Accounting for Large Amplitude Protein Deformation during in Silico Macromolecular Docking
Abstract
:1. Introduction
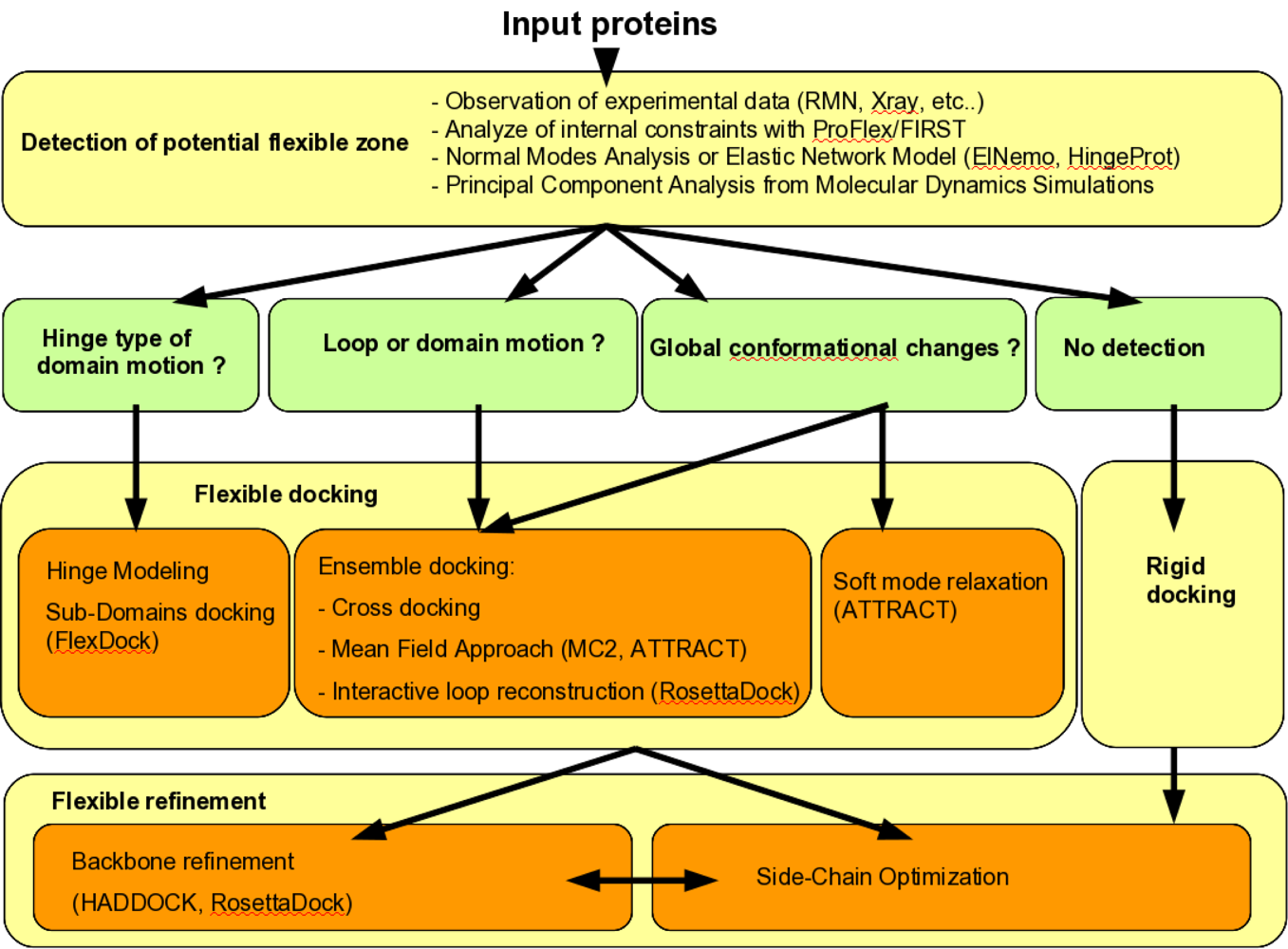
2. The Flexible Docking Problem
2.1. Flexible Refinement
2.2. Characteristics of Protein Flexibility
3. Methods for Flexible Docking Search
3.1. Continuous Approaches
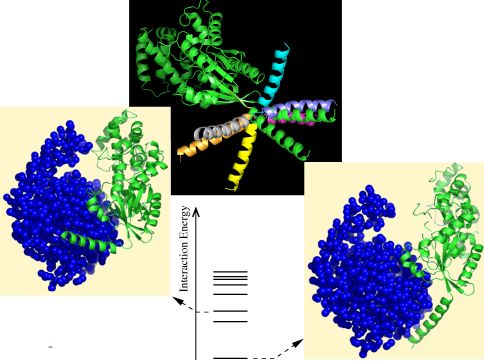
3.1.1. Soft Mode Relaxation
3.2. Discrete Approaches
3.2.1. Cross-Docking
3.2.2. Interface Remodeling
3.2.3. Multi-Copy/Mean Field Approach
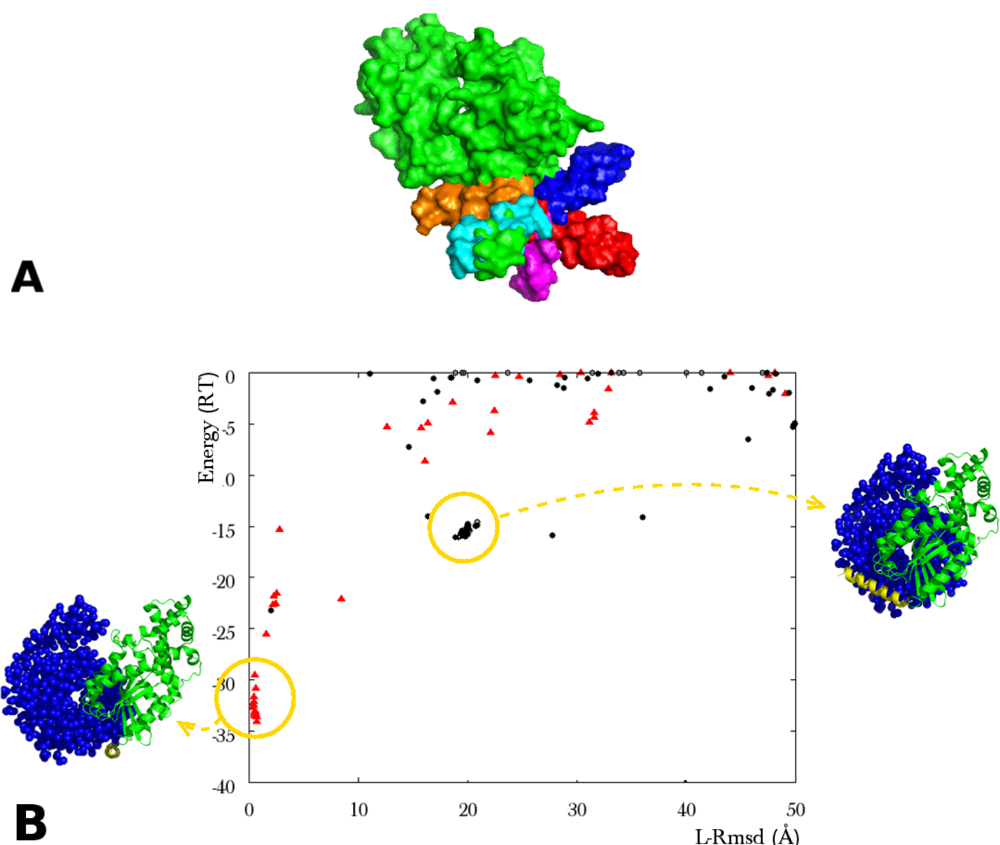
3.2.4. Multi-Component Docking
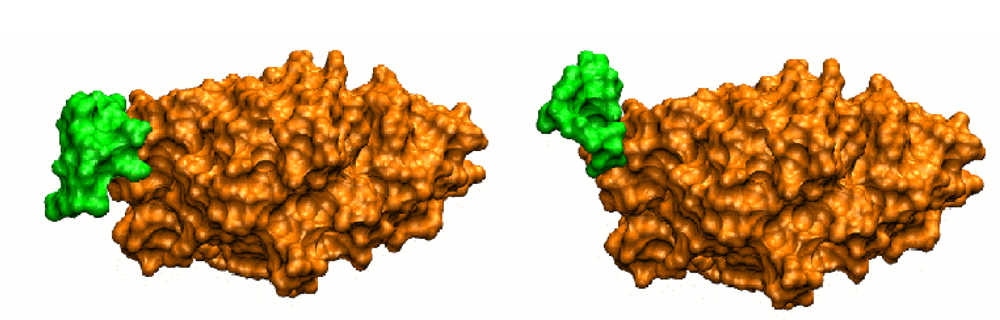
3.2.4.1. Interactive Loop Reconstruction
4. Conclusions
References
- Wodak, SJ; Janin, J. Computer analysis of protein-protein interaction. J. Mol. Biol 1978, 124, 323–342. [Google Scholar]
- Janin, J; Henrick, K; Moult, J; Eyck, LT; Sternberg, MJE; Vajda, S; Vakser, I; Wodak, SJ. of PRedicted Interactions, C.A.. CAPRI: A Critical Assessment of PRedicted Interactions. Proteins 2003, 52, 2–9. [Google Scholar]
- Mendez, R; Leplae, R; Maria, LD; Wodak, SJ. Assessment of blind predictions of protein-protein interactions: current status of docking methods. Proteins 2003, 52, 51–67. [Google Scholar]
- Janin, J. Assessing predictions of protein-protein interaction: the CAPRI experiment. Protein Sci 2005, 14, 278–283. [Google Scholar]
- Mendez, R; Leplae, R; Lensink, MF; Wodak, SJ. Assessment of CAPRI predictions in rounds 3–5 shows progress in docking procedures. Proteins 2005, 60, 150–169. [Google Scholar]
- Lensink, MF; Mndez, R; Wodak, SJ. Docking and scoring protein complexes: CAPRI 3rd Edition. Proteins 2007, 69, 704–718. [Google Scholar]
- Lensink, MF; Wodak, SJ. Docking and scoring protein interactions: CAPRI 2009. Proteins 2010, 78, 3073–3084. [Google Scholar]
- Bonvin, AMJJ. Flexible protein-protein docking. Curr. Opin. Struct. Biol 2006, 16, 194–200. [Google Scholar]
- Andrusier, N; Mashiach, E; Nussinov, R; Wolfson, HJ. Principles of flexible protein-protein docking. Proteins 2008, 73, 271–289. [Google Scholar]
- Ritchie, DW. Recent progress and future directions in protein-protein docking. Curr. Protein Pept. Sci 2008, 9, 1–15. [Google Scholar]
- Zacharias, M. Accounting for conformational changes during protein-protein docking. Curr. Opin. Struct. Biol 2010, 20, 180–186. [Google Scholar]
