L1210 Cells Overexpressing ABCB1 Drug Transporters Are Resistant to Inhibitors of the N- and O-glycosylation of Proteins
Abstract
:1. Introduction
2. Results
2.1. Characterization of P-gp Positive Variants of L1210 Cells
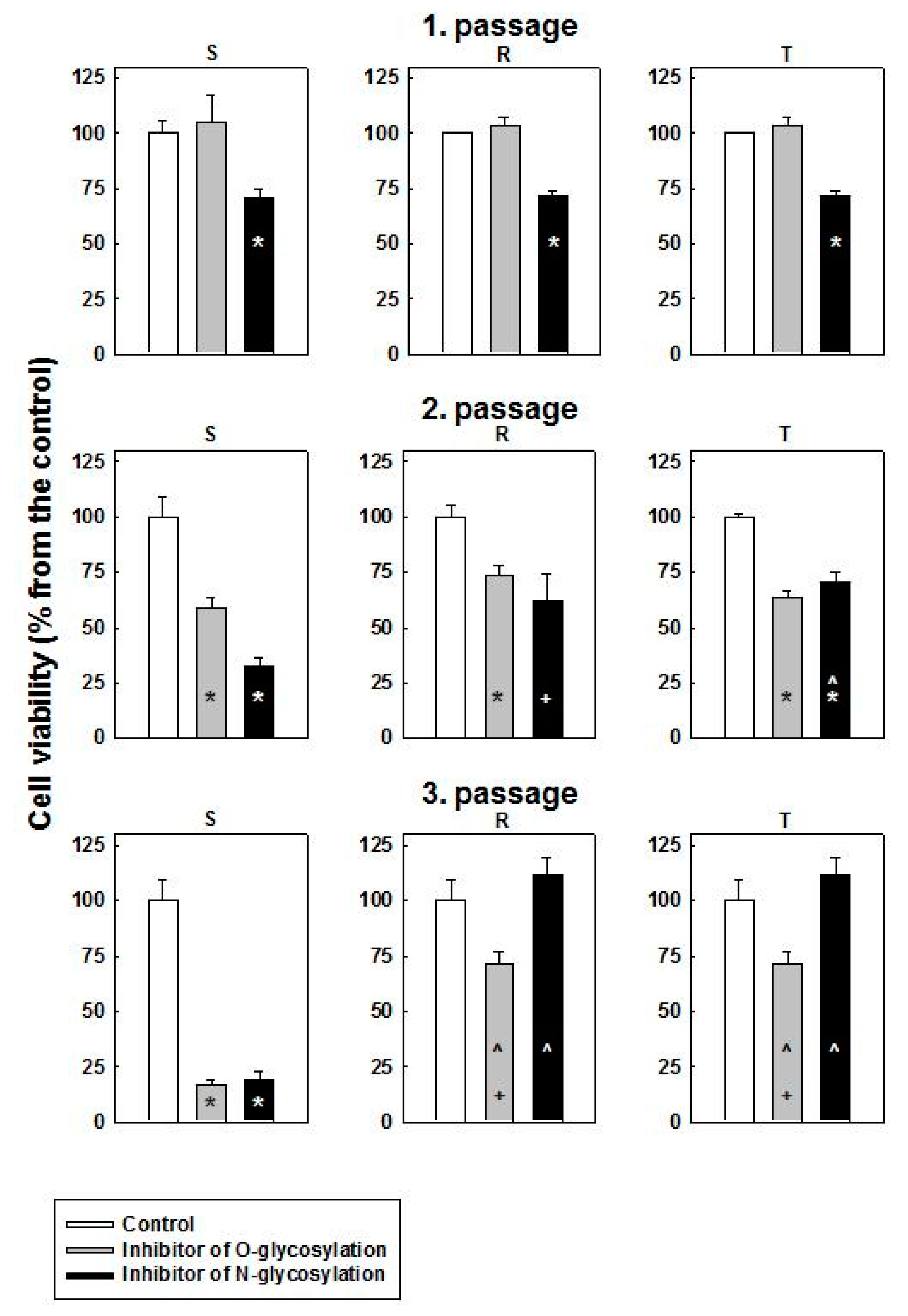
2.2. Cytotoxic Effect of O- and N-Glycosylation Inhibitors on S, R and T Cells
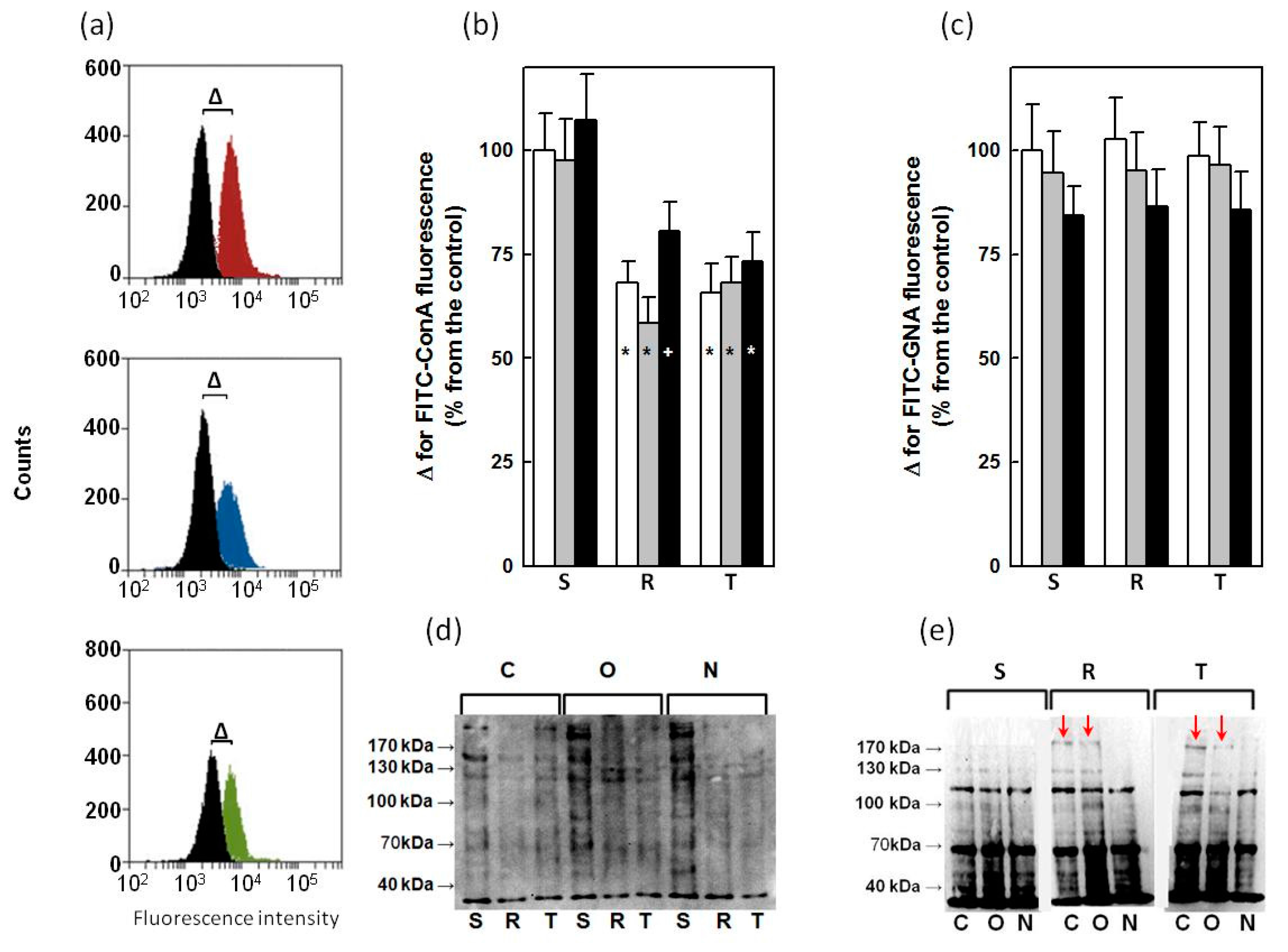
2.3. Effect of GalNAc-α-O-benzyl and Tunicamycin on P-gp Glycosylation
2.4. Binding of ConA and GNA to Glycoprotein in S, R and T Cells
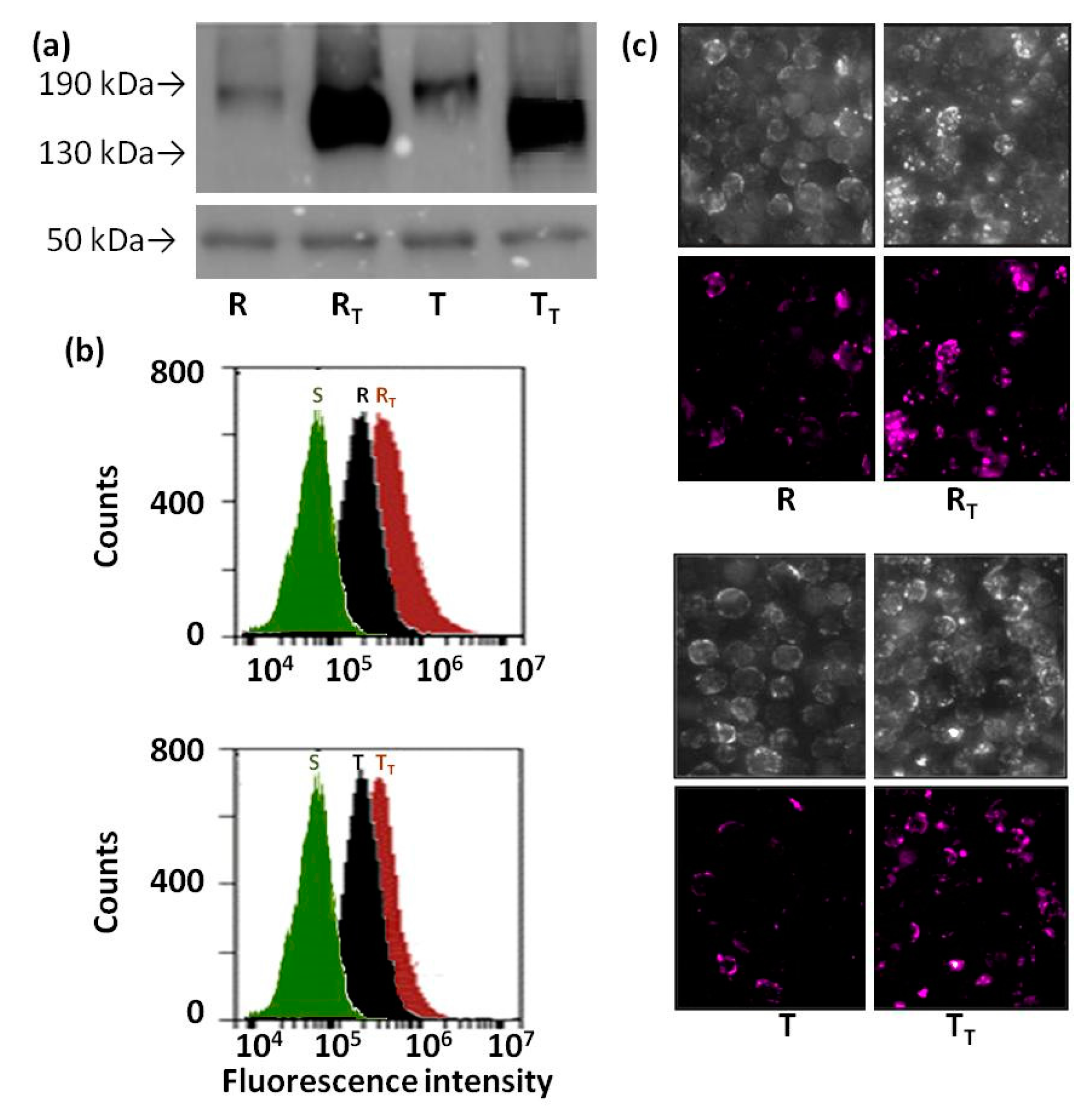
2.5. Effect of Tunicamycin on Protein Ubiquitination in R and T Cells
3. Discussion
- (a)
- Tunicamycin may be a substrate for P-gp efflux and therefore could effectively be eliminated from the intracellular volume of P-gp-positive cells. While this idea has previously been discussed [32], direct evidence for such P-gp activity is lacking. However, the fact that tunicamycin also blocks the glycosylation of several proteins, including P-gp, in P-gp-positive cells (as shown in Figure 2 and described elsewhere [27,33,34]) is contradictory to this speculation. Moreover, resistance of R and T cells to tunicamycin could not be reversed by verapamil (a known P-gp inhibitor) even if this substance completely blocked P-gp efflux activity [27].
- (b)
