Organic Analysis of Peridotite Rocks from the Ashadze and Logatchev Hydrothermal Sites
Abstract
:1. Introduction
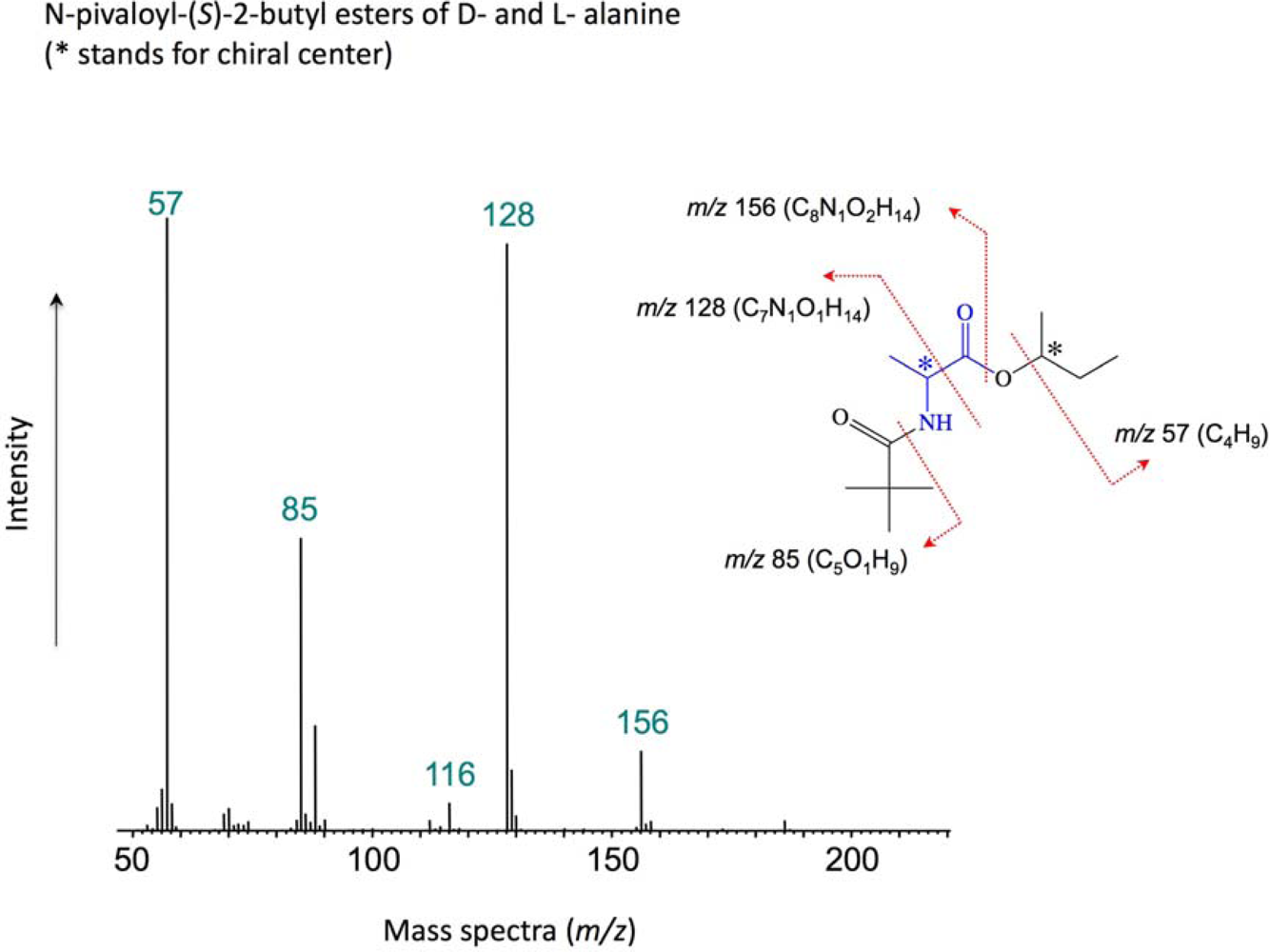
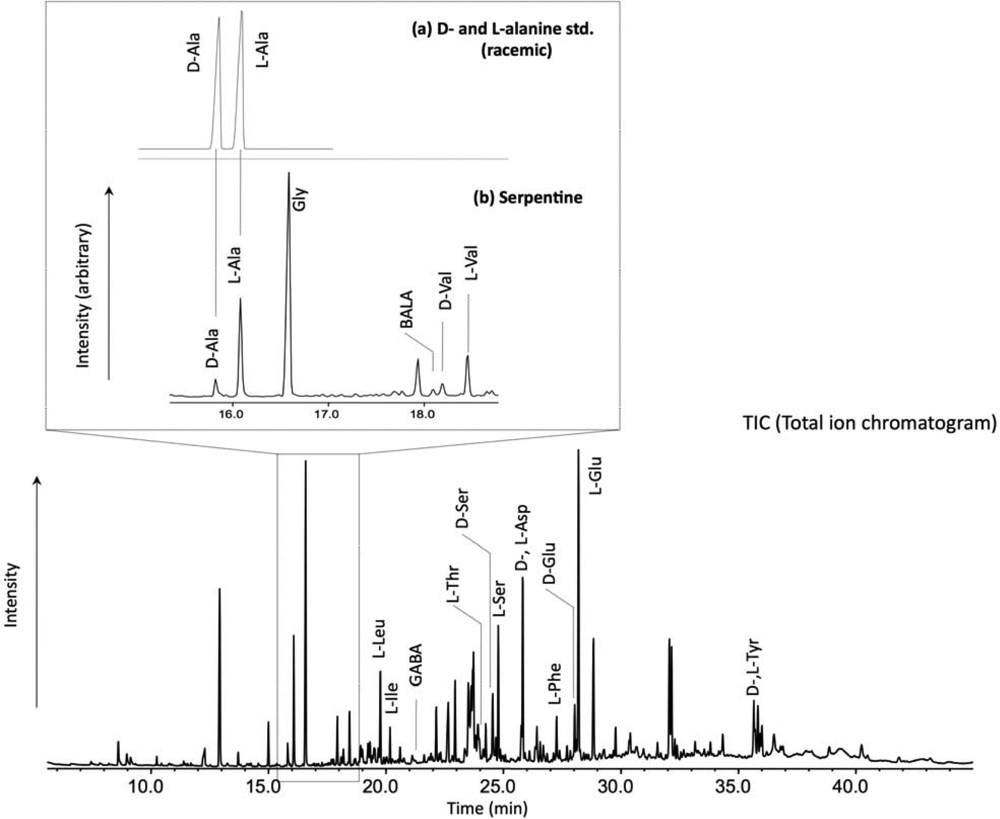
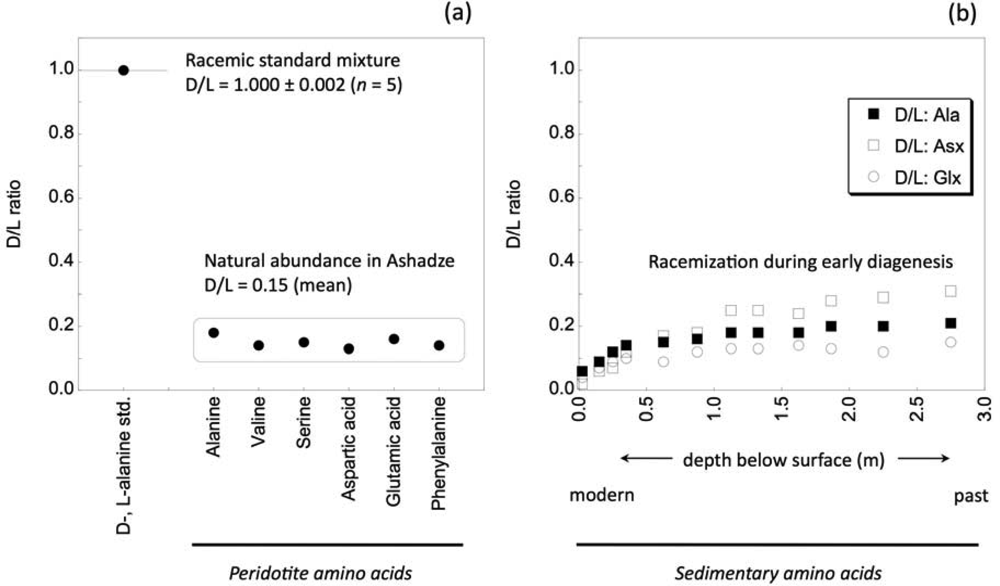
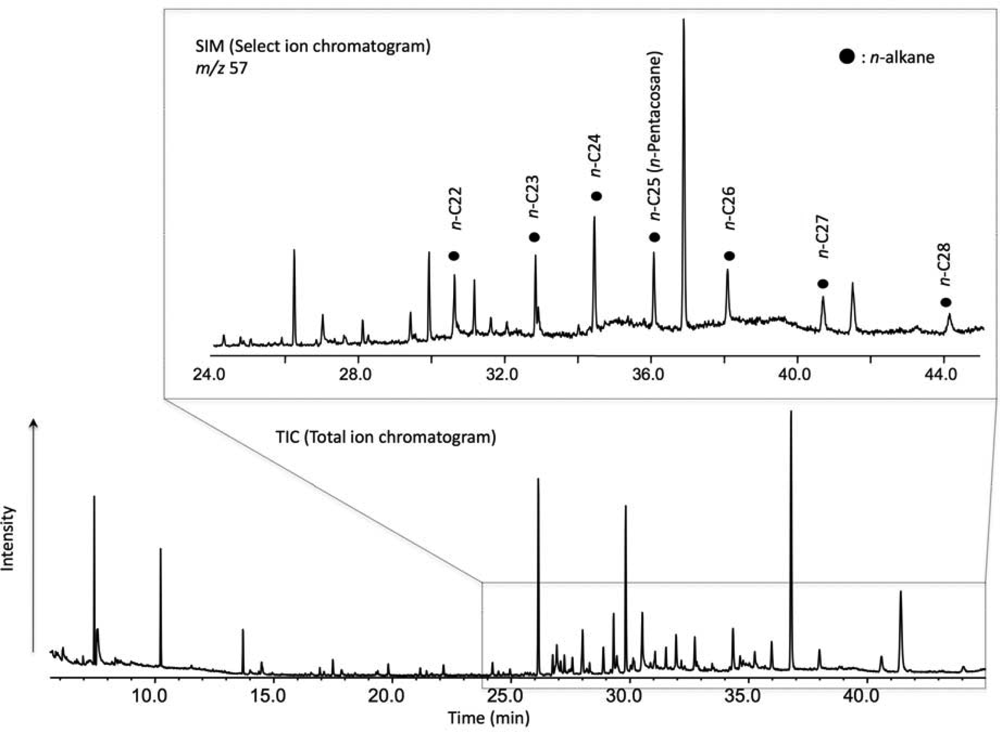
2. Experimental Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Altérations hydrothermales de la lithosphère océanique. In Comptes Rendus Geosciences; Honnorez, J (Ed.) Elsevier: Paris, France, 2003; Volume 335, pp. 777–864.
