Protein Interaction and Na/K-ATPase-Mediated Signal Transduction
Abstract
:1. Introduction
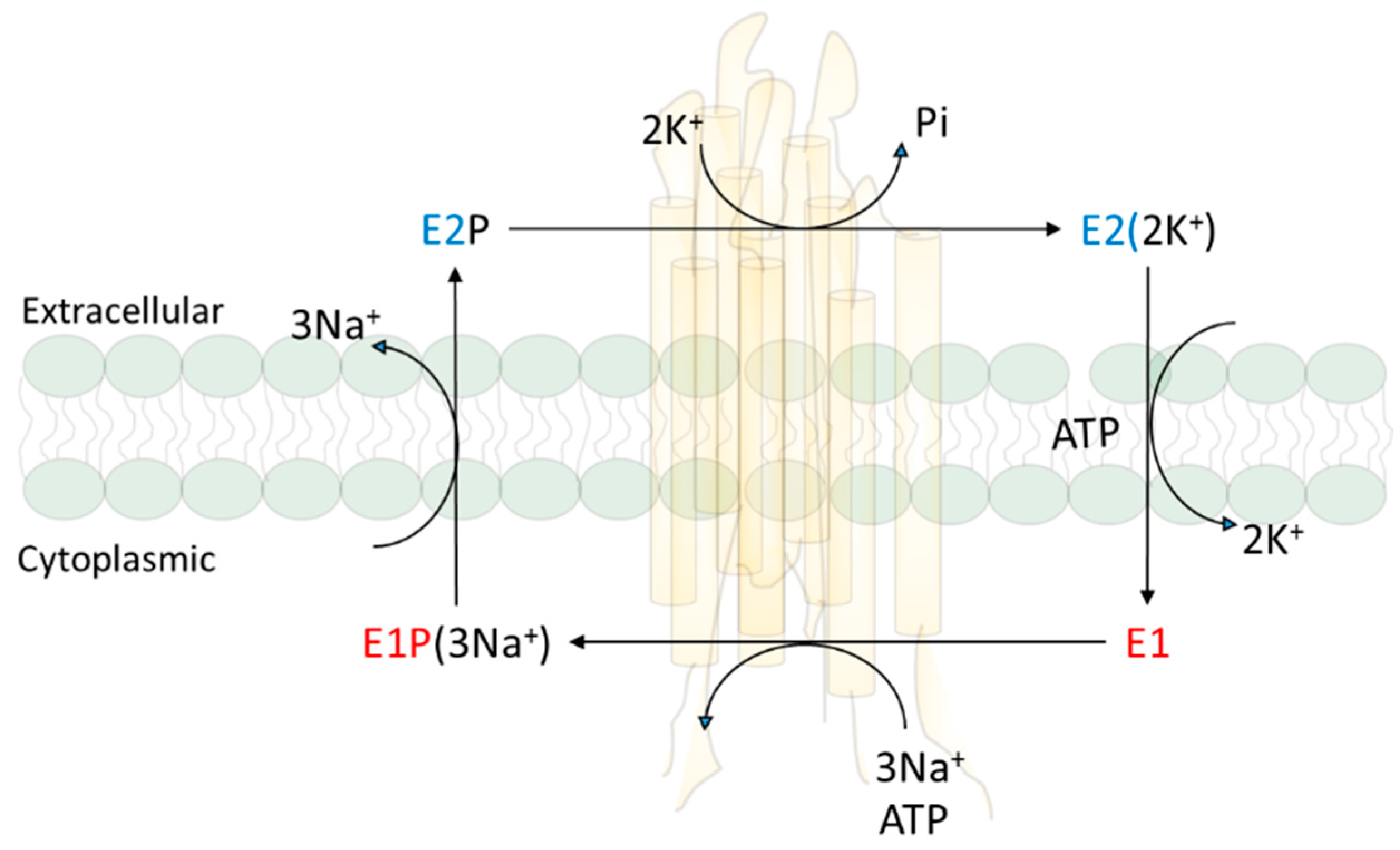
2. Na/K-ATPase and Active Ion Transport
3. Na/K-ATPase and Signal Transduction
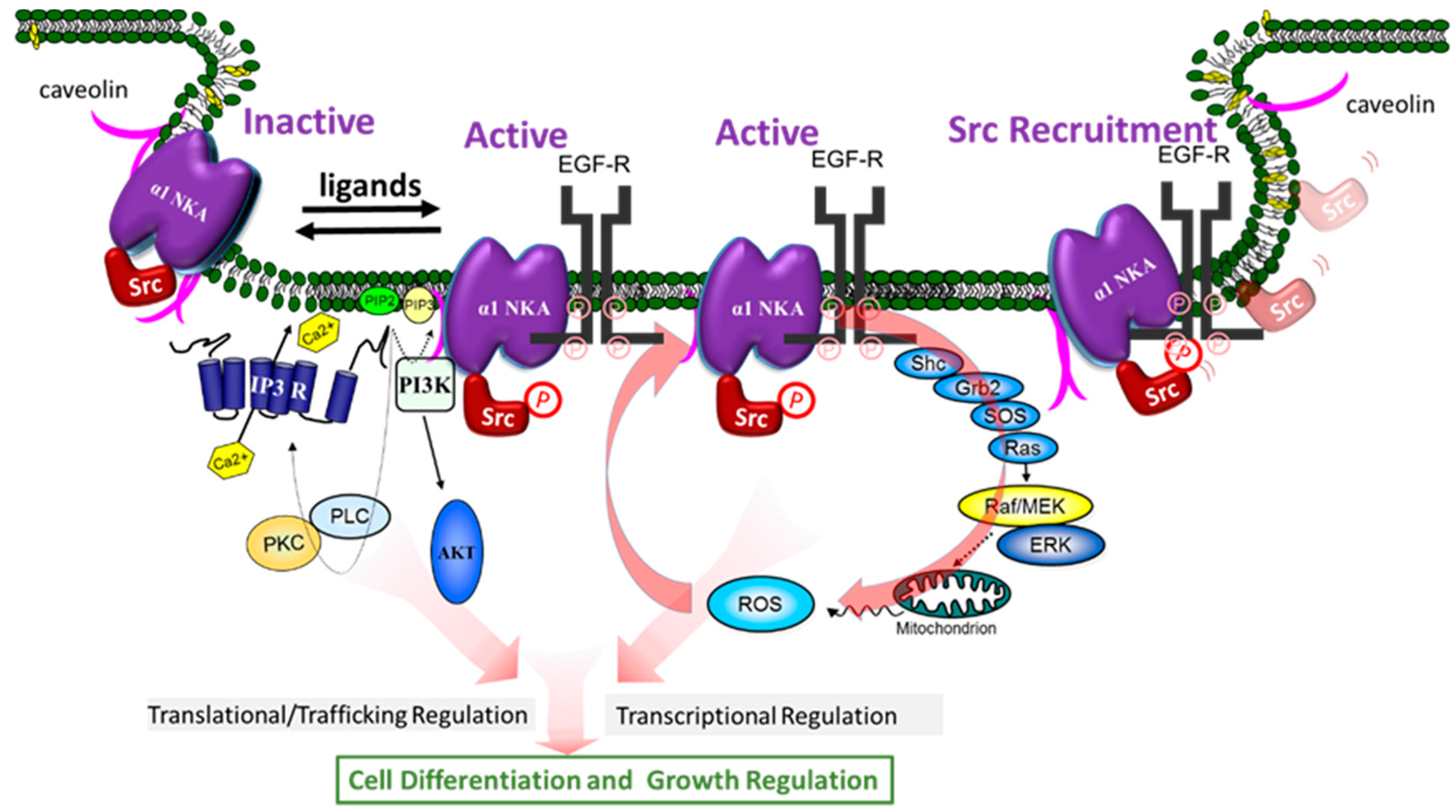
3.1. Na/K-ATPase: More Than a Pump
3.2. Protein Interaction in Signal Transduction
3.3. Src Kinase in NKA-Mediated Signal Transduction
3.4. Evidence of NKA/Src Interaction
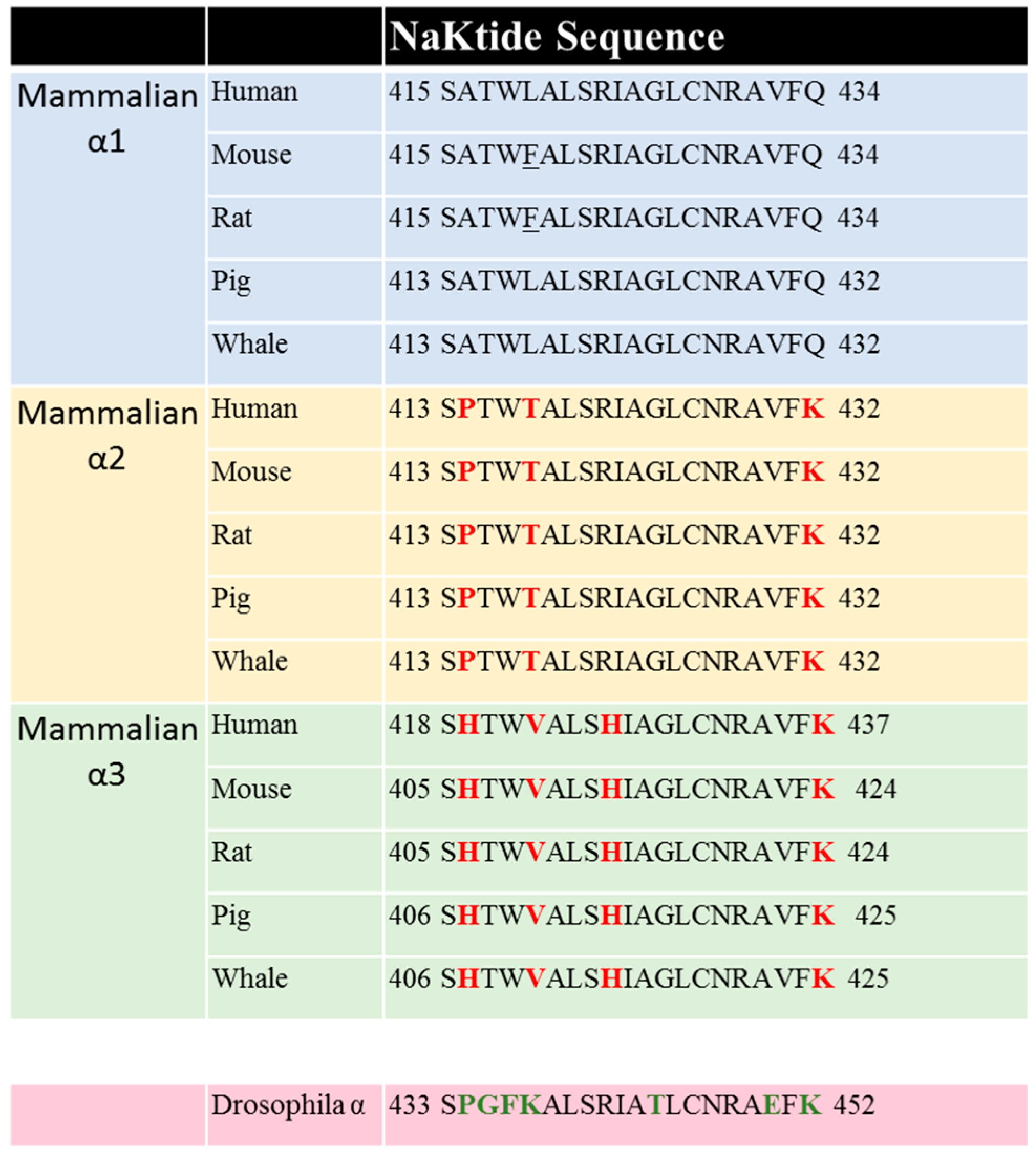
3.5. The Identification of Putative Src Binding Sites and the Discovery of NaKtide as a Specific Inhibitor of NKA-Mediated Signal Transduction
3.6. Identification of CD2 as an Important Src SH2 Ligand
3.7. The Identification of a Mutant α1 NKA That Pumps but Is Null in Src Interaction
3.8. NKA/Src Interactions Are Isoform-Specific
3.9. NKA/Src Complex as a Receptor
4. Conformation-Dependent Regulation of Src by α1 NKA, a New Hypothesis
4.1. NKA/Src/ROS Loop and Disease Progression
4.2. NKA/Src Interaction as a Drug Target
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skou, J.C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta 1957, 23, 394–401. [Google Scholar] [CrossRef]
- Quastel, M.R.; Kaplan, J.G. Inhibition by ouabain of human lymphocyte transformation induced by phytohaemagglutinin in vitro. Nature 1968, 219, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.G. Membrane cation transport and the control of proliferation of mammalian cells. Annu. Rev. Physiol. 1978, 40, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, O. Growth inhibitory effect of cardiac glycosides and aglycones on neoplastic cells: In vitro and in vivo studies. GANN Jpn. J. Cancer Res. 1967, 58, 521–528. [Google Scholar]
- Xie, Z.; Cai, T. Na+-K+–ATPase-Mediated signal transduction: From protein interaction to cellular function. Mol. Interv. 2003, 3, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Aperia, A. New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J. Intern. Med. 2007, 261, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zaleski, S. Carl schmidt. Chem. Ber. 1894, 27, 963–978. [Google Scholar] [CrossRef]
- Heidenhain, R. Neue versuche über die aufsaugung im dünndarm. Pflügers Arch. Eur. J. Physiol. 1894, 56, 579–631. [Google Scholar] [CrossRef]
- Overton, E. Ueber die allgemeinen osmotischen eigenschaften der zelle, ihre vermutlichen ursachen und ihre bedeutung für die physiologie. Vierteljahrsschr. Nat. Ges. Zürich. 1899, 88–135. [Google Scholar]
- Overton, E. Beiträge zur allgemeinen muskel und nervenphysilogie. II. Ueber die unentbehrlichkeit von natrium- (oder lithium) ionen für dencontractionsactdesmuskels. Pflügers Arch. Ges. Physiol. 1902, 346–386. [Google Scholar] [CrossRef]
- Heppel, L.A. The diffusion of radioactive sodium into the muscles of potassium-deprivedrats. Am. J. Physiol. 1940, 128, 449–454. [Google Scholar]
- Steinbach, H.B. Sodium and potassium in frog muscle. J. Biol. Chem. 1940, 133, 695–701. [Google Scholar]
- Dean, R.B. Theories of electrolyte equilibrium in muscle. Biol. Symp. 1941, 3, 331–348. [Google Scholar]
- Solomon, R.Z.; Hald, P.M.; Peters, J.P. The state of the inorganic components of human red blood cells. J. Biol. Chem. 1940, 132, 732–738. [Google Scholar]
- Danowski, T.S. The transfer of potassium across the human blood cell membrane. J. Biol. Chem. 1941, 139, 693–705. [Google Scholar]
- Harris, J.E. The influence of the metabolism of human erythrocytes on their potassiumcontent. J. Biol. Chem. 1941, 141, 579–595. [Google Scholar]
- Schatzmann, H.J. Cardiac glycosides as inhibitors of active potassium and sodium transport by erythrocyte membrane. Helv. Physiol. Pharmacol. Acta 1953, 11, 346–354. [Google Scholar] [PubMed]
- Engelhardt, W.A.; Ljubimowa, M.N. Myosine and adenosinetriphosphatase. Nature 1939, 144, 668–669. [Google Scholar] [CrossRef]
- Gardos, G. Accumulation of potassium ions by human blood corpuscles. Acta Physiol. Hung. 1954, 6, 191–199. [Google Scholar] [PubMed]
- Clarke, R.J.; Fan, X. Pumping ions. Clin. Exp. Pharmacol. Physiol. 2011, 38, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.J.; Albers, R.W. Sodium-Potassium-Activated adenosine triphosphatase of electrophorus electric organ. IV. Modification of responses to sodium and potassium by arsenite plus 2,3-dimercaptopropanol. J. Biol. Chem. 1967, 242, 4972–4979. [Google Scholar] [PubMed]
- Post, R.L.; Kume, S.; Tobin, T.; Orcutt, B.; Sen, A.K. Flexibility of an active center in sodium-plus-potassium adenosine triphosphatase. J. Gen. Physiol. 1969, 54, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.L. Isolation and characterization of the components of the sodium pump. Q. Rev. Biophys. 1974, 7, 239–274. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.L. Purification and characterization of (Na+ + K+)-ATPase. VI. Differential tryptic modification of catalytic functions of the purified enzyme in presence of NaCL and KCL. Biochim. Biophys. Acta 1977, 466, 97–108. [Google Scholar] [CrossRef]
