechanism of action. Eur. J. Med. Chem 2009, 44, 1823–1829. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem 1985, 150, 76–85. [Google Scholar]
- Walker, J.M. The Bicinchoninic Acid (BCA) Assay for Protein Quantitation. In The Protein Protocols Handbook, 3rd ed; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 2009; pp. 11–15. [Google Scholar]
- Nguyen, V.; Zharikova, A.D.; Prokai-Tatrai, K.; Prokai, L. [Glu2]TRH dose-dependently attenuates TRH-evoked analeptic effect in the mouse brain. Brain Res. Bull. 2010, 82, 83–86. [Google Scholar]

| Equation Number | False Positives a | False Negatives b | Correctly Predicted c |
|---|---|---|---|
| 1 | 6 | 1 | 21 |
| 2 | 19 | 1 | 8 |
| 3 | 17 | 1 | 10 |
| 4 | 2 | 3 | 23 |
| 5 | 5 | 1 | 22 |
| 6 | 6 | 2 | 20 |
| 7 | 1 | 7 | 20 |
| 8 | 7 | 3 | 18 |
© 2013 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prokai, L.; Rivera-Portalatin, N.M.; Prokai-Tatrai, K. Quantitative Structure-Activity Relationships Predicting the Antioxidant Potency of 17β-Estradiol-Related Polycyclic Phenols to Inhibit Lipid Peroxidation. Int. J. Mol. Sci. 2013, 14, 1443-1454. https://doi.org/10.3390/ijms14011443
Prokai L, Rivera-Portalatin NM, Prokai-Tatrai K. Quantitative Structure-Activity Relationships Predicting the Antioxidant Potency of 17β-Estradiol-Related Polycyclic Phenols to Inhibit Lipid Peroxidation. International Journal of Molecular Sciences. 2013; 14(1):1443-1454. https://doi.org/10.3390/ijms14011443
Chicago/Turabian StyleProkai, Laszlo, Nilka M. Rivera-Portalatin, and Katalin Prokai-Tatrai. 2013. "Quantitative Structure-Activity Relationships Predicting the Antioxidant Potency of 17β-Estradiol-Related Polycyclic Phenols to Inhibit Lipid Peroxidation" International Journal of Molecular Sciences 14, no. 1: 1443-1454. https://doi.org/10.3390/ijms14011443