- Mével, C. Serpentinization of abyssal peridotites at mid-ocean ridges. C. R. Geoscience 2003, 335, 825–852. [Google Scholar]
- Fouquet, Y; Cherkashov, G; Charlou, J; Ondreas, H; Cannat, M; Bortnikov, N; Silantiev, S; Etoubleau, J. Diversity of ultramafic hosted hydrothermal deposits on the Mid Atlantic Ridge: First submersible studies on Ashadze, Logatchev 2 and Krasnov vent Fields during the Serpentine cruise. AGU Fall Meeting, San Francisco, CA, USA, 10–14 December 2007. Abstract T51F–03.
- Schmidt, K; Koschinsky, A; Garbe-Schönberg, D; de Carvalho, LM; Seifert, R. Geochemistry of hydrothermal fluids from the ultramafic-hosted Logatchev hydrothermal field, 15°N on the Mid-Atlantic Ridge: Temporal and spatial investigation. Chem. Geolog 2007, 242, 1–21. [Google Scholar]
- Charlou, J; Donval, J; Konn, C; Birot, D; Sudaikov, S; Jean-Baptiste, P; Fouquet, Y. High hydrogen and abiotic hydrocarbons from new ultramafic hydrothermal sites between 12°N and 15°N on the Mid Atlantic Ridge-results of the Serpentine cruise (march 2007). AGU-Fall Meeting, San Francisco, CA, USA, 10–14 December 2007. Abstract T51F–04.
