2.1. The Encapsulation of Anthocyanins from Maqui Juice (MJ) by SD and FD
In order to find the optimal conditions for encapsulation by SD, a response surface methodology (RSM) experiment was carried out. The experimental data are shown in
Table 1. Equations were fitted to predict yield, recovery, and encapsulation efficiency (EE) of total anthocyanins (TA) and specific anthocyanins.
Table 2 shows the predictive models following the general equation:
where
are the regression coefficients for each response variable
,
is the inlet air temperature, and
the MJ/maltodextrin (MD)-soy protein isolate (SPI) ratio.
The coefficients of determination (R2) and R2-adjusted for the predictive models were higher than 0.90 and 0.82, respectively, for each response variable except for recovery (0.42 and 0.0, respectively), suggesting that the predictive models seemed to reasonably represent the observed values, with the exception of recovery. Therefore, 8 of the 9 responses could be sufficiently explained by the models, while for recovery, results suggest that its optimal condition is outside the experimental zone. Anthocyanin recovery reveals the effect of the drying process on anthocyanin content. In this study, high recovery results (≥82.6%) indicate that the temperatures used in SD did not have a great effect on the degradation of anthocyanins.
Multiple response optimization methodology was performed in order to obtain optimal conditions that maximize all variables. These optimal conditions were an inlet air temperature of 186 °C and a MJ/MD-SPI ratio of 1:5. The corresponding predictive values were a yield of 73.4%, a recovery of 93.5%, and an EE of TA of 90.9%. The predictive yield was relatively low but in line with previous reports [
6], whereas recovery (93.5%) and anthocyanin EE (>87%) were higher than expected from previous research [
10]. In order to compare the properties of SD powders obtained under optimal conditions with FD powders, a MJ/MD-SPI ratio of 1:5 was used to formulate the FD powders.
2.2. Characterization of the MJ Powders Obtained by SD and FD
The anthocyanin profile of MJ was similar than those previously described [
4,
11,
12,
13,
14,
15], showing eight anthocyanins (del-3-sa-5-glu, del-3,5-diglu, cy-3,5-diglu+cy-3-sa-5-glu (coeluted in peak 3–4), del-3-sa, del-3-glu, cy-3-sa and cy-3-glu). Additionally, cy-3-sa was found under the limit of quantification (0.16 µg/mL). The anthocyanin profiles for MJ powders (SD and FD) were similar to MJ where 3,5-
O-diglycosylated anthocyanins (≈70%) predominated over 3-
O-glycosylated anthocyanins (≈30%) in both MJ and the MJ powders (
Table 3). In concordance with the high anthocyanin content of MJ (17.5 mg/g) as a raw material, both MJ powders showed high anthocyanin content (SD, 3.3 mg/g and FD, 3.3 mg/g) in comparison to microparticles in powder from other raw materials such as Andes berry (0.1 mg/g) [
16] and bayberry (0.6 mg/g) [
17].
The EE has been used to determine if the anthocyanins were encapsulated. EE represents anthocyanin-biopolymer interaction due to electrostatic interactions, hydrogen bonding, hydrophobic interactions, or Van der Waals forces [
7]. However, encapsulation by spray-drying is considered an immobilization technology rather than a true encapsulation technology because some active compounds remain exposed on the microparticle surface [
6]. Thus, the EE is a way to gauge successful anthocyanin encapsulation which should result in a powder that has minimum surface anthocyanin content on the microparticles and maximum retention of active compounds [
5]. According to our results, and in line with the predictive value for SD, the EE of TA was high for SD (92.5%) and FD (>93.0%) powders (
Table 4) where the encapsulation method did not significantly affect the EE. Similar results showing high EE of TA were described by Santana et al. [
18] for jussara microparticles (88.3–99.3%) using a mixture of arabic gum/modified starch/SPI and SD. Furthermore, our results showed that the mixture of polysaccharides and proteins improved the EE of TA, as compared to using either MD (58.5%) or SPI (86.6%) alone, in the microencapsulation of pomegranate juice using SD [
10]. In line with the EE of TA results, the EE of specific anthocyanins did not show significant differences between SD and FD methods, although the EE of 3,5-
O-diglycosylated anthocyanins was significantly higher than EE of 3-
O-monoglycosylated anthocyanins (
Table 4). This suggests a better interaction between the EA (MD-SPI) and 3,5-
O-diglycosylated anthocyanins by hydrogen bonding, which could be attributed to the greatest number of hydroxyl groups for 3,5-
O-diglycosylated anthocyanins.