- Saladin, A; Prévost, C. Protein-Protein Docking. In Protein-Protein Complexes Analysis, Modeling and Drug Design; Zacharias, M, Ed.; Imperial College Press: London, UK, 2010; pp. 147–181. [Google Scholar]
- Zacharias, M. Scoring and Refinement of Predicted Protein-Protein Complexes. In Protein-Protein Complexes Analysis, Modeling and Drug Design; Zacharias, M, Ed.; Imperial College Press: London, UK, 2010; pp. 236–271. [Google Scholar]
- Pons, C; Grosdidier, S; Solernou, A; Pérez-Cano, L; Fernàndez-Recio, J. Present and future challenges and limitations in protein-protein docking. Proteins 2010, 78, 95–108. [Google Scholar]
- Bastard, K; Prévost, C. Recent Research Advances in Structural BioInformatics. In Flexible Macromolecular Docking: An Overview of Recent Progress; de Brevern, AG, Ed.; Research Signpost: Trivandrum, India, 2007; pp. 249–274. [Google Scholar]
- Vajda, S; Kozakov, D. Convergence and combination of methods in protein-protein docking. Curr. Opin. Struct. Biol 2009, 19, 164–170. [Google Scholar]
- Norel, R; Lin, SL; Wolfson, HJ; Nussinov, R. Shape complementarity at protein-protein interfaces. Biopolymers 1994, 34, 933–940. [Google Scholar]
- Ritchie, DW; Kemp, GJ. Protein docking using spherical polar Fourier correlations. Proteins 2000, 39, 178–194. [Google Scholar]
- Katchalski-Katzir, E; Shariv, I; Eisenstein, M; Friesem, AA; Aflalo, C; Vakser, IA. Molecular surface recognition: determination of geometric fit between proteins and their ligands by correlation techniques. Proc. Natl. Acad. Sci. USA 1992, 89, 2195–2199. [Google Scholar]
- Zacharias, M. Protein-protein docking with a reduced protein model accounting for side-chain flexibility. Protein Sci 2003, 12, 1271–1282. [Google Scholar]
- Schueler-Furman, O; Wang, C; Baker, D. Progress in protein-protein docking: atomic resolution predictions in the CAPRI experiment using RosettaDock with an improved treatment of side-chain flexibility. Proteins 2005, 60, 187–194. [Google Scholar]
- Fernández-Recio, J; Totrov, M; Abagyan, R. ICM-DISCO docking by global energy optimization with fully flexible side-chains. Proteins 2003, 52, 113–117. [Google Scholar]
- Król, M; Tournier, AL; Bates, PA. Flexible relaxation of rigid-body docking solutions. Proteins 2007, 68, 159–169. [Google Scholar]