- Jorgensen, P.L. Purification and characterization of (Na+, K+)-ATPase. V. Conformational changes in the enzyme transitions between the Na-form and the K-form studied with tryptic digestion as a tool. Biochim. Biophys. Acta 1975, 401, 399–415. [Google Scholar] [CrossRef]
- Jorgensen, P.L.; Klodos, I. Purification and characterization of (Na+ + K+)-ATPase. VII. Tryptic degradation of the Na-form of the enzyme protein resulting in selective modification of dephosphorylation reactions of the (Na+ + K+)-ATPase. Biochim. Biophys. Acta 1978, 507, 8–16. [Google Scholar] [CrossRef]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am. J. Physiol. 1998, 275, F633–F650. [Google Scholar] [PubMed]
- Sweadner, K.J. Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta 1989, 988, 185–220. [Google Scholar] [CrossRef]
- Marks, M.J.; Seeds, N.W. A heterogeneous ouabain-ATPase interaction in mouse brain. Life Sci. 1978, 23, 2735–2744. [Google Scholar] [CrossRef]
- Sweadner, K.J. Two molecular forms of (Na+ + K+)-stimulated ATPase in brain. Separation, and difference in affinity for strophanthidin. J. Biol. Chem. 1979, 254, 6060–6067. [Google Scholar] [PubMed]
- Shull, G.E.; Schwartz, A.; Lingrel, J.B. Amino-Acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature 1985, 316, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Shull, G.E.; Greeb, J.; Lingrel, J.B. Molecular cloning of three distinct forms of the Na+,K+-ATPase α-subunit from rat brain. Biochemistry 1986, 25, 8125–8132. [Google Scholar] [CrossRef] [PubMed]
- Shamraj, O.I.; Lingrel, J.B. A putative fourth Na+,K(+)-ATPase α-subunit gene is expressed in testis. Proc. Natl. Acad. Sci. USA 1994, 91, 12952–12956. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.; Nguyen, A.N.; Timmerberg, B.; Tash, J.S.; Blanco, G. The Na,K-ATPase α4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol. Hum. Reprod. 2006, 12, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.L.; Andersen, J.P. Structural basis for E1-E2 conformational transitions in Na,K-pump and Ca-pump proteins. J. Membr. Biol. 1988, 103, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Morth, J.P.; Pedersen, B.P.; Toustrup-Jensen, M.S.; Sorensen, T.L.; Petersen, J.; Andersen, J.P.; Vilsen, B.; Nissen, P. Crystal structure of the sodium-potassium pump. Nature 2007, 450, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Nyblom, M.; Poulsen, H.; Gourdon, P.; Reinhard, L.; Andersson, M.; Lindahl, E.; Fedosova, N.; Nissen, P. Crystal structure of Na+, K(+)-ATPase in the Na(+)-bound state. Science 2013, 342, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Morth, J.P.; Pedersen, B.P.; Buch-Pedersen, M.J.; Andersen, J.P.; Vilsen, B.; Palmgren, M.G.; Nissen, P. A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 2011, 12, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Cornelius, F.; Hirata, A.; Toyoshima, C. Sequential substitution of K(+) bound to Na(+),K(+)-ATPase visualized by X-ray crystallography. Nat. Commun. 2015, 6, 8004. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.; Gregersen, J.L.; Yatime, L.; Nissen, P.; Fedosova, N.U. Structures and characterization of digoxin- and bufalin-bound Na+, K+-ATPase compared with the ouabain-bound complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.; Yatime, L.; Nissen, P.; Fedosova, N.U. Crystal structure of the high-affinity Na+ K+-ATPase-ouabain complex with Mg2+ bound in the cation binding site. Proc. Natl. Acad. Sci. USA 2013, 110, 10958–10963. [Google Scholar] [CrossRef] [PubMed]
- Akera, T.; Brody, T.M. Inotropic action of digitalis and ion transport. Life Sci. 1976, 18, 135–144. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides and their mechanisms of action. Am. J. Cardiovasc. Drugs 2007, 7, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, S.D.; Bucci, A.; Manunta, P.; Rivera, R.; Ferrandi, M.; Hamlyn, J.M.; Lapenna, D.; Cuccurullo, F.; Mezzetti, A. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am. J. Hypertens. 2001, 14, 44–50. [Google Scholar] [CrossRef]
- Wang, J.G.; Staessen, J.A.; Messaggio, E.; Nawrot, T.; Fagard, R.; Hamlyn, J.M.; Bianchi, G.; Manunta, P. Salt, endogenous ouabain and blood pressure interactions in the general population. J. Hypertens. 2003, 21, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Manunta, P.; Stella, P.; Rivera, R.; Ciurlino, D.; Cusi, D.; Ferrandi, M.; Hamlyn, J.M.; Bianchi, G. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension 1999, 34, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Cuff, J.M.; Lichtman, A. The early effects of ouabain on potassium metabolism and rate of proliferation of mouse lymphoblasts. J. Cell Physiol. 1975, 85, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Pollack, L.R.; Tate, E.H.; Cook, J.S. Na+,K+-ATPase in hela cells after prolonged growth in low K+ or ouabain. J. Cell Physiol. 1981, 106, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Touza, N.A.; Pocas, E.S.; Quintas, L.E.; Cunha-Filho, G.; Santos, M.L.; Noel, F. Inhibitory effect of combinations of digoxin and endogenous cardiotonic steroids on Na+/K+-ATPase activity in human kidney membrane preparation. Life Sci. 2011, 88, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, Y.; Petrovich, E.; Haviv, H.; Goldshleger, R.; Tal, D.M.; Garty, H.; Karlish, S.J. Purification of the human α2 isoform of Na,K-ATPase expressed in pichia pastoris. Stabilization by lipids and FXYD1. Biochemistry 2007, 46, 14937–14950. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G. The Na/K-ATPase and its isozymes: What we have learned using the baculovirus expression system. Front. Biosci. 2005, 10, 2397–2411. [Google Scholar] [CrossRef] [PubMed]