The recovery of anthocyanins in the SD powders (99.8%) was in line with the predictive value and significantly higher than the FD powders (91.8–91.9%) (
Table 4). However, these values were higher than those reported by Laokuldilok and Kanha [
8] for black glutinous rice bran microparticles using SD (47.7%) and FD (71.9%).
The SD yield (64.1%) was 10% lower than the predictive value and significantly lower than FD (94.6%). Similarly, Laokuldilok and Kanha [
8] reported differences in the yield between SD (>64%) and FD (85%). This can be explained by the high viscosity of the feed solution when SPI is used as an EA, which causes more solids to stick to the wall of the dryer chamber, resulting in less powder at the end of the SD process.
The physical characteristics (
Table 4) showed that the water activity (a
w) of SD powders was significantly higher than FD powders, but all of the values were low (<0.3), therefore avoiding potential microbial food spoilage [
16]. Additionally, the moisture content results were in the range acceptable for food powders (3–10%) but they were also significantly higher in SD powders than FD powders. In concordance with a high moisture content, SD powders had significantly lower hygroscopicity and higher bulk density than FD powders. Moreover, it is important to notice that no significant differences between FD powders obtained by grinded with coffee grinder (FDc) and porcelain mortar (FDm) for any of these physical parameters were found (
Table 4), reflecting that the grinding process did not affect a
w, moisture content, hygroscopicity, nor the bulk density of the two types of FD powders.
The ability of the powders to reconstitute in water is significant for the development of food ingredients. As expected, including SPI in the MJ powders decreased solubility in comparison to using only MD [
18]. The solubility of SD powders (70.4%) was significantly higher than FD powders (59.9–59.1%), which may be attributed to differences in the morphology of powders and the corresponding contact surface with water. In line with physical parameters, no significant difference in solubility between FDc and FDm were found. Furthermore, the solubility results may also be attributed to the high total sugar content of MJ.