- Wang, C; Bradley, P; Baker, D. Protein-protein docking with backbone flexibility. J. Mol. Biol 2007, 373, 503–519. [Google Scholar]
- Andrusier, N; Nussinov, R; Wolfson, HJ. FireDock: Fast interaction refinement in molecular docking. Proteins 2007, 69, 139–159. [Google Scholar]
- Kozakov, D; Hall, DR; Beglov, D; Brenke, R; Comeau, SR; Shen, Y; Li, K; Zheng, J; Vakili, P; Paschalidis, IC; Vajda, S. Achieving reliability and high accuracy in automated protein docking: ClusPro, PIPER, SDU, and stability analysis in CAPRI rounds 13–19. Proteins 2010, 78, 3124–3130. [Google Scholar]
- Noy, E; Goldblum, A. Flexible protein-protein docking based on Best-First search algorithm. J. Comput. Chem 2010, 31, 1929–1943. [Google Scholar]
- Hwang, H; Vreven, T; Janin, J; Weng, Z. Protein-protein docking benchmark version 4.0. Proteins 2010, 78, 3111–3114. [Google Scholar]
- Jackson, RM; Gabb, HA; Sternberg, MJ. Rapid refinement of protein interfaces incorporating solvation: application to the docking problem. J. Mol. Biol 1998, 276, 265–285. [Google Scholar]
- Kimura, SR; Brower, RC; Vajda, S; Camacho, CJ. Dynamical view of the positions of key side chains in protein-protein recognition. Biophys. J 2001, 80, 635–642. [Google Scholar]
- Rajamani, D; Thiel, S; Vajda, S; Camacho, CJ. Anchor residues in protein-protein interactions. Proc. Natl. Acad. Sci. USA 2004, 101, 11287–11292. [Google Scholar]
- Bastard, K; Thureau, A; Lavery, R; Prévost, C. Docking macromolecules with flexible segments. J. Comput. Chem 2003, 24, 1910–1920. [Google Scholar]
- Dominguez, C; Boelens, R; Bonvin, AMJJ. HADDOCK: A protein-protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc 2003, 125, 1731–1737. [Google Scholar]
- de Vries, SJ; van Dijk, ADJ; Krzeminski, M; van Dijk, M; Thureau, A; Hsu, V; Wassenaar, T; Bonvin, AMJJ. HADDOCK versus HADDOCK: New features and performance of HADDOCK2.0 on the CAPRI targets. Proteins 2007, 69, 726–733. [Google Scholar]
- May, A; Zacharias, M. Protein-ligand docking accounting for receptor side chain and global flexibility in normal modes: Evaluation on kinase inhibitor cross docking. J. Med. Chem 2008, 51, 3499–3506. [Google Scholar]
- Mashiach, E; Nussinov, R; Wolfson, HJ. FiberDock: Flexible induced-fit backbone refinement in molecular docking. Proteins 2010, 78, 1503–1519. [Google Scholar]