- El-Okdi, N.; Smaili, S.; Raju, V.; Shidyak, A.; Gupta, S.; Fedorova, L.; Elkareh, J.; Periyasamy, S.; Shapiro, A.P.; Kahaleh, M.B.; et al. Effects of cardiotonic steroids on dermal collagen synthesis and wound healing. J. Appl. Physiol. 2008, 105, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, H.; Xie, Z. Ouabain-Induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J. Mol. Cell Cardiol. 1997, 29, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kometiani, P.; Li, J.; Gnudi, L.; Kahn, B.B.; Askari, A.; Xie, Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of ras and mitogen-activated protein kinases. J. Biol. Chem. 1998, 273, 15249–15256. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Kometiani, P.; Liu, J.; Li, J.; Shapiro, J.I.; Askari, A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J. Biol. Chem. 1999, 274, 19323–19328. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Askari, A.; Xie, Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J. Biol. Chem. 2000, 275, 27832–27837. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Wang, H.; Tian, J.; Xie, Z. Src-Mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J. Biol. Chem. 2002, 277, 18694–18702. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, R.I.; Doris, P.A. Ouabain is a potent promoter of growth and activator of ERK1/2 in ouabain-resistant rat renal epithelial cells. J. Biol. Chem. 2003, 278, 28160–28166. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, M.; Molinari, I.; Barassi, P.; Minotti, E.; Bianchi, G.; Ferrari, P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J. Biol. Chem. 2004, 279, 33306–33314. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, L.; Visentin, B.; Cusinato, F.; Pighin, I.; Luciani, S. Antiapoptotic effect of ouabain on human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2004, 321, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M.; Pastor, N.; Azrak, S.S.; Ayuso, M.J.; Austin, C.A.; Cortes, F. Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients. J. Nat. Prod. 2005, 68, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Bielawski, K.; Winnicka, K.; Bielawska, A. Inhibition of DNA topoisomerases I and II, and growth inhibition of breast cancer MCF-7 cells by ouabain, digoxin and proscillaridin A. Biol. Pharm. Bull. 2006, 29, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Golden, W.C.; Martin, L.J. Low-Dose ouabain protects against excitotoxic apoptosis and up-regulates nuclear Bcl-2 in vivo. Neuroscience 2006, 137, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zelenin, S.; Aperia, A.; Aizman, O. Low doses of ouabain protect from serum deprivation-triggered apoptosis and stimulate kidney cell proliferation via activation of NF-κB. J. Am. Soc. Nephrol. 2006, 17, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ortega, M.; Maldonado-Lagunas, V.; Melendez-Zajgla, J.; Carrillo-Hernandez, J.F.; Pastelin-Hernandez, G.; Picazo-Picazo, O.; Ceballos-Reyes, G. Proliferation and apoptosis of hela cells induced by in vitro stimulation with digitalis. Eur. J. Pharmacol. 2006, 534, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.; Eva, A.; Kirch, U.; Boldyrev, A.; Scheiner-Bobis, G. Ouabain activates signaling pathways associated with cell death in human neuroblastoma. Biochim. Biophys. Acta 2007, 1768, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Q.; Guan, L. Effects of ouabain on proliferation, intracellular free calcium and c-myc mRNA expression in vascular smooth muscle cells. J. Comp. Physiol. B 2007, 177, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; Roland, I.; van Quaquebeke, E.; Nilsson, B.; Mathieu, A.; van Vynckt, F.; Darro, F.; Blanco, G.; Facchini, V.; Kiss, R. The α subunit of the sodium pump could represent a novel target to combat non-small cell lung cancers. J. Pathol. 2007, 212, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, T.; de Neve, N.; Gailly, P.; Mathieu, V.; Haibe-Kains, B.; Bontempi, G.; Lapeira, J.; Decaestecker, C.; Facchini, V.; Kiss, R. Nucleolus and c-myc: Potential targets of cardenolide-mediated antitumor activity. Mol. Cancer Ther. 2008, 7, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, M.; Li, Z.; Li, R.; Jia, L.; Xiong, X.; Southall, N.; Wang, S.; Xia, M.; Austin, C.P.; et al. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res. 2009, 69, 6556–6564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhan, Y.; Xu, R.; Shao, R.; Jiang, J.; Wang, Z. Src mediates extracellular signal-regulated kinase 1/2 activation and autophagic cell death induced by cardiac glycosides in human non-small cell lung cancer cell lines. Mol. Carcinog. 2015, 54, E26–E34. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Plant-Derived cardiac glycosides: Role in heart ailments and cancer management. Biomed. Pharmacother. 2016, 84, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, F.; Kanai, R.; Toyoshima, C. A structural view on the functional importance of the sugar moiety and steroid hydroxyls of cardiotonic steroids in binding to Na,K-ATPase. J. Biol. Chem. 2013, 288, 6602–6616. [Google Scholar] [CrossRef] [PubMed]
