Antimicrobial Activity of Truncated and Polyvalent Peptides Derived from the FKCRRQWQWRMKKGLA Sequence against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
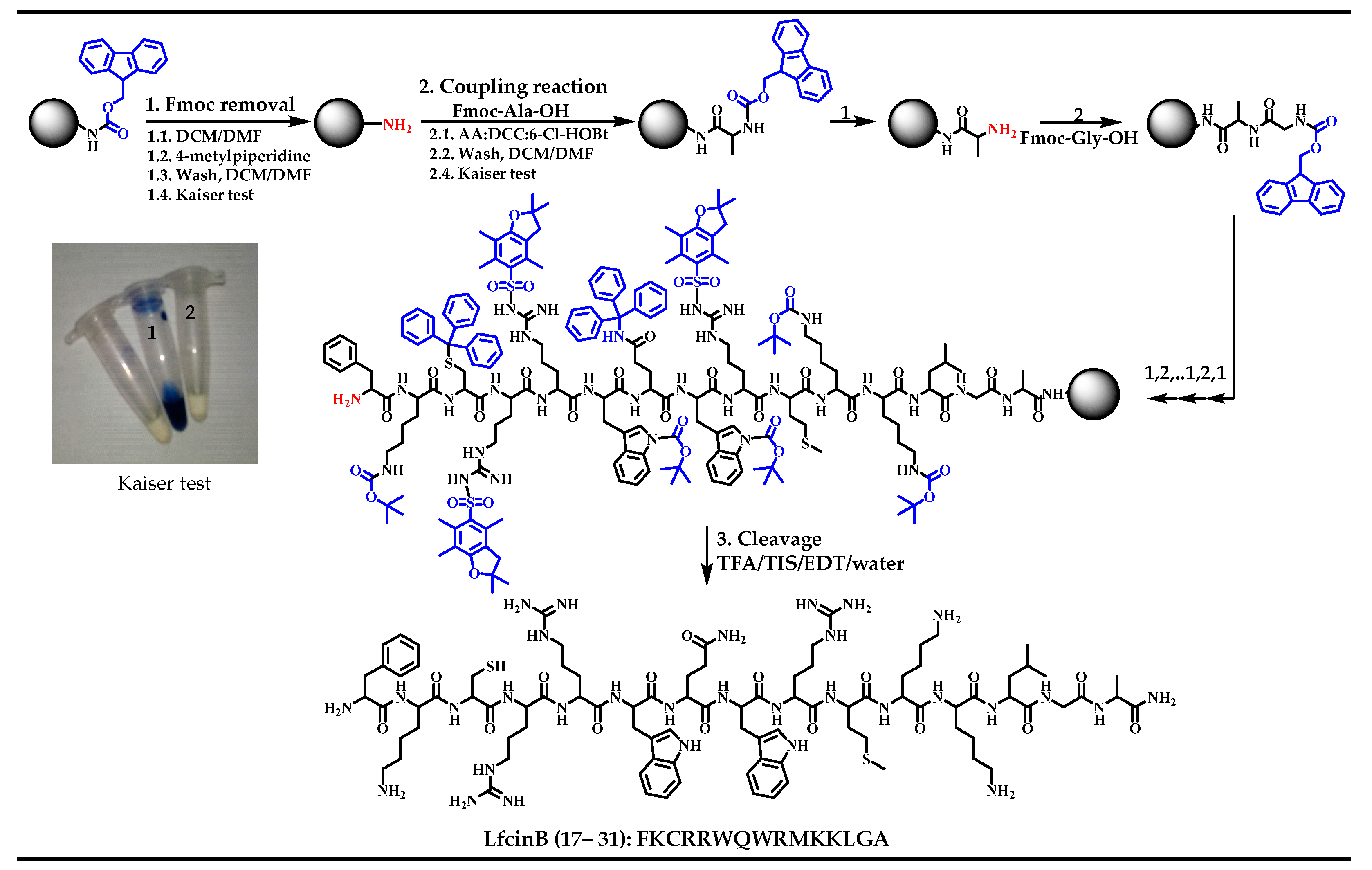
3.1. LfcinB-Derived Peptide Synthesis
3.2. Analytical Methods
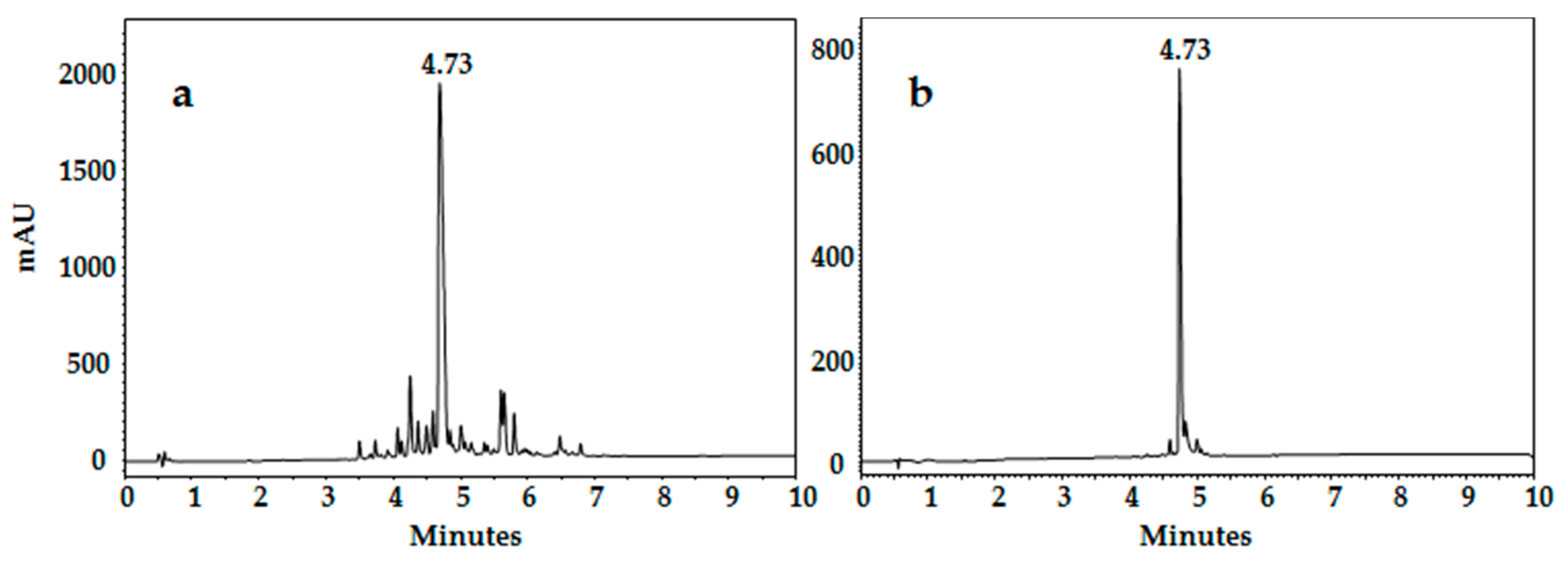
3.2.1. Reverse Phase HPLC
3.2.2. Peptide Purification
3.2.3. MALDI-TOF MS
3.3. LfcinB-Derived Peptide Antibacterial Activity
3.3.1. Antibacterial Activity
3.3.2. Hemolytic Activity Assay
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization (WHO): Geneva, Switzerland, 2014; pp. 1–205. Available online: http://www.who.int/drugresistance/documents/surveillancereport/en/ (accessed on 18 October 2016).