- Tunicamycin induced the global inhibition of glycoprotein synthesis (Hiss et al., 2007) associated with the elevation of immature proteins cell content leading to endoplasmic reticulum stress and apoptosis [35]. P-gp-positive cells with altered regulation of apoptosis initiation and progression [13,17] could survive better under this condition.
- (c)
- The application of tunicamycin increases the cellular level of UDP-N-acetylglucosamine, associated with different features of cell damage visible using electron microscopy, namely, in the membranes of the endoplasmic reticulum and nuclei [34]. Significantly decreased intracellular levels of UDP-sugars and UDP-glucose have been detected in P-gp-positive R and T cells compared with P-gp-negative S cells [14,23]. Therefore, the resistance of P-gp-positive L1210 cells to tunicamycin may reflect the decreased levels of UDP-sugars in these cells.
4. Materials and Methods
4.1. Cell Culture Conditions
4.2. Western and Eastern Blot Procedures
4.3. Deglycosydation of Membrane Proteins with PNGase and Endo H
4.4. Detection of ConA and GNA Binding to the Surface of S, R and T Cells by Flow Cytometry
4.5. Immunoprecipitation of Proteins by Antiubiquitin Antibody
4.6. Detection of Ubiquitin on Cell Surface of S, R and T Cells by Confocal Microscopy and Flow Cytometry