- Zeino, M.; Brenk, R.; Gruber, L.; Zehl, M.; Urban, E.; Kopp, B.; Efferth, T. Cytotoxicity of cardiotonic steroids in sensitive and multidrug-resistant leukemia cells and the link with Na(+)/K(+)-ATPase. J. Steroid Biochem. Mol. Biol. 2015, 150, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Estape, E.S.; Torres-Negron, I.; Gonzalez, L.; Martinez-Maldonado, M. A new animal model to study endogenous cardiotonic steroids and the progression of cardiovascular events in salt-sensitive hypertension. Int. Arch. Trans. Med. 2015, 1, 002. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Juhaszova, M.; Golovina, V.A. The cellular mechanism of action of cardiotonic steroids: A new hypothesis. Clin. Exp. Hypertens. 1998, 20, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Langer, G.A. Effects of digitalis on myocardial ionic exchange. Circulation 1972, 46, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.D.; Etter, E.F.; Philipson, K.D.; Carrington, W.A.; Fogarty, K.E.; Lifshitz, L.M.; Fay, F.S. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature 1993, 365, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Juhaszova, M.; Blaustein, M.P. Distinct distribution of different Na+ pump α subunit isoforms in plasmalemma. Physiological implications. Ann. N. Y. Acad. Sci. 1997, 834, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lee, M.Y.; Kinsey, S.P.; Weber, D.J.; Blaustein, M.P. An N-terminal sequence targets and tethers Na+ pump α2 subunits to specialized plasma membrane microdomains. J. Biol. Chem. 2006, 281, 12929–12940. [Google Scholar] [CrossRef] [PubMed]
- Pawson, T. Protein modules and signalling networks. Nature 1995, 373, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Yang, G.; He, Y.; Wang, Y.; Li, Y.; Chen, Z. The conservation pattern of short linear motifs is highly correlated with the function of interacting protein domains. BMC Genom. 2008, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Veshnock, P.J. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature 1987, 328, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, M.; Salardi, S.; Tripodi, G.; Barassi, P.; Rivera, R.; Manunta, P.; Goldshleger, R.; Ferrari, P.; Bianchi, G.; Karlish, S.J. Evidence for an interaction between adducin and Na(+)-K(+)-ATPase: Relation to genetic hypertension. Am. J. Physiol. 1999, 277, H1338–H1349. [Google Scholar] [PubMed]
- Tripodi, G.; Valtorta, F.; Torielli, L.; Chieregatti, E.; Salardi, S.; Trusolino, L.; Menegon, A.; Ferrari, P.; Marchisio, P.C.; Bianchi, G. Hypertension-Associated point mutations in the adducin α and β subunits affect actin cytoskeleton and ion transport. J. Clin. Investig. 1996, 97, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Sweadner, K.J.; Rael, E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 2000, 68, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. FXYD proteins: New regulators of Na-K-ATPase. Am. J. Physiol. Renal. Physiol. 2006, 290, F241–F250. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Shinoda, T.; Cornelius, F.; Toyoshima, C. Crystal structure of the sodium-potassium pump (Na+, K+-ATPase) with bound potassium and ouabain. Proc. Natl. Acad. Sci. USA 2009, 106, 13742–13747. [Google Scholar] [CrossRef] [PubMed]
- Yatime, L.; Laursen, M.; Morth, J.P.; Esmann, M.; Nissen, P.; Fedosova, N.U. Structural insights into the high affinity binding of cardiotonic steroids to the Na+, K+-ATPase. J. Struct. Biol. 2011, 174, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.J.; Scott, B.T.; Jones, L.R. Purification and complete sequence determination of the major plasma membrane substrate for cAMP-dependent protein kinase and protein kinase C in myocardium. J. Biol. Chem. 1991, 266, 11126–11130. [Google Scholar] [PubMed]
- Walaas, S.I.; Czernik, A.J.; Olstad, O.K.; Sletten, K.; Walaas, O. Protein kinase C and cyclic AMP-dependent protein kinase phosphorylate phospholemman, an insulin and adrenaline-regulated membrane phosphoprotein, at specific sites in the carboxy terminal domain. Biochem. J. 1994, 304, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Lansbery, K.L.; Burcea, L.C.; Mendenhall, M.L.; Mercer, R.W. Cytoplasmic targeting signals mediate delivery of phospholemman to the plasma membrane. Am. J. Physiol. Cell Physiol. 2006, 290, C1275–C1286. [Google Scholar] [CrossRef] [PubMed]