- Konn, C; Charlou, JL; Donval, JP; Holm, NG; Dehairs, F; Bouillon, S. Hydrocarbons and oxydized organic compounds in hydrothermal fluids from Rainbow and Lost City ultramafic-hosted vents. Chem. Geolog 2009, 258, 299–314. [Google Scholar]
- Berndt, ME; Allen, DE; Seyfried, WE, Jr. Reduction of CO2 during serpentinization of olivine at 300 °C and 500 bar. Geology 1996, 24, 351–354. [Google Scholar]
- Foustoukos, DI; Seyfried, WE, Jr. Hydrocarbons in hydrothermal vent fluids: the role of chromium-bearing catalysts. Science 2004, 304, 1002–1005. [Google Scholar]
- Seyfried, WE, Jr; Foustoukos, DI; Fu, Q. Redox evolution and mass transfer during serpentinization: An experimental and theoretical study at 200 °C, 500 bar with implications for ultramafic-hosted hydrothermal systems at Mid-Ocean Ridges. Geochim. Cosmochim. Acta 2007, 71, 3872–3886. [Google Scholar]
- McCollom, TM; Seewald, JS. Carbon isotope composition of organic compounds produced by abiotic synthesis under hydrothermal conditions. Earth Planet. Sci. Lett 2006, 243, 74–84. [Google Scholar]
- Shock, LE; Schulte, MD. Organic synthesis during fluid mixing in hydrothermal systems. J. Geophys. Res 1998, 103, 28513–28527. [Google Scholar]
- McCollom, T; Bach, W. Thermodynamic constraints of hydrogen generation during serpentinization of ultramafic rocks. Geochim. Cosmochim. Acta 2009, 73, 856–875. [Google Scholar]
- Bach, W; Garrido, CJ; Paulick, H; Harvey, J; Rosner, M. Seawater-peridotite interactions: First insights from ODP Leg 209, MAR 15°N. Geochem. Geoph. Geosyst 2004, 5, 1–22. [Google Scholar]
- Miller, SL; Orgel, LE. The Origin of Life on the Earth; Prentice-Hall: Englewoods Cliffs, NJ, USA, 1974. [Google Scholar]
- Yanagawa, H; Kobayashi, K. An experimental approach to chemical evolution in submarine hydrothermal systems. Orig. Life Evol. Biosphere 1992, 22, 147–159. [Google Scholar]
- Holm, NG; Andersson, EM. Abiotic synthesis of organic compounds under the conditions of submarine hydrothermal systems: a perspective. Planet. Space Sci 1995, 43, 153–159. [Google Scholar]
- Imai, E; Honda, H; Hatori, K; Brack, A; Matsuno, K. Elongation of oligoeptides in a simulated submarine hydrothermal system. Science 1999, 283, 831–833. [Google Scholar]
- Islam, MN; Kaneko, T; Kobayashi, K. Reaction of amino acids in a supercritical water-flow reactor simulating submarine hydrothermal systems. Bull Chem Soc Jpn 2003, 76, 1171–1178. [Google Scholar]
- Simoneit, BRT. Prebiotic organic synthesis under hydrothermal conditions: an overview. Adv. Space Res 2004, 33, 88–94. [Google Scholar]
- Pascal, R; Boiteau, L; Commeyras, A. From the prebiotic synthesis of α-amino acids towards a primitive translation apparatus for the synthesis of peptides. Top. Curr. Chem 2005, 259, 69–122. [Google Scholar]
- Zaia, DAM; Zaia, TBV; De Santana, H. Which amino acids should be used in prebiotic chemistry studies? Orig. Life Evol. Biosphere 2008, 38, 469–488. [Google Scholar]
- LaRowe, DE; Regnier, P. Thermodynamic potential for the abiotic synthesis of adenine, cytosine, guanine, thymine, uracil, ribose and desoxyribose in hydrothermal systems. Orig. Life Evol. Biosphere 2008, 38, 383–397. [Google Scholar]
- Brearley, AJ. Nebular versus parent-body processing. In Meteorites, Comets and Planets Treatise on Geochemistry; Davis, AM, Ed.; Elsevier: Oxford, U.K, 2005; Volume 1. [Google Scholar]