- Haug, B.; Strom, M.; Svendsen, J. The medicinal chemistry of short Lactoferricin-based antibacterial peptides. Curr. Med. Chem. 2007, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Poole, S. Pyrogen testing of polypeptide and protein drugs. In Polypeptide and Protein Drugs: Production, Characterization and Formulation; Hider, R.C., Barlow, D., Eds.; Ellis Horwood: Chichester, UK, 1991; pp. 146–153. [Google Scholar]
- Brogden, N.; Brogden, K. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int. J. Antimicrob. Agents 2011, 38, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.; Gooderham, W.; Hancock, R. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Hancock, R. Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 2006, 18, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Hurst, M.; Fujii, C.; Kung, A.; Ho, J.; Cheng, F. Protegrin-1: A broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997, 41, 1738–1742. [Google Scholar] [PubMed]
- Gaspar, D.; Veiga, A.; Castanho, M. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Bocchinfuso, G.; Palleschi, A.; Orioni, B.; Grande, G.; Formaggio, F.; Toniolo, C.; Park, Y.; Hahm, Y.; Stella, L. Different mechanisms of action of antimicrobial peptides: Insights from fluorescence spectroscopy experiments and molecular dynamics simulations. J. Pept. Sci. 2009, 15, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. Mode of Action of Membrane Active Antimicrobial Peptides. Biopolymers 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Dhople, V.; Krukemeyer, A.; Ayyalusami, R. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Tomita, M.; Gieh, T.; Ellison, R. Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect. Immun. 1993, 61, 719–728. [Google Scholar] [PubMed]
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta 1992, 1121, 130–136. [Google Scholar] [CrossRef]
- Haney, E.F.; Nazmi, K.; Bolscher, J.G.; Vogel, H.J. Structural and biophysical characterization of an antimicrobial peptide chimera comprised of lactoferricin and lactoferrampin. Biochim. Biophys. Acta 2012, 1818, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.M.; Zhou, N.; Shan, X.; Arrowsmith, C.H.; Vogel, H.J. Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin. Biochemistry 1998, 37, 4288–4298. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.H.; Osbakk, S.A.; Vorland, L.H.; Traavik, T.; Gutteberg, T.J. Lactoferrin and cyclic lactoferricin inhibit the entry of human cytomegalovirus into human fibroblasts. Antivir. Res. 2001, 51, 141–149. [Google Scholar] [CrossRef]
- Andersen, J.H.; Jenssen, H.; Gutteberg, T.J. Lactoferrin and lactoferricin inhibit Herpes simplex 1 and 2 infection and exhibit synergy when combined with acyclovir. Antivir. Res. 2003, 58, 209–215. [Google Scholar] [CrossRef]