- Bibert, S.; Roy, S.; Schaer, D.; Horisberger, J.D.; Geering, K. Phosphorylation of phospholemman (FXYD1) by protein kinases A and C modulates distinct Na,K-ATPase isozymes. J. Biol. Chem. 2008, 283, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.Y.; Rothblum, L.I.; Moorman, J.R.; Tucker, A.L.; Song, J.; Ahlers, B.A.; Carl, L.L.; Wang, J.; Zhang, X.Q. Regulation of cardiac Na+/Ca2+ exchanger by phospholemman. Ann. N. Y. Acad. Sci. 2007, 1099, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Despa, S.; Bossuyt, J.; Han, F.; Ginsburg, K.S.; Jia, L.G.; Kutchai, H.; Tucker, A.L.; Bers, D.M. Phospholemman-Phosphorylation mediates the β-adrenergic effects on Na/K pump function in cardiac myocytes. Circ. Res. 2005, 97, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Efendiev, R.; Chen, Z.; Krmar, R.T.; Uhles, S.; Katz, A.I.; Pedemonte, C.H.; Bertorello, A.M. The 14-3-3 protein translates the Na+,K+-ATPase α1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J. Biol. Chem. 2005, 280, 16272–16277. [Google Scholar] [CrossRef] [PubMed]
- Yudowski, G.A.; Efendiev, R.; Pedemonte, C.H.; Katz, A.I.; Berggren, P.O.; Bertorello, A.M. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase α subunit and regulates its trafficking. Proc. Natl. Acad. Sci. USA 2000, 97, 6556–6561. [Google Scholar] [CrossRef] [PubMed]
- Efendiev, R.; Budu, C.E.; Bertorello, A.M.; Pedemonte, C.H. G-Protein-Coupled receptor-mediated traffic of Na,K-ATPase to the plasma membrane requires the binding of adaptor protein 1 to a Tyr-255-based sequence in the α-subunit. J. Biol. Chem. 2008, 283, 17561–17567. [Google Scholar] [CrossRef] [PubMed]
- Lauf, P.K.; Alqahtani, T.; Flues, K.; Meller, J.; Adragna, N.C. Interaction between Na-K-ATPase and Bcl-2 proteins BclXL and BAK. Am. J. Physiol. Cell Physiol. 2015, 308, C51–C60. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Brugge, J.S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997, 13, 513–609. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cai, T.; Yuan, Z.; Wang, H.; Liu, L.; Haas, M.; Maksimova, E.; Huang, X.Y.; Xie, Z.J. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell 2006, 17, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.G.; Shoshani, L.; Flores-Maldonado, C.; Lazaro, A.; Cereijido, M. Relationship between Na(+),K(+)-ATPase and cell attachment. J. Cell Sci. 1999, 112, 4223–4232. [Google Scholar] [PubMed]
- Aydemir-Koksoy, A.; Abramowitz, J.; Allen, J.C. Ouabain-Induced signaling and vascular smooth muscle cell proliferation. J. Biol. Chem. 2001, 276, 46605–46611. [Google Scholar] [CrossRef] [PubMed]
- Kometiani, P.; Liu, L.; Askari, A. Digitalis-Induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol. Pharmacol. 2005, 67, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Kotova, O.; Al-Khalili, L.; Talia, S.; Hooke, C.; Fedorova, O.V.; Bagrov, A.Y.; Chibalin, A.V. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J. Biol. Chem. 2006, 281, 20085–20094. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Vetteth, S.; Periyasamy, S.M.; Kanj, M.; Fedorova, L.; Khouri, S.; Kahaleh, M.B.; Xie, Z.; Malhotra, D.; Kolodkin, N.I.; et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension 2006, 47, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Haas, M.; Liang, M.; Cai, T.; Tian, J.; Li, S.; Xie, Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J. Biol. Chem. 2004, 279, 17250–17259. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, M.; Molinari, I.; Torielli, L.; Padoani, G.; Salardi, S.; Rastaldi, M.P.; Ferrari, P.; Bianchi, G. Adducin- and ouabain-related gene variants predict the antihypertensive activity of rostafuroxin, part 1: Experimental studies. Sci. Trans. Med. 2010, 2, 59ra86. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.-N.T.; Wallace, D.P.; Blanco, G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells. J. Am. Soc. Nephrol. 2007, 18, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.N.; Jansson, K.; Sanchez, G.; Sharma, M.; Reif, G.A.; Wallace, D.P.; Blanco, G. Ouabain activates the Na-K-ATPase signalosome to induce autosomal dominant polycystic kidney disease cell proliferation. AJP Renal Physiol. 2011, 301, F897–F906. [Google Scholar] [CrossRef] [PubMed]
- Clifford, R.J.; Kaplan, J.H. Human breast tumor cells are more resistant to cardiac glycoside toxicity than non-tumorigenic breast cells. PLoS ONE 2013, 8, e84306. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, T.; Tian, J.; Xie, J.X.; Zhao, X.; Liu, L.; Shapiro, J.I.; Xie, Z. Naktide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J. Biol. Chem. 2009, 284, 21066–21076. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Xie, J.X.; Li, X.; Tian, J.; Cai, T.; Cui, H.; Ding, H.; Shapiro, J.I.; Xie, Z. Na/k-ATPase mimetic pnaktide peptide inhibits the growth of human cancer cells. J. Biol. Chem. 2011, 286, 32394–32403. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Duan, Q.; Xie, Z. SH2 ligand-like effects of second cytosolic domain of Na/K-ATPase α1 subunit on Src kinase. PLoS ONE 2015, 10, e0142119. [Google Scholar] [CrossRef] [PubMed]
- Yosef, E.; Katz, A.; Peleg, Y.; Mehlman, T.; Karlish, S.J. Do Src kinase and caveolin interact directly with Na,K-ATPase? J. Biol. Chem. 2016, 291, 11736–11750. [Google Scholar] [CrossRef] [PubMed]