- Zolensky, M; Barrett, R; Browning, L. Mineralogy and composition of matrix and chondrule rims in carbonaceous chondrites. Geochim. Cosmochim. Acta 1993, 57, 3123–3148. [Google Scholar]
- El Amri, C; Maurel, MC; Sagon, G; Baron, MH. The micro-distribution of carbonaceous matter in the Murchison meteorite as investigated by Raman imaging. Spectrochim. Acta A 2005, 61, 2049–2056. [Google Scholar]
- Zinner, E; Amari, S; Anders, E; Lewis, R. Large amounts of extinct 26Al in interstellar grains from the Murchison meteorite. Nature 1991, 349, 51–54. [Google Scholar]
- Carlson, RW; Boyet, M. Short-lived radionuclides as monitors of early crust-mantle differentiation on the terrestrial planets. Earth Planet Sci. Lett 2009, 279, 147–156. [Google Scholar]
- Bunch, TE; Chang, S. Carbonaceous chondrites: II. Carbonaceous chondrite phyllosilicates and light element geochemistry as indicators of parent body processes and surface conditions. Geochim. Cosmochim. Acta 1980, 44, 1543–1577. [Google Scholar]
- Schulte, M; Shock, E. Coupled organic synthesis and mineral alteration on meteorite parent bodies. Meteorit. Planet. Sci 2004, 39, 1577–1590. [Google Scholar]
- Botta, O; Bada, JL. Extraterrestrial organic compounds in meteorites. Surv. Geophys 2002, 23, 411–467. [Google Scholar]
- Gilmour, I. Structural and isotopic analysis of organic matter in carbonaceaous chondrites. In Meteorites, Comets and Planets Treatise on Geochemistry; Davis, AM, Ed.; Elsevier: Oxford, U.K., 2005; Volume 1, pp. 269–290. [Google Scholar]
- Martins, Z; Botta, O; Fogel, ML; Sephton, MA; Glavin, DP; Watson, JS; Dworkin, JP; Schwartz, AW; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett 2008, 270, 130–136. [Google Scholar]
- Cronin, JR; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar]
- Engel, MH; Macko, SA. The stereochemistry of amino acids in the Murchison meteorite. Precambrian Res 2001, 106, 35–45. [Google Scholar]
- Glavin, DP; Dworkin, JP. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar]
- Kobayashi, K; Kaneko, T; Saito, T; Ohima, T. Amino acids formation in gas mixtures by high energy particle irradiation. Orig. Life Evol. Biosph 1998, 28, 155–165. [Google Scholar]
- Kerridge, JF. Formation and processing of organics in the early solar system. Space Science Rev 1999, 90, 275–288. [Google Scholar]
- Takano, Y; Ohashi, A; Kaneko, T; Kobayashi, K. Abiotic synthesis of high-molecular-weight organics from an inorganic gas mixture of carbon monoxide, ammonia and water by 3 MeV proton irradiation. Appl. Phys. Lett 2004, 84, 1410–1412. [Google Scholar]
- Takano, Y; Kaneko, T; Kobayashi, K; Hiroishi, D; Ikeda, H; Marumo, K. Experimental verification of photostability for free- and bound amino acids exposed to γ-rays and UV irradiation. Earth Planets Space 2004, 56, 669–674. [Google Scholar]
- Bernstein, M. Prebiotic material from on and off the early Earth. Phil. Trans. R. Soc. B 2006, 361, 1689–1702. [Google Scholar]
- Ehrenfreund, P; Sephton, MA. Carbon molecules in space: from astrochemistry to astrobiology. Faraday Discuss 2006, 133, 277–288. [Google Scholar]
- Elsila, JE; Dworkin, JP; Bernstein, MP; Martin, MP; Sandford, SA. Mechanisms of amino acid formation in interstellar ice analogs. Astrophys. J 2007, 660, 911–918. [Google Scholar]
- Takano, Y; Takahashi, J; Kaneko, T; Marumo, K; Kobayashi, K. Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet Sci Lett 2007, 254, 106–114. [Google Scholar]
- Exploring Organic Environments in the Solar System; The National Academies Press: Washington, D.C., USA, 2007.