- Tomita, M.; Wakabayashi, H.; Shin, K.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Twenty-five years of research on bovine lactoferrin applications. Biochimie 2009, 91, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.B.; Haug, B.E.; Skar, M.L.; Stensen, W.; Stiberg, T.; Svendsen, J.S. The Pharmacophore of Short Cationic Antibacterial Peptides. J. Med. Chem. 2003, 46, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Farnaud, S.; Evans, R. Lactoferrin a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Mader, J.S.; Richardson, A.; Salsman, J.; Top, D.; de Antueno, R.; Duncan, R.; Hoskin, D.W. Bovine lactoferricin causes apoptosis in Jurkat T-leukemia cells by sequential permeabilization of the cell membrane and targeting of mitochondria. Exp. Cell. Res. 2007, 313, 2634–2650. [Google Scholar] [CrossRef] [PubMed]
- Kondori, N.; Baltzer, L.; Dolphin, G.T.; Mattsby-Baltzer, I. Fungicidal activity of human lactoferrin-derived peptides based on the antimicrobial αβ region. Int. J. Antimicrob. Agents 2011, 37, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.; Hunter, H.; Vogel, H. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Shestakov, A.; Jenssen, H.; Nordström, I.; Eriksson, K. Lactoferricin but not lactoferrin inhibit herpes simplex virus type 2 infection in mice. Antivir. Res. 2012, 93, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Elass-Rochard, E.; Roseanu, A.; Legrand, D.; Trif, M.; Salmon, V.; Motas, C.; Montreuil, J.; Spik, G. Lactoferrin-lipopolysaccharide interaction: Involvement of the 28–34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 1995, 312, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Shai, Y. From Innate Immunity to de-Novo Designed Antimicrobial Peptides. Curr. Pharm. Des. 2002, 8, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Dur, U.; Sudheendra, U.S.; Ayyalusamy, R. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Ayyalusamy, R. Beyond NMR Spectra of Antimicrobial Peptides: Dynamical Images at Atomic Resolution and Functional Insights. Solid State Nucl. Magn. Reson. 2009, 35, 201–207. [Google Scholar]
- Umeyama, M.; Kira, A.; Nishimura, K.; Naito, A. Interactions of bovine lactoferricin with acidic phospholipid bilayers and its antimicrobial activity as studied by solid-state NMR. Biochim. Biophys. Acta 2006, 1758, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Romo, T.D.; Bradney, L.A.; Greathouse, D.V.; Grossfield, A. Membrane binding of an acyl-lactoferricin B antimicrobial peptide from solid-state NMR experiments and molecular dynamics simulations. Biochim. Biophys. Acta 2011, 1808, 2019–2130. [Google Scholar] [CrossRef] [PubMed]
- Haukland, H.H.; Ulvatne, H.; Sandvik, K.; Vorland, L.H. The antimicrobial peptides lactoferricin B and magainin 2 cross over the bacterial cytoplasmic membrane and reside in the cytoplasm. FEBS Lett. 2001, 508, 389–393. [Google Scholar] [CrossRef]