- Ehrlich, LP; Nilges, M; Wade, RC. The impact of protein flexibility on protein-protein docking. Proteins 2005, 58, 126–133. [Google Scholar]
- Suhre, K; Sanejouand, YH. ElNemo: A normal mode web server for protein movement analysis and the generation of templates for molecular replacement. Nucleic Acids Res 2004, 32, W610–W614. [Google Scholar]
- Dobbins, SE; Lesk, VI; Sternberg, MJE. Insights into protein flexibility: The relationship between normal modes and conformational change upon protein-protein docking. Proc. Natl. Acad. Sci. USA 2008, 105, 10390–10395. [Google Scholar]
- Navizet, I; Cailliez, F; Lavery, R. Probing protein mechanics: residue-level properties and their use in defining domains. Biophys. J 2004, 87, 1426–1435. [Google Scholar]
- Sacquin-Mora, S; Lavery, R. Modeling the mechanical response of proteins to anisotropic deformation. Chemphyschem 2009, 10, 115–118. [Google Scholar]
- Sacquin-Mora, S; Laforet, E; Lavery, R. Locating the active sites of enzymes using mechanical properties. Proteins 2007, 67, 350–359. [Google Scholar]
- Lavery, R; Sacquin-Mora, S. Protein mechanics: A route from structure to function. J. Biosci 2007, 32, 891–898. [Google Scholar]
- Emekli, U; Schneidman-Duhovny, D; Wolfson, HJ; Nussinov, R; Haliloglu, T. HingeProt: Automated prediction of hinges in protein structures. Proteins 2008, 70, 1219–1227. [Google Scholar]
- Jacobs, DJ; Rader, AJ; Kuhn, LA; Thorpe, MF. Protein flexibility predictions using graph theory. Proteins 2001, 44, 150–165. [Google Scholar]
- Keating, KS; Flores, SC; Gerstein, MB; Kuhn, LA. StoneHinge: Hinge prediction by network analysis of individual protein structures. Protein Sci 2009, 18, 359–371. [Google Scholar]
- Koshland, DE. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc. Natl. Acad. Sci. USA 1958, 44, 98–104. [Google Scholar]
- Kumar, S; Ma, B; Tsai, CJ; Sinha, N; Nussinov, R. Folding and binding cascades: Dynamic landscapes and population shifts. Protein Sci 2000, 9, 10–19. [Google Scholar]
- May, A; Zacharias, M. Accounting for global protein deformability during protein-protein and protein-ligand docking. Biochim. Biophys. Acta 2005, 1754, 225–231. [Google Scholar]