The dispersibility of the MJ powders was over 97.7% and no significant differences among MJ powders were found. The high dispersibility indicates a low tendency to form lumps when MJ powders are added to water, facilitating their reconstitution. Furthermore, it is important to notice that the addition of the MJ powders did not modify the pH of the water.
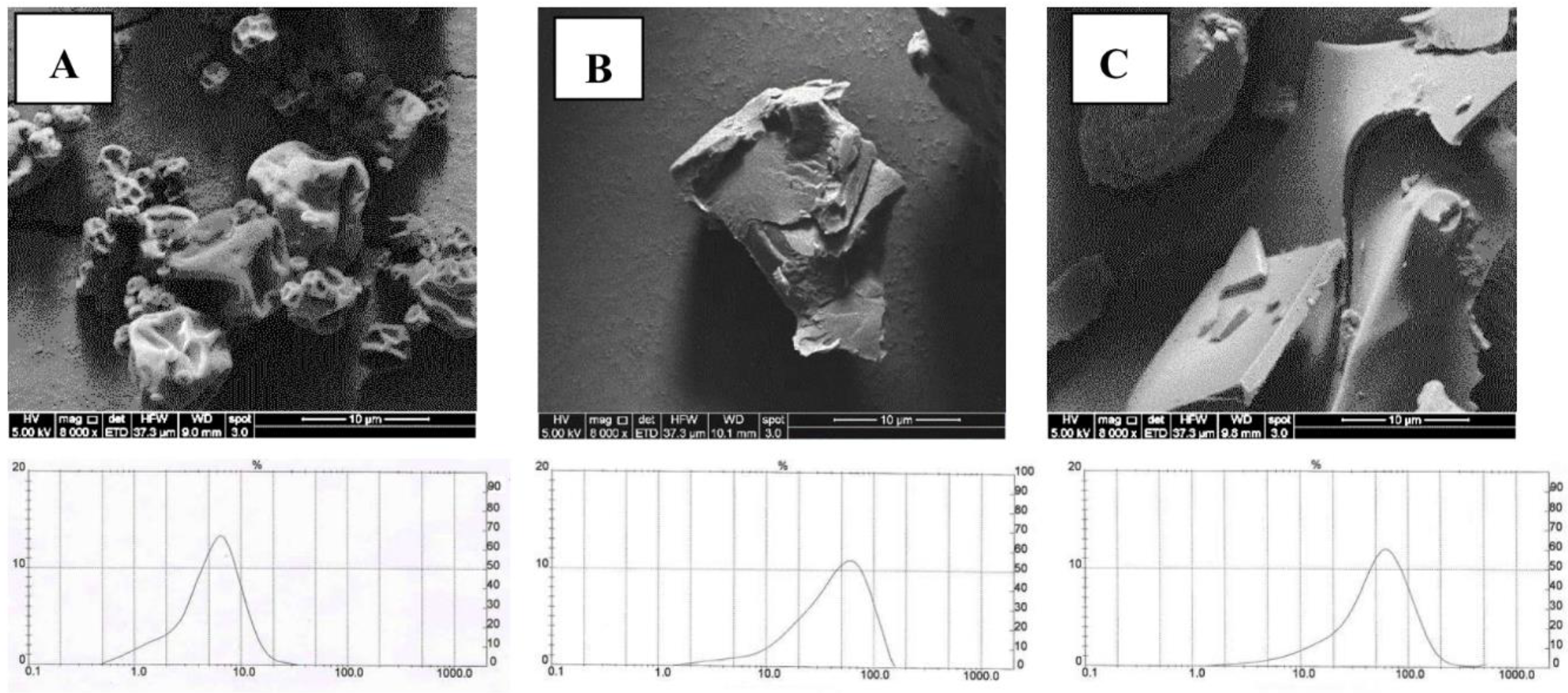
Scanning electron microscopic photographs showed that SD powders (
Figure 1A) have spherical shapes and particles with indented surfaces, obviating their agglomerating tendency. For FDc (
Figure 1B) and FDm (
Figure 1C) powders, SEM photographs showed undefined forms with more intended surfaces than SD powders, in agreement with the description by Fang and Bhandari [
19] who suggested that the encapsulation method as well as the grinding process directly affects both the shape and size of powders.
The particle size measurement assumes powders have a spherical shape [
20]. The particle size of SD powders was 6.4 µm, and 90% of the total sample had a diameter below 10.9 µm (
Figure 1A) in line with the particle size of SD powders [
16]. The particle size from FD powders (49.4 and 64.0 µm for FDc and FDm, respectively) was almost 10 times higher than the SD powder particles, and 90% of the total sample had a diameter below 93.9 and 117.1 µm, respectively. Therefore, in terms of the characteristics of MJ powders, the most relevant differences between SD and FD powders were the morphology and particle size that affect their solubility in water. However, the differences in particle size between FD powders did not have any impact on their solubility. MD is a water soluble biopolymer but SPI is partially soluble in water. However, MD blend with SPI represents a new system with different properties with respect to each single biopolymer [
21], which explains why the solubility of maqui-juice microparticles with MD blend with SPI is lower than MD.
2.3. Stability of MJ Powders in Yogurt
Previous studies [
10,
22] on the evaluation of anthocyanin stability in yogurt has been focused on the retention of TA by spectrometry where the stability of anthocyanins comparing encapsulated with non-encapsulated anthocyanins has been scarcely studied [
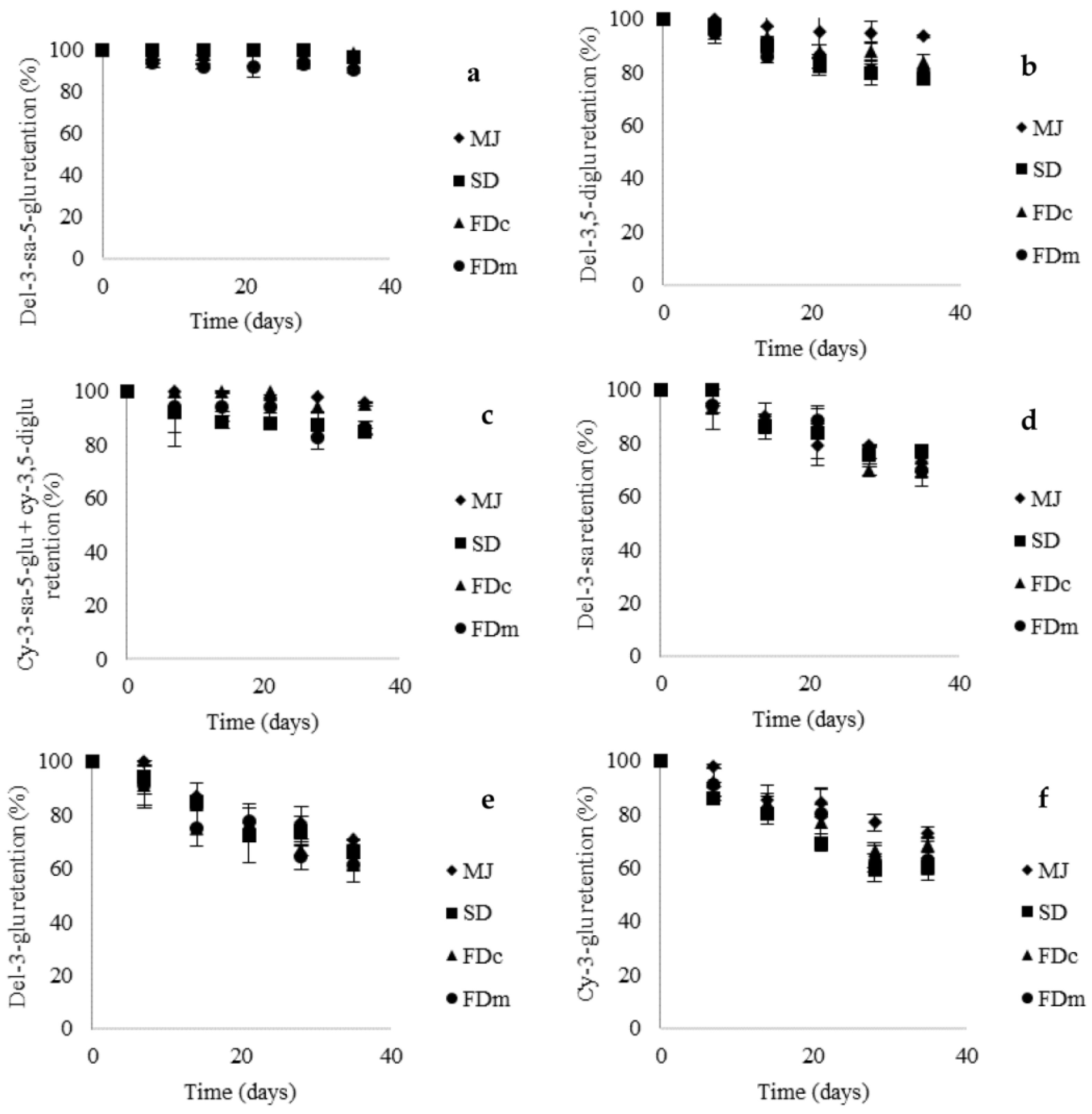
10]. The evolution of TA retention (
Figure 2) was similar among MJ powders and MJ in agreement with results reported by Robert et al. [