- Tu, Y.H.; Ho, Y.H.; Chuang, Y.C.; Chen, P.C.; Chen, C.S. Identification of lactoferricin B intracellular targets using an Escherichia coli proteome chip. PLoS ONE 2011, 6, e28197. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Matsumoto, H.; Hashimoto, K.; Teraguchi, S.; Takase, M.; Hayasawa, H. N-Acylated and D enantiomer derivatives of a nonamer core peptide of lactoferricin B showing improved antimicrobial activity. Antimicrob. Agents Chemother. 1999, 43, 1267–1269. [Google Scholar] [PubMed]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; de Boer, L.; Zaat, S.A.; Vogel, H.J. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef] [PubMed]
- León, M.A.; Leal, A.L.; Almanzar, G.; Rosas, J.E.; García, J.E.; Rivera, Z.J. Antibacterial activity of synthetic peptides derived from Lactoferricin against Escherichia coli ATCC 25922 and Enterococcus faecalis ATCC 29212. Biomed. Res. Int. 2015, 2015, 1–8. [Google Scholar]
- Huertas-Méndez, N.J.; Vargas-Casanova, Y.; Gómez-Chimbi, A.K.; Hernández, E.; Leal-Castro, A.L.; Melo-Diaz, J.M.; Rivera-Monroy, Z.J.; García-Castañeda, J.E. Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Antimicrobial Activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076. Molecules 2017, 22, 452. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.B.; Haug, B.E.; Rekdal, O.; Skar, M.L.; Stensen, W.; Svendsen, J.S. Important structural features of 15-residue lactoferricin derivatives and methods for improvement of antimicrobial activity. Biochem. Cell Biol. 2002, 80, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, F.; Xie, Y.; Wang, Y. Comparative antimicrobial activity and mechanism of action of bovine lactoferricin-derived synthetic peptides. Biometals 2011, 24, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Hoek, K.S.; Milne, J.M.; Grieve, P.A.; Dionysius, D.A.; Smith, R. Antibacterial activity in bovine lactoferrin-derived peptides. Antimicrob. Agents Chemother. 1997, 41, 54–59. [Google Scholar] [PubMed]
- Haug, B.E.; Skar, M.L.; Svendsen, J.S. Bulky aromatic amino acids increase the antibacterial activity of 15-residue bovine lactoferricin derivatives. J. Pept. Sci. 2001, 7, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Guo, H.Y.; Zhang, M.; Jiang, L.; Ren, F.Z. The six amino acid antimicrobial peptide bLFcin6 penetrates cells and delivers siRNA. FEBS J. 2013, 280, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.; de Antueno, R.; Duncan, R.; Hoskin, D.W. Intracellular delivery of bovine lactoferricin's antimicrobial core (RRWQWR) kills T-leukemia cells. Biochem. Biophys. Res. Commun. 2009, 388, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Solarte, V.; Rosas, J.E.; Rivera, Z.J.; Arango, M.L.; García, J.E.; Vernot, J.A. A Tetrameric Peptide Derived from Bovine Lactoferricin Exhibits Specific Cytotoxic Effects against Oral Squamous-Cell Carcinoma Cell Lines. Biomed. Res. Int. 2015, 2015, 630179. [Google Scholar] [CrossRef] [PubMed]