- Grunberg, R; Leckner, J; Nilges, M. Complementarity of structure ensembles in protein-protein binding. Structure 2004, 12, 2125–2136. [Google Scholar]
- Mustard, D; Ritchie, DW. Docking essential dynamics eigenstructures. Proteins 2005, 60, 269–274. [Google Scholar]
- Smith, GR; Sternberg, MJE; Bates, PA. The relationship between the flexibility of proteins and their conformational states on forming protein-protein complexes with an application to protein-protein docking. J. Mol. Biol 2005, 347, 1077–1101. [Google Scholar]
- Chaudhury, S; Gray, JJ. Conformer selection and induced fit in flexible backbone protein-protein docking using computational and NMR ensembles. J. Mol. Biol 2008, 381, 1068–1087. [Google Scholar]
- Ritchie, DW; Venkatraman, V. Ultra-fast FFT protein docking on graphics processors. Bioinformatics 2010, 26, 2398–2405. [Google Scholar]
- Cortés, J; Siméon, T; de Angulo, VR; Guieysse, D; Remaud-Siméon, M; Tran, V. A path planning approach for computing large-amplitude motions of flexible molecules. Bioinformatics 2005, i116–i125. [Google Scholar]
- Regad, L; Martin, J; Nuel, G; Camproux, AC. Mining protein loops using a structural alphabet and statistical exceptionality. BMC Bioinforma 2010, 11, 75. [Google Scholar] [Green Version]
- Shehu, A; Clementi, C; Kavraki, LE. Modeling protein conformational ensembles: from missing loops to equilibrium fluctuations. Proteins 2006, 65, 164–179. [Google Scholar]
- Mandell, DJ; Kortemme, T. Backbone flexibility in computational protein design. Curr. Opin. Biotechnol 2009, 20, 420–428. [Google Scholar]
- Bastard, K; Prévost, C; Zacharias, M. Accounting for loop flexibility during protein-protein docking. Proteins 2006, 62, 956–969. [Google Scholar]
- Loriot, S; Sachdeva, S; Bastard, K; Prévost, C; Cazals, F. On the Characterization and Selection of Diverse Conformational Ensembles, with Applications to Flexible Docking. IEEE/ACM Trans.Comput. Biol. Bioinf 2011, 8, 487–498. [Google Scholar]
- Schneidman-Duhovny, D; Inbar, Y; Nussinov, R; Wolfson, HJ. Geometry-based flexible and symmetric protein docking. Proteins 2005, 60, 224–231. [Google Scholar]
- Ben-Zeev, E; Kowalsman, N; Ben-Shimon, A; Segal, D; Atarot, T; Noivirt, O; Shay, T; Eisenstein, M. Docking to single-domain and multiple-domain proteins: Old and new challenges. Proteins 2005, 60, 195–201. [Google Scholar]