10] for anthocyanins from pomegranate juice and pomegranate microparticles. Furthermore, our results showed high retention of TA (SD, 82%, FDc, 88%, and FDm, 81%) after 35 days in line with a study carried out with a freeze-dried mulberry fruit juice [
23]. Contrary Coisson et al. [
22] and Robert et al. [
10] reported that anthocyanins from acai and pomegranate juices, disappeared after 2 and 7 days of storage at 4 °C, respectively. This may be explained by the presence of different types of anthocyanins in fruits justifying the study of the stability of specific anthocyanins in yogurt.
Ribeiro et al. [
24] indicated that in aqueous solutions, anthocyanins undergo structural rearrangements in response to changes in pH. Anthocyanins are most stable in acidic solutions (pH 1–3), whereas at a pH above 4, anthocyanins undergo chemical degradations to produce phenolic acids. However, as shown in
Figure 2, the anthocyanins did not react uniformly; del-3-sa-5-glu showed the highest retention (90–99%) whereas cy-3-glu showed the lowest retention (61–69%) during storage at 5 °C after 35 days. Because the addition of MJ and MJ microparticles into yogurt did not modify the pH of the food matrix (4.3), anthocyanin degradation may be mainly attributed to the pH of the food matrix. Structural features of the anthocyanins influenced their stability in yogurt as reflected in their half-life values (
Table 5). In this context, the
kobs and the corresponding half-life values were calculated only for 3-
O-monoglycosylated anthocyanins that reached a degradation of over 30%. The half-life of del-3-sa was significantly higher than del-3-glu and cy-3-glu, suggesting that the extra xylose as a sugar component of del-3-sa favors its stability in yogurt. On the other hand, the encapsulation method did not affect the stability of 3-
O-monoglycosylated anthocyanins as reflected in the half-life values. This suggests that the differences in particle size and morphology between SD and FD powders in this study did not affect the stability of anthocyanins as other research [
8,
19] have proposed. The results also showed that MJ powders protected anthocyanins during the shelf-life of yogurt, preserving their stability, suggesting that yogurt might be a good carrier for functionalization.