- Svenson, J.; Vergote, V.; Karstad, R.; Burvenich, C.; Svendsen, J.S.; Spiegeleer, B. Metabolic fate of lactoferricin-based antimicrobial peptides: Effect of truncation and incorporation of amino acid analogs on the in vitro metabolic stability. J. Pharmacol. Exp. Ther. 2010, 332, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Dathe, M.; Nikolenko, H.; Klose, J.; Bienert, M. Cyclization increases the antimicrobial activity and selectivity of arginine- and tryptophan-containing hexapeptides. Biochemistry 2004, 43, 9140–9150. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.W.; Shyu, C.L.; Mao, F.C. Antibacterial activity of short hydrophobic and basic-rich peptides. Am. J. Vet. Res. 2003, 64, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Strøm, M.B.; Rekdal, O.; Svendsen, J.S. Antibacterial activity of 15-residue lactoferricin derivatives. J. Pept. Res. 2000, 56, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Takeda, A.; Tachi, T.; Matsuzaki, K. Dimer structure of magainin 2 bound to phospholipid vesicles. Biopolymers 2002, 64, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, F.; Buck-Koehntop, B.A.; Thennarasu, S.; Ramamoorthy, A.; Veglia, G. Structures of the dimeric and monomeric variants of magainin antimicrobial peptides (MSI-78 and MSI-594) in micelles and bilayers, determined by NMR spectroscopy. Biochemistry 2006, 45, 5793–5799. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Matsushita, Y.; Niidome, T.; Hatekeyama, T.; Aoyag, H. Parallel and antiparallel dimers of magainin 2: Their interaction with phospholipid membrane and antibacterial activity. J. Pept. Sci. 2002, 8, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Tencza, S.B.; Creighton, D.J.; Yuan, T.; Vogel, H.J.; Montelaro, R.C.; Mietzner, T.A. Lentivirus-derived antimicrobial peptides: Increased potency by sequence engineering and dimerization. J. Antimicrob. Chemother. 1999, 44, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Schibli, D.; Hwang, P.; Vogel, H. The structure of the antimicrobial active center of lactoferricin B bound to sodium dodecyl sulfate micelles. FEBS Lett. 1999, 446, 213–217. [Google Scholar] [CrossRef]
- Lloyd-Williams, P.; Albericio, F.; Giralt, E. Chemical Approaches to the Synthesis of Peptides and Proteins, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Vergel, C.; Rivera, Z.J.; Rosas, J.E.; García, J.E. Efficient Synthesis of Peptides with 4-Methylpiperidine as Fmoc Removal Reagent by Solid Phase Synthesis. J. Mex. Chem. Soc. 2014, 58, 386–392. [Google Scholar]
- Yang, Y. Side Reactions in Peptide Synthesis, 1st ed.; Elsevier: London, UK, 2016; pp. 53–55. [Google Scholar]
- Rekdal, O.; Andersen, J.; Vorland, L.H.; Svendsen, J.S. Construction and synthesis of lactoferricin derivatives with enhanced antibacterial activity. J. Pept. Sci. 1999, 5, 32–45. [Google Scholar] [CrossRef]
- Azuma, M.; Kojima, T.; Yokoyama, I.; Tajiri, H.; Yoshikawa, K.; Saga, S.; Del Carpio, C.A. Antibacterial activity of multiple antigen peptides homologous to a loop region in human lactoferrin. J. Pept. Res. 1999, 54, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color test for detection of free terminal amino groups in the solid phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Code | Sequence | Purified Product | |||
|---|---|---|---|---|---|
| Yield (%) | Characterization | ||||