- Brott, B.K.; Decker, S.; O’Brien, M.C.; Jove, R. Molecular features of the viral and cellular Src kinases involved in interactions with the gtpase-activating protein. Mol. Cell Biol. 1991, 11, 5059–5067. [Google Scholar] [CrossRef] [PubMed]
- Shvartsman, D.E.; Donaldson, J.C.; Diaz, B.; Gutman, O.; Martin, G.S.; Henis, Y.I. Src kinase activity and SH2 domain regulate the dynamics of Src association with lipid and protein targets. J. Cell Biol. 2007, 178, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Madan, N.; Ye, Q.; Duan, Q.; Li, Z.; Wang, S.; Si, S.; Xie, Z. Identification of a mutant α1 Na/K-ATPase that pumps but is defective in signal transduction. J. Biol. Chem. 2013, 288, 13295–13304. [Google Scholar] [CrossRef] [PubMed]
- Madan, N.; Xu, Y.; Duan, Q.; Banerjee, M.; Larre, I.; Pierre, S.V.; Xie, Z. Src-Independent ERK signaling through the rat α3 isoform of Na/K-ATPase. Am. J. Physiol. Cell Physiol. 2017, 312, C222–C232. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ye, Q.; Cui, X.; Madan, N.; Yi, Q.; Pierre, S.V.; Xie, Z. Expression of rat Na-K-ATPase α2 enables ion pumping but not ouabain-induced signaling in α1-deficient porcine renal epithelial cells. Am. J. Physiol. 2015, 309, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Gable, M.E.; Abdallah, S.L.; Najjar, S.M.; Liu, L.; Askari, A. Digitalis-Induced cell signaling by the sodium pump: On the relation of Src to Na(+)/K(+)-ATPase. Biochem. Biophys. Res. Commun. 2014, 446, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Weigand, K.M.; Swarts, H.G.; Fedosova, N.U.; Russel, F.G.; Koenderink, J.B. Na,K-ATPase activity modulates Src activation: A role for ATP/ADP ratio. Biochim. Biophys. Acta 2012, 1818, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Mohammadi, K.; Aynafshar, B.; Wang, H.; Li, D.; Liu, J.; Ivanov, A.V.; Xie, Z.; Askari, A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am. J. Physiol. Cell Physiol. 2003, 284, C1550–C1560. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Cai, T.; Tian, J.; Ivanov, A.V.; Giovannucci, D.R.; Xie, Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol. Biol. Cell 2005, 16, 4034–4045. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shapiro, A.P.; Haller, S.; Katragadda, V.; Liu, L.; Tian, J.; Basrur, V.; Malhotra, D.; Xie, Z.J.; Abraham, N.G.; et al. Involvement of reactive oxygen species in a feed-forward mechanism of Na/K-ATPase-mediated signaling transduction. J. Biol. Chem. 2013, 288, 34249–34258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, Q.; Liu, C.; Xie, J.X.; Yan, Y.; Lai, F.; Duan, Q.; Li, X.; Tian, J.; Xie, Z. Involvement of Na/K-ATPase in hydrogen peroxide-induced activation of the Src/ERK pathway in LLC-PK1 cells. Free Radic. Biol. Med. 2014, 71, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Lingrel, J.B. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu. Rev. Physiol. 2010, 72, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Quintas, L.E.; Pierre, S.V.; Liu, L.; Bai, Y.; Liu, X.; Xie, Z.J. Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts. J. Mol. Cell Cardiol. 2010, 49, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.M.; Burlaka, I.; Khodus, G.; Brismar, H.; Aperia, A. Calcium oscillations triggered by cardiotonic steroids. FEBS J. 2013, 280, 5450–5455. [Google Scholar] [CrossRef] [PubMed]
- Dvela, M.; Rosen, H.; Ben-Ami, H.C.; Lichtstein, D. Endogenous ouabain regulates cell viability. Am. J. Physiol. Cell Physiol. 2012, 302, C442–C452. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kesiry, R.; Periyasamy, S.M.; Malhotra, D.; Xie, Z.; Shapiro, J.I. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004, 66, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, X.; Pierre, S.V.; Askari, A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am. J. Physiol. Cell Physiol. 2007, 293, C1489–C1497. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Akkuratov, E.E.; Bai, Y.; Gaskill, C.M.; Askari, A.; Liu, L. Cell signaling associated with Na(+)/K(+)-ATPase: Activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry 2013, 52, 9059–9067. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Lai, F.; Banerjee, M.; Duan, Q.; Li, Z.; Si, S.; Xie, Z. Expression of mutant α1 Na/K-ATPase defective in conformational transition attenuates Src-mediated signal transduction. J. Biol. Chem. 2013, 288, 5803–5814. [Google Scholar] [CrossRef] [PubMed]