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drake, R.R. Glycosylation and cancer: Moving glycomics to the forefront. Adv. Cancer Res. 2015, 126, 1–10. [Google Scholar] [PubMed]
- Mechref, Y.; Hu, Y.; Garcia, A.; Hussein, A. Identifying cancer biomarkers by mass spectrometry-based glycomics. Electrophoresis 2012, 33, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Gemeiner, P.; Mislovicova, D.; Tkac, J.; Svitel, J.; Patoprsty, V.; Hrabarova, E.; Kogan, G.; Kozar, T. Lectinomics ii. A highway to biomedical/clinical diagnostics. Biotechnol. Adv. 2009, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Murphy, P.V.; Gabius, H.J. Multivalent carbohydrate-lectin interactions: How synthetic chemistry enables insights into nanometric recognition. Molecules 2016, 21, 629. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Spengler, G.; Molnar, J. Identification of important compounds isolated from natural sources that have activity against multidrug-resistant cancer cell lines: Effects on proliferation, apoptotic mechanism and the efflux pump responsible for multi-resistance phenotype. Anticancer Res. 2016, 36, 5665–5672. [Google Scholar] [CrossRef] [PubMed]
- Kibria, G.; Hatakeyama, H.; Harashima, H. Cancer multidrug resistance: Mechanisms involved and strategies for circumvention using a drug delivery system. Arch. Pharm. Res. 2014, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Barancik, M.; Sulova, Z.; Uhrik, B. P-glycoprotein--implications