- Inbar, Y; Benyamini, H; Nussinov, R; Wolfson, HJ. Prediction of multimolecular assemblies by multiple docking. J. Mol. Biol 2005, 349, 435–447. [Google Scholar]
- Schneidman-Duhovny, D; Nussinov, R; Wolfson, HJ. Automatic prediction of protein interactions with large scale motion. Proteins 2007, 69, 764–773. [Google Scholar]
- Inbar, Y; Benyamini, H; Nussinov, R; Wolfson, HJ. Combinatorial docking approach for structure prediction of large proteins and multi-molecular assemblies. Phys. Biol 2005, 2, S156–S165. [Google Scholar]
- van Dijk, ADJ; de Vries, SJ; Dominguez, C; Chen, H; Zhou, HX; Bonvin, AMJJ. Data-driven docking: HADDOCK’s adventures in CAPRI. Proteins 2005, 60, 232–238. [Google Scholar]
- Karaca, E; Melquiond, ASJ; de Vries, SJ; Kastritis, PL; Bonvin, AMJJ. Building macromolecular assemblies by information-driven docking: Introducing the HADDOCK multibody docking server. Mol. Cell Proteomics 2010, 9, 1784–1794. [Google Scholar]
- Saladin, A; Fiorucci, S; Poulain, P; Prévost, C; Zacharias, M. PTools: An opensource molecular docking library. BMC Struct. Biol 2009, 9, 27. [Google Scholar]
- Mandell, DJ; Coutsias, EA; Kortemme, T. Sub-angstrom accuracy in protein loop reconstruction by robotics-inspired conformational sampling. Nat. Methods 2009, 6, 551–552. [Google Scholar]
- Saladin, A; Amourda, C; Poulain, P; Férey, N; Baaden, M; Zacharias, M; Delalande, O; Prévost, C. Modeling the early stage of DNA sequence recognition within RecA nucleoprotein filaments. Nucleic Acids Res 2010, 38, 6313–6323. [Google Scholar]



© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bastard, K.; Saladin, A.; Prévost, C. Accounting for Large Amplitude Protein Deformation during in Silico Macromolecular Docking. Int. J. Mol. Sci. 2011, 12, 1316-1333. https://doi.org/10.3390/ijms12021316
Bastard K, Saladin A, Prévost C. Accounting for Large Amplitude Protein Deformation during in Silico Macromolecular Docking. International Journal of Molecular Sciences. 2011; 12(2):1316-1333. https://doi.org/10.3390/ijms12021316
Chicago/Turabian StyleBastard, Karine, Adrien Saladin, and Chantal Prévost. 2011. "Accounting for Large Amplitude Protein Deformation during in Silico Macromolecular Docking" International Journal of Molecular Sciences 12, no. 2: 1316-1333. https://doi.org/10.3390/ijms12021316