- Cleaves, HJ. The prebiotic geochemistry of formaldehyde. Precambrian Res 2008, 164, 111–118. [Google Scholar]
- Nuevo, M; Auger, G; Blanot, D; d’Hendecourt, L. A detailed analysis of the amino acids produced after the vacuum UV irradiation of ice analogs. Orig. Life Evol. Biosph 2008, 38, 37–56. [Google Scholar]
- Brown, RD; Godfrey, PD; Storey, JW; Bassez, M-P; Robinson, BJ; Batchelor, RA; Mc. Culloch, MG; Rydbeck, OE; Hjalmarson, AJ. A search for interstellar glycine. Mon. Not. R astr. Soc 1979, 186, 5–8. [Google Scholar]
- Bassez, MP. La structure de l’eau supercritique et l’origine de la vie. In Science et Technologie: Regards Croisés; L’Harmattan: Paris, 1999; pp. 583–591. [Google Scholar]
- Takai, K; Nakamura, K; Suzuki, K; Inagaki, F; Nealson, KH; Kumagai, H. Ultramafics-Hydrothermalism-Hydrogenesis-HyperSLIME (UltraH3 microbial ecosystem in the Archean deep-sea hydrothermal systems. Paleontolog. Res 2006, 10, 269–282. [Google Scholar]
- Bassez, MP. Synthèse prébiotique dans les conditions hydrothermales. Comptes Rendus Chimie 2009, 12, 801–807. [Google Scholar]
- Aubrey, AD; Cleaves, HJ; Bada, JL. The role of submarine hydrothermal systems in the synthesis of amino acids. Orig. Life Evol. Biosph 2009, 39, 91–108. [Google Scholar]
- Fujioka, K; Futamura, Y; Shiohara, T; Hoshino, A; Kanaya, F; Manome, Y; Yamamoto, K. Amino acid synthesis in a supercritical carbon dioxide-water mixture. Int. J. Mol. Sci 2009, 10, 2722–2732. [Google Scholar]
- Delacour, A. Institut de Physique du Globe de Paris; Mineral composition of the Logatchev and Ashadze serpentine samples. Private communication 2008.
- Takano, Y; Kobayashi, K; Yamanaka, T; Marumo, K; Urabe, T. Amino acids in the 308 °C deep-sea hydrothermal system of the Suiyo Seamount, Izu-Bonin Arc, Pacific Ocean. Earth Planet Sci. Lett 2004, 219, 147–153. [Google Scholar]
- Amelung, W; Zhang, X. Determination of amino acid enantiomers in soils. Soil Biol. Biochem 2001, 33, 553–562. [Google Scholar]
- Takano, Y; Kobayashi, K; Ishikawa, Y; Marumo, K. Emergence of the inflection point on racemization rate constants for d- and l-amino acids in the early stages of terrestrial diagenesis. Org. Geochem 37, 334–341.
- Glynn, S; Mills, RA; Palmer, MR; Pancost, RD; Severmann, S; Boyce, AJ. The role of prokaryotes in supergene alteration of submarine hydrothermal sulfides. Earth Planet. Sci. Lett 2006, 244, 170–185. [Google Scholar]
- Proskurowski, G; Lilley, MD; Seewald, JS; Früh-Green, GL; Olson, EJ; Lupton, JE; Sylva, SP; Kelley, DS. Abiogenis hydrocarbon production at Lost City hydrothermal field. Science 2008, 319, 604–607. [Google Scholar]
- Delacour, A; Früh-Green, GL; Bernasconi, SM; Schaeffer, P; Kelley, DS. Carbon geochemistry of serpentinites in the Lost City hydrothermal system (30°N, MAR). Geochim. Cosmochim. Acta 2008, 72, 3681–3702. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bassez, M.-P.; Takano, Y.; Ohkouchi, N. Organic Analysis of Peridotite Rocks from the Ashadze and Logatchev Hydrothermal Sites. Int. J. Mol. Sci. 2009, 10, 2986-2998. https://doi.org/10.3390/ijms10072986
Bassez M-P, Takano Y, Ohkouchi N. Organic Analysis of Peridotite Rocks from the Ashadze and Logatchev Hydrothermal Sites. International Journal of Molecular Sciences. 2009; 10(7):2986-2998. https://doi.org/10.3390/ijms10072986
Chicago/Turabian StyleBassez, Marie-Paule, Yoshinori Takano, and Naohiko Ohkouchi. 2009. "Organic Analysis of Peridotite Rocks from the Ashadze and Logatchev Hydrothermal Sites" International Journal of Molecular Sciences 10, no. 7: 2986-2998. https://doi.org/10.3390/ijms10072986
APA StyleBassez, M.-P., Takano, Y., & Ohkouchi, N. (2009). Organic Analysis of Peridotite Rocks from the Ashadze and Logatchev Hydrothermal Sites. International Journal of Molecular Sciences, 10(7), 2986-2998. https://doi.org/10.3390/ijms10072986