- Elkareh, J.; Kennedy, D.J.; Yashaswi, B.; Vetteth, S.; Shidyak, A.; Kim, E.G.; Smaili, S.; Periyasamy, S.M.; Hariri, I.M.; Fedorova, L.; et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension 2007, 49, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Chen, Y.; Huang, W.; Viterna, J.; Liu, J.; Westfall, K.; Tian, J.; Bartlett, D.J.; Tang, W.H.; Xie, Z.; et al. CD36 and Na/K-ATPase-α1 form a proinflammatory signaling loop in kidney. Hypertension 2013, 61, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Shidyak, A.; Periyasamy, S.M.; Haller, S.; Taleb, M.; El-Okdi, N.; Elkareh, J.; Gupta, S.; Gohara, S.; Fedorova, O.V.; et al. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension 2009, 54, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, Y.; Liu, L.; Xie, Z.; Malhotra, D.; Joe, B.; Shapiro, J.I. Impairment of Na/K-ATPase signaling in renal proximal tubule contributes to Dahl salt-sensitive hypertension. J. Biol. Chem. 2011, 286, 22806–22813. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kennedy, D.J.; Ramakrishnan, D.P.; Yang, M.; Huang, W.; Li, Z.; Xie, Z.; Chadwick, A.C.; Sahoo, D.; Silverstein, R.L. Oxidized LDL-bound CD36 recruits an Na+/K+-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci. Signal. 2015, 8, ra91. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.; Maxwell, K.; Yan, Y.; Liu, J.; Chaudhry, M.A.; Getty, M.; Xie, Z.; Abraham, N.G.; Shapiro, J.I. Pnaktide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis. Sci. Adv. 2015, 1, e1500781. [Google Scholar] [CrossRef] [PubMed]
- Dvela-Levitt, M.; Cohen-Ben Ami, H.; Rosen, H.; Ornoy, A.; Hochner-Celnikier, D.; Granat, M.; Lichtstein, D. Reduction in maternal circulating ouabain impairs offspring growth and kidney development. J. Am. Soc. Nephrol. 2015, 26, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Khodus, G.R.; Kruusmagi, M.; Kamali-Zare, P.; Liu, X.L.; Eklof, A.C.; Zelenin, S.; Brismar, H.; Aperia, A. Ouabain protects against adverse developmental programming of the kidney. Nat. Commun. 2010, 1, 42. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Karashima, E.; Hamlyn, J.M.; Blaustein, M.P. Ouabain-Digoxin antagonism in rat arteries and neurones. J. Physiol. 2014, 592, 941–969. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Xie, J. The Na/K-ATPase-mediated signal transduction as a target for new drug development. Front. Biosci. 2005, 10, 3100–3109. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Pitt, B.; Rahimtoola, S.H.; Waagstein, F.; White, M.; Love, T.E.; Braunwald, E. Effects of digoxin at low serum concentrations on mortality and hospitalization in heart failure: A propensity-matched study of the dig trial. Int. J. Cardiol. 2008, 123, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Pasdois, P.; Quinlan, C.L.; Rissa, A.; Tariosse, L.; Vinassa, B.; Costa, A.D.; Pierre, S.V.; Dos Santos, P.; Garlid, K.D. Ouabain protects rat hearts against ischemia-reperfusion injury via pathway involving Src kinase, mitoKATP, and ROS. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1470–H1478. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, G.; Frascarelli, S.; Zucchi, R.; Biver, T.; Montali, U. Cardioprotection by ouabain and digoxin in perfused rat hearts. J. Cardiovasc. Pharmacol. 2008, 52, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Pierre, S.V.; Yang, C.; Yuan, Z.; Seminerio, J.; Mouas, C.; Garlid, K.D.; Dos-Santos, P.; Xie, Z. Ouabain triggers preconditioning through activation of the Na+,K+-ATPase signaling cascade in rat hearts. Cardiovasc. Res. 2007, 73, 488–496. [Google Scholar] [CrossRef] [PubMed]
- McConkey, D.J.; Lin, Y.; Nutt, L.K.; Ozel, H.Z.; Newman, R.A. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000, 60, 3807–3812. [Google Scholar] [PubMed]
- Wang, Y.; Qiu, Q.; Shen, J.J.; Li, D.D.; Jiang, X.J.; Si, S.Y.; Shao, R.G.; Wang, Z. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. Int. J. Biochem. Cell Biol. 2012, 44, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Prassas, I.; Karagiannis, G.S.; Batruch, I.; Dimitromanolakis, A.; Datti, A.; Diamandis, E.P. Digitoxin-Induced cytotoxicity in cancer cells is mediated through distinct kinase and interferon signaling networks. Mol. Cancer Ther. 2011, 10, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Wallace, D.P. Novel role of ouabain as a cystogenic factor in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2013, 305, F797–F812. [Google Scholar] [CrossRef] [PubMed]
- Jansson, K.; Magenheimer, B.S.; Maser, R.L.; Calvet, J.P.; Blanco, G. Overexpression of the polycystin-1 C-tail enhances sensitivity of M-1 cells to ouabain. J. Membr. Biol. 2013, 246, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, M.; Liu, L.; Malhotra, D.; Xie, Z.; Shapiro, J.I. Ouabain-Induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005, 67, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Yan, Y.; Malhotra, D.; Liu, J.; Xie, Z.; Najjar, S.M.; Shapiro, J.I. Ouabain and insulin induce sodium pump endocytosis in renal epithelium. Hypertension 2012, 59, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, Z.J. The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim. Biophys. Acta 2010, 1802, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Digby, G.J.; Lober, R.M.; Sethi, P.R.; Lambert, N.A. Some G protein heterotrimers physically dissociate in living cells. Proc. Natl. Acad. Sci. USA 2006, 103, 17789–17794. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.A.; Zauhar, R.J.; Lanzara, R.G. Molecular dynamics of a biophysical model for β2-adrenergic and G protein-coupled receptor activation. J. Mol. Graph. Model. 2006, 25, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Z.; Tian, J.; Jiang, W.; Wang, Y.; Zhang, X.; Li, Z.; You, Q.; Shapiro, J.I.; Si, S.; et al. Identification of hydroxyxanthones as Na/K-ATPase ligands. Mol. Pharmacol. 2010, 77, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Kemble, D.J. To C or not to C: Direct and indirect redox regulation of Src protein tyrosine kinase. Cell Cycle 2009, 8, 2353–2355. [Google Scholar] [CrossRef] [PubMed]