of metabolism of neoplastic cells and cancer therapy. Curr. Cancer Drug Targets 2005, 5, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Gibalova, L.; Seres, M.; Barancik, M.; Sulova, Z. New insight into P-glycoprotein as a drug target. Anticancer Agents Med. Chem. 2013, 13, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, L.M.; da Silva, V.A.; Freire-de-Lima, L.; Previato, J.O.; Mendonca-Previato, L.; Capella, M.A. Glycosylation in cancer: Interplay between multidrug resistance and epithelial-to-mesenchymal transition? Front. Oncol. 2016, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.M. Abc transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Sharom, F.J. Abc multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Scala, S.; Akhmed, N.; Rao, U.S.; Paull, K.; Lan, L.B.; Dickstein, B.; Lee, J.S.; Elgemeie, G.H.; Stein, W.D.; Bates, S.E. P-glycoprotein substrates and antagonists cluster into two distinct groups. Mol. Pharmacol. 1997, 51, 1024–1033. [Google Scholar] [PubMed]
- Zu, Y.; Yang, Z.; Tang, S.; Han, Y.; Ma, J. Effects of P-glycoprotein and its inhibitors on apoptosis in k562 cells. Molecules 2014, 19, 13061–13075. [Google Scholar] [CrossRef] [PubMed]