| RP-HPLC | MALDI-TOF | ||||
| tR (min) | (m/z) [M + H]+ | ||||
| Lineal peptides | LfcinB 17–31 | FKCRRWQWRMKKLGA | 16 | 4.98 | 1994.71 |
| LfcinB 18–31 | KCRRWQWRMKKLGA | 10 | 4.71 | 1849.12 | |
| LfcinB 19–31 | CRRWQWRMKKLGA | 25 | 4.99 | 1718.00 | |
| LfcinB 20–31 | RRWQWRMKKLGA | 9 | 4.95 | 1617.38 | |
| LfcinB 17–30 | FKCRRWQWRMKKLG | 12 | 4.96 | 1922.48 | |
| LfcinB 17–29 | FKCRRWQWRMKKL | 5 | 5.06 | 1865.73 | |
| LfcinB 17–28 | FKCRRWQWRMKK | 5 | 4.68 | 1752.55 | |
| LfcinB 17–27 | FKCRRWQWRMK | 13 | 4.87 | 1625.18 | |
| LfcinB 17–26 | FKCRRWQWRM | 7 | 5.17 | 1497.06 | |
| LfcinB 17–25 | FKCRRWQWR | 11 | 4.73 | 1365.82 | |
| LfcinB 20–25 | RRWQWR | 37 | 4.19 | 986.66 | |
| [Ala19]-LfcinB 17–31 | FKARRWQWRMKKLGA | 20 | 4.94 | 1961.99 | |
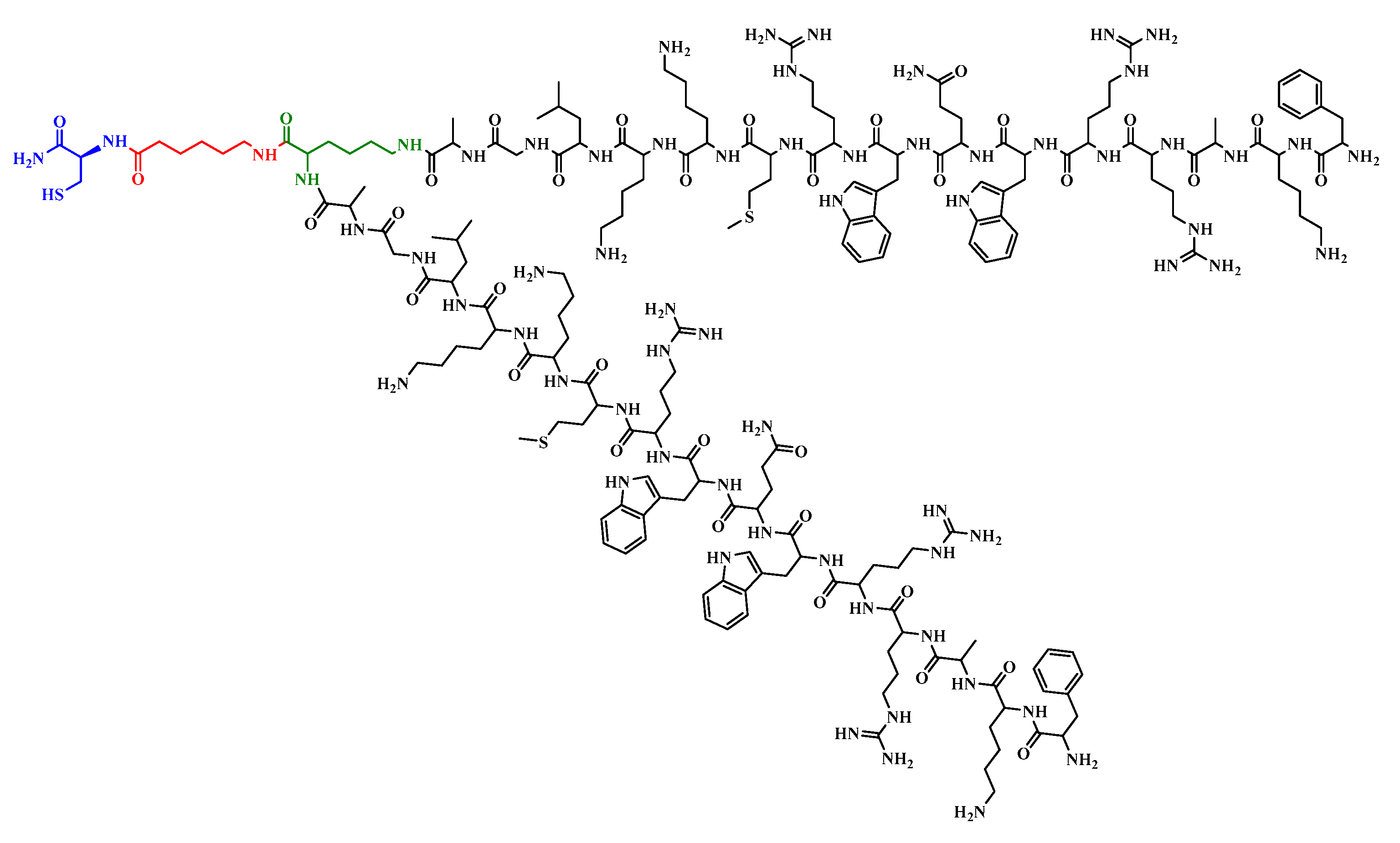
| Polyvalent peptides | ([Ala19]-LfcinB 17–31)2 | (FKARRWQWRMKKLGA)2K-Ahx-C | 18 | 5.52 | 4255.51 |
| (LfcinB 21–25)Pal | RWQWRWQWR | 30 | 5.95 | 1488.58 | |
| Code | E. coli | S. aureus | |||
|---|---|---|---|---|---|
| ATCC 25922 | ATCC 25923 | ||||
| MIC | MBC | MIC | MBC | ||
| Lineal peptides | 200 | >200 | >200 | >200 | |
| >200 | >200 | >200 | >200 | ||
| 100 | 100 | >200 | >200 | ||
| 200 | >200 | >200 | >200 | ||
| 200 | 200 | >200 | >200 | ||
| 200 | >200 | - | - | ||
| 200 | 200 | >200 | >200 | ||
| 100 | 200 | >200 | >200 | ||
| 100 | 200 | 200 | 200 | ||
| 100 | 100 | >200 | >200 | ||
| >200 | >200 | >200 | >200 | ||
| - | - | >200 | >200 | ||
| Polyvalent peptides | - | - | 12.5 | 12.5 | |
| (LfcinB 21–25)Pal | 12.5 | 25 | - | - | |
| (LfcinB 20–25)4 | 6.25 | 12.5 | 25 | 25 | |
| Protein | BLF | >50 | >50 | >50 | >50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huertas, N.D.J.; Monroy, Z.J.R.; Medina, R.F.; Castañeda, J.E.G. Antimicrobial Activity of Truncated and Polyvalent Peptides Derived from the FKCRRQWQWRMKKGLA Sequence against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923. Molecules 2017, 22, 987. https://doi.org/10.3390/molecules22060987
Huertas NDJ, Monroy ZJR, Medina RF, Castañeda JEG. Antimicrobial Activity of Truncated and Polyvalent Peptides Derived from the FKCRRQWQWRMKKGLA Sequence against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923. Molecules. 2017; 22(6):987. https://doi.org/10.3390/molecules22060987
Chicago/Turabian StyleHuertas, Nataly De Jesús, Zuly Jenny Rivera Monroy, Ricardo Fierro Medina, and Javier Eduardo García Castañeda. 2017. "Antimicrobial Activity of Truncated and Polyvalent Peptides Derived from the FKCRRQWQWRMKKGLA Sequence against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923" Molecules 22, no. 6: 987. https://doi.org/10.3390/molecules22060987
APA StyleHuertas, N. D. J., Monroy, Z. J. R., Medina, R. F., & Castañeda, J. E. G. (2017). Antimicrobial Activity of Truncated and Polyvalent Peptides Derived from the FKCRRQWQWRMKKGLA Sequence against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923. Molecules, 22(6), 987. https://doi.org/10.3390/molecules22060987