- MacKay, C.E.; Knock, G.A. Control of vascular smooth muscle function by Src-family kinases and reactive oxygen species in health and disease. J. Physiol. 2015, 593, 3815–3828. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Nasrallah, R.; Touyz, R.M.; Hebert, R.L. Nadph oxidases, reactive oxygen species, and the kidney: Friend and foe. J. Am. Soc. Nephrol. 2013, 24, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Redox signaling in cardiovascular health and disease. Free Radic. Biol. Med. 2013, 61, 473–501. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, A.; Cotter, T.G. Redox regulation of protein kinases. FEBS J. 2013, 280, 1944–1965. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, J.; Haas, M.; Shapiro, J.I.; Askari, A.; Xie, Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J. Biol. Chem. 2000, 275, 27838–27844. [Google Scholar] [PubMed]
- Xie, Z.J.; Wang, Y.H.; Askari, A.; Huang, W.H.; Klaunig, J.E.; Askari, A. Studies on the specificity of the effects of oxygen metabolites on cardiac sodium pump. J. Mol. Cell. Cardiol. 1990, 22, 911–920. [Google Scholar] [CrossRef]
- Huang, W.H.; Wang, Y.; Askari, A. (Na+ + K+)-ATPase: Inactivation and degradation induced by oxygen radicals. Int. J. Biochem. 1992, 24, 621–626. [Google Scholar] [PubMed]
- Yan, Y.; Shapiro, A.P.; Mopidevi, B.R.; Chaudhry, M.A.; Maxwell, K.; Haller, S.T.; Drummond, C.A.; Kennedy, D.J.; Tian, J.; Malhotra, D.; et al. Protein carbonylation of an amino acid residue of the Na/K-ATPase α1 subunit determines Na/K-ATPase signaling and sodium transport in renal proximal tubular cells. J. Am. Heart Assoc. 2016, 5, e003675. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Garcia, A.; Mahmmoud, Y.A.; Hamilton, E.J.; Galougahi, K.K.; Fry, N.A.; Figtree, G.A.; Cornelius, F.; Clarke, R.J.; Rasmussen, H.H. Susceptibility of β1 Na+-K+ pump subunit to glutathionylation and oxidative inhibition depends on conformational state of pump. J. Biol. Chem. 2012, 287, 12353–12364. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Liu, C.C.; Garcia, A.; Hamilton, E.J.; Huang, Y.; Chia, K.K.; Hunyor, S.N.; Figtree, G.A.; Rasmussen, H.H. β(3) Adrenergic stimulation of the cardiac Na+-K+ pump by reversal of an inhibitory oxidative modification. Circulation 2010, 122, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- White, C.N.; Liu, C.C.; Garcia, A.; Hamilton, E.J.; Chia, K.K.; Figtree, G.A.; Rasmussen, H.H. Activation of camp-dependent signaling induces oxidative modification of the cardiac Na+-K+ pump and inhibits its activity. J. Biol. Chem. 2010, 285, 13712–13720. [Google Scholar] [CrossRef] [PubMed]
- Goldshleger, R.; Karlish, S.J. Fe-Catalyzed cleavage of the α subunit of Na/K-ATPase: Evidence for conformation-sensitive interactions between cytoplasmic domains. Proc. Natl. Acad. Sci. USA 1997, 94, 9596–9601. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.X.; Li, X.; Xie, Z. Regulation of renal function and structure by the signaling Na/K-ATPase. IUBMB Life 2013, 65, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, J.; Chaudhry, M.; Maxwell, K.; Yan, Y.; Wang, X.; Shah, P.T.; Khawaja, A.A.; Martin, R.; Robinette, T.J.; et al. Attenuation of Na/K-ATPase mediated oxidant amplification with pnaktide ameliorates experimental uremic cardiomyopathy. Sci. Rep. 2016, 6, 34592. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.; Srikanthan, K.; Goguet-Rubio, P.; Nichols, A.; Mallick, A.; Nawab, A.; Martin, R.; Shah, P.T.; Chaudhry, M. pNaKtide Attenuates steatohepatitis and atherosclerosis by blocking Na/K-ATPase/ROS amplification in C57Bl6 and ApoE knockout mice fed a western diet. Sci. Rep. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Garver, W.S.; Hossain, G.S.; Winscott, M.M.; Heidenreich, R.A. The Npc1 mutation causes an altered expression of caveolin-1, annexin II and protein kinases and phosphorylation of caveolin-1 and annexin II in murine livers. Biochim. Biophys. Acta 1999, 1453, 193–206. [Google Scholar] [CrossRef]
- Lockwich, T.P.; Liu, X.; Singh, B.B.; Jadlowiec, J.; Weiland, S.; Ambudkar, I.S. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 2000, 275, 11934–11942. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Wang, H.; Chen, Y.; Liu, L.; Gunning, W.T.; Quintas, L.E.; Xie, Z.J. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J. Cell Biol. 2008, 182, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, T.; Wang, H.; Li, Z.; Loreaux, E.; Lingrel, J.B.; Xie, Z. Regulation of intracellular cholesterol distribution by Na/K-ATPase. J. Biol. Chem. 2009, 284, 14881–14890. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Tian, J.; Liu, L.; Pierre, S.; Liu, J.; Shapiro, J.; Xie, Z.J. Identification of a pool of non-pumping Na/K-ATPase. J. Biol. Chem. 