- Fiala, R.; Sulova, Z.; El-Saggan, A.H.; Uhrik, B.; Liptaj, T.; Dovinova, I.; Hanusovska, E.; Drobna, Z.; Barancik, M.; Breier, A. P-glycoprotein-mediated multidrug resistance phenotype of l1210/vcr cells is associated with decreases of oligo- and/or polysaccharide contents. Biochim. Biophys. Acta 2003, 1639, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Sulova, Z.; Ditte, P.; Kurucova, T.; Polakova, E.; Rogozanova, K.; Gibalova, L.; Seres, M.; Skvarkova, L.; Sedlak, J.; Pastorek, J.; et al. The presence of P-glycoprotein in l1210 cells directly induces down-regulation of cell surface saccharide targets of concanavalin a. Anticancer Res. 2010, 30, 3661–3668. [Google Scholar] [PubMed]
- Sulova, Z.; Mislovicova, D.; Gibalova, L.; Vajcnerova, Z.; Polakova, E.; Uhrik, B.; Tylkova, L.; Kovarova, A.; Sedlak, J.; Breier, A. Vincristine-induced overexpression of P-glycoprotein in l1210 cells is associated with remodeling of cell surface saccharides. J. Proteome Res. 2009, 8, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Imrichova, D.; Paulikova, H.; Barancik, M.; Sulova, Z. Vincristine as an inductor of drug resistance marker expression in neoplastic cells. In Vincristine: Clinical Uses, Pharmacokinetics and Impacts on Health; Coello, J.M., Sabres, Y.D., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 1–31. Available online: https://www.novapublishers.com/catalog/product_info.php?products_id=46763 (accessed on 1 June 2017).