2.4. Bioaccessibility of MJ Powders after an In Vitro Digestion
The BA has been defined as the amount of compound (anthocyanins) that was released from the food matrix after digestion [
25]. In this study, BA was calculated in order to quantify the inhibition of anthocyanin degradation, comparing encapsulated (MJ powders) with non-encapsulated MJ anthocyanins. BA of TA in MJ powders (44.1% for SD and 43.8% for FD powders) was significantly higher than in MJ (35.2%) (
Table 3). Nevertheless, no significant differences in the BA of TA between SD and FD powders were found, suggesting that their physical differences would not have an effect on the stability of anthocyanins.
Lucas-González et al. [
26] demonstrated that the stability of maqui anthocyanins from a lyophilized fruit was greatly affected in the intestinal phase during in vitro GI digestion. Therefore, in our study, the expected low BA of TA in the MJ can be attributed to the high degradation of anthocyanins in GI conditions. It is well known that anthocyanins are unstable at high pH, and the shift from the acidic pH (pH 2) of the stomach to the almost neutral pH in the duodenum (pH 6) may be responsible for their specific hydrolysis and/or degradation [
25,
26,
27,
28]. Mouth conditions were considered in this study, although Mosele et al. [
28] indicated that anthocyanins suffer negligible modifications due to the short exposure time in the mouth, resulting in a marginal effect of the α-amylase.
When anthocyanins are encapsulated, they are more protected in the GI tract, as was shown in our study. Oidtmann et al. [
29] indicated that in bilberry microparticles encapsulated with polysaccharides (pectin amide) or proteins (whey protein isolate), anthocyanins were not degraded under simulated gastric conditions despite the fact that they had a competitive release from the microparticles. The same study indicated that in intestinal conditions, anthocyanins were also released from the microparticles (after 30 min), with degradation occurring after their release [
29]. From another point of view, Flores et al. [
27] indicated that the enzymatic and pH conditions are highly extractive in the stomach, which may affect the integrity of microparticles, favoring the release of anthocyanins. Nevertheless, the mixture of MD and SPI as EA demonstrated a protective effect, resulting in the enhancement of BA of TA in both SD and FD powders. Thus, our results suggest that this high BA is due to lower exposure times to gut conditions because of the protective effect of MD and SPI.
In concordance with the results of TA, BA of specific anthocyanins from MJ powders was significantly higher than MJ (
Table 3). Moreover, there were significant differences in the BA among anthocyanins, where cy-3-sa-5-glu and cy-3,5-diglu had the highest BA (78.5% for SD and 76.5% for FD) and del-3-glu had the lowest BA (20.8% for SD and 22.6% for FD), demonstrating differences in their stability after their release from MJ powders. In agreement with our results, Lila et al. [
25] suggest that the fact that cy-3-sa-5-glu plus cy-3,5-diglu demonstrated the highest BA is because two cyanidins combined with two linked sugars generate a more stable anthocyanin structure.
Despite the fact that changes in pH are involved in the degradation of anthocyanins in the GI tract and significant differences between the stability of 3-
O-monoglycosylated and 3,5-
O-diglycosylated anthocyanins as in yogurt were found (
Table 3), the anthocyanins did not show the same behavior after the in vitro digestion. This can be contributed to the more complex reactions that anthocyanins experience during the digestion process, where the action of different enzymes in combination with different temperatures and time of exposure are all involved.
In this study, the results showed that the BA of both MJ microparticles (44.1–43.8%) and MJ (35.2%) were higher than that described by Lila et al. [
25] for a semi-purified maqui extract (4%). These differences may be explained by the additional protector effect that MJ, as a food matrix, gives to anthocyanins. McDougall et al. [
30] suggested that polyphenols generate linkages with the food matrix during digestion that may protect more labile anthocyanins against degradation.