- Bubencikova, T.; Cholujova, D.; Messingerova, L.; Mislovicova, D.; Seres, M.; Breier, A.; Sulova, Z. Detection of glycomic alterations induced by overexpression of P-glycoprotein on the surfaces of l1210 cells using sialic acid binding lectins. Int. J. Mol. Sci. 2012, 13, 15177–15192. [Google Scholar] [CrossRef] [PubMed]
- Pavlikova, L.; Seres, M.; Imrichova, D.; Hano, M.; Rusnak, A.; Zamorova, M.; Katrlik, J.; Breier, A.; Sulova, Z. The expression of P-gp in leukemia cells is associated with cross-resistance to protein n-glycosylation inhibitor tunicamycin. Gen. Physiol. Biophys. 2016, 35, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.A.; Ivey, S. Distinct n-glycan glycosylation of P-glycoprotein isolated from the human uterine sarcoma cell line mes-sa/dx5. Biochim. Biophys. Acta 2007, 1770, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Loo, T.W.; Bartlett, M.C.; Clarke, D.M. Thapsigargin or curcumin does not promote maturation of processing mutants of the abc transporters, cftr, and p-glycoprotein. Biochem. Biophys. Res. Commun. 2004, 325, 580–585. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, Q.; Chen, F.; Zhang, J.; Li, H.; Lin, J.-M. DNA-mediated cell surface engineering for multiplexed glycan profiling using maldi-tof mass spectrometry. Chem. Sci. 2016, 7, 5448–5452. [Google Scholar] [CrossRef]
- Turakova, K.; Pavlikova, L.; Messingerova, L.; Lakatos, B.; Breier, A.; Sulova, Z. Reduced udp-glucose levels are associated with P-glycoprotein over-expression in l1210 cells and limit glucosylceramide synthase activity. Anticancer Res. 2015, 35, 2627–2634. [Google Scholar] [PubMed]
- Kramer, R.; Weber, T.K.; Arceci, R.; Ramchurren, N.; Kastrinakis, W.V.; Steele, G., Jr.; Summerhayes, I.C. Inhibition of n-linked glycosylation of P-glycoprotein by tunicamycin results in a reduced multidrug resistance phenotype. Br. J. Cancer 1995, 71, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Yoshimura, A.; Furukawa, T.; Sumizawa, T.; Nakazima, Y.; Akiyama, S. Glycosylation of P-glycoprotein in a multidrug-resistant kb cell line, and in the human tissues. Biochim. Biophys. Acta 1991, 1073, 309–315. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.Y.; Hait, W.N.; Yang, J.M. Regulation of the stability of P-glycoprotein by ubiquitination. Mol. Pharmacol. 2004, 66, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Seres, M.; Cholujova, D.; Bubencikova, T.; Breier, A.; Sulova, Z. Tunicamycin depresses P-glycoprotein glycosylation without an effect on its membrane localization and drug efflux activity in l1210 cells. Int. J. Mol. Sci. 2011, 12, 7772–7784. [Google Scholar] [CrossRef] [PubMed]
- Pasquier, J.; Rioult, D.; Abu-Kaoud, N.; Marie, S.; Rafii, A.; Guerrouahen, B.S.; Le Foll, F. P-glycoprotein-activity measurements in multidrug resistant cell lines: Single-cell versus single-well population fluorescence methods. Biomed Res. Int. 2013, 2013, 676845. [Google Scholar] [CrossRef] [PubMed]
- Kuan, S.F.; Byrd, J.C.; Basbaum, C.; Kim, Y.S. Inhibition of mucin glycosylation by aryl-n-acetyl-alpha-galactosaminides in human colon cancer cells. J. Biol. Chem. 1989, 264, 19271–19277. [Google Scholar] [PubMed]
- Kalra, A.V.; Campbell, R.B. Mucin impedes cytotoxic effect of 5-fu against growth of human pancreatic cancer cells: Overcoming cellular barriers for therapeutic gain. Br. J. Cancer 2007, 97, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.P.; Haq, B.; Carraway, K.L.; Savaraj, N.; Lampidis, T.J. Multidrug resistance correlates with overexpression of muc4 but inversely with P-glycoprotein and multidrug resistance related protein in transfected human melanoma cells. Biochem. Pharmacol. 2003, 65, 1419–1425. [Google Scholar] [CrossRef]
- Hiss, D.; Gabriels, G.; Jacobs, P.; Folb, P. Tunicamycin potentiates drug cytotoxicity and vincristine retention in multidrug resistant cell lines. Eur. J. Cancer 1996, 32A, 2164–2172. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. Functional consequences of proline mutations in the predicted transmembrane domain of P-glycoprotein. J. Biol. Chem. 1993, 268, 3143–3149. [Google Scholar] [PubMed]
- Morin, M.J.; Bernacki, R.J. Biochemical effects and therapeutic potential of tunicamycin in murine l1210 leukemia. Cancer Res. 1983, 43, 1669–1674. [Google Scholar] [PubMed]
- Schonthal, A.H. Endoplasmic reticulum stress: Its role in disease and novel prospects for therapy. Scientifica (Cairo) 2012, 2012, 857516. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Goldstein, I.J.; Van Damme, E.J.; Peumans, W.J. Binding properties of a mannose-specific lectin from the snowdrop (galanthus nivalis) bulb. J. Biol. Chem. 1988, 263, 728–734. [Google Scholar] [PubMed]
- Tarentino, A.L.; Trimble, R.B.; Plummer, T.H., Jr. Enzymatic approaches for studying the structure, synthesis, and processing of glycoproteins. Methods Cell Biol. 1989, 32, 111–139. [Google Scholar] [PubMed]
- Plummer, T.H., Jr.; Phelan, A.W.; Tarentino, A.L. Detection and quantification of peptide-n4-(n-acetyl-beta-glucosaminyl) asparagine amidases. Eur. J. Biochem. 1987, 163, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Freeze, H.H.; Kranz, C. Endoglycosidase and glycoamidase release of n-linked glycans. Curr. Protoc. Mol. Biol. 2010. [Google Scholar] [CrossRef]
- Xu, G.; Jaffrey, S.R. Proteomic identification of protein ubiquitination events. Biotechnol. Genet. Eng. Rev. 2013, 29, 73–109. [Google Scholar] [CrossRef] [PubMed]
- Ronai, Z.A. Monoubiquitination in proteasomal degradation. Proc. Natl. Acad. Sci. USA 2016, 113, 8894–8896. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Chen, Z.J. Diversity of polyubiquitin chains. Dev. Cell 2009, 16, 485–486. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Duong, D.M.; Seyfried, N.T.; Cheng, D.; Xie, Y.; Robert, J.; Rush, J.; Hochstrasser, M.; Finley, D.; Peng, J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 2009, 137, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Polekova, L.; Barancik, M.; Mrazova, T.; Pirker, R.; Wallner, J.; Sulova, Z.; Breier, A. Adaptation of mouse leukemia cells l1210 to vincristine. Evidence for expression of P-glycoprotein. Neoplasma 1992, 39, 73–77. [Google Scholar] [PubMed]
- Pastan, I.; Gottesman, M.M.; Ueda, K.; Lovelace, E.; Rutherford, A.V.; Willingham, M.C. A retrovirus carrying an mdr1 cdna confers multidrug resistance and polarized expression of P-glycoprotein in mdck cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4486–4490. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage t4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlikova, L.; Seres, M.; Hano, M.; Bohacova, V.; Sevcikova, I.; Kyca, T.; Breier, A.; Sulova, Z. L1210 Cells Overexpressing ABCB1 Drug Transporters Are Resistant to Inhibitors of the N- and O-glycosylation of Proteins. Molecules 2017, 22, 1104. https://doi.org/10.3390/molecules22071104
Pavlikova L, Seres M, Hano M, Bohacova V, Sevcikova I, Kyca T, Breier A, Sulova Z. L1210 Cells Overexpressing ABCB1 Drug Transporters Are Resistant to Inhibitors of the N- and O-glycosylation of Proteins. Molecules. 2017; 22(7):1104. https://doi.org/10.3390/molecules22071104
Chicago/Turabian StylePavlikova, Lucia, Mario Seres, Milan Hano, Viera Bohacova, Ivana Sevcikova, Tomas Kyca, Albert Breier, and Zdena Sulova. 2017. "L1210 Cells Overexpressing ABCB1 Drug Transporters Are Resistant to Inhibitors of the N- and O-glycosylation of Proteins" Molecules 22, no. 7: 1104. https://doi.org/10.3390/molecules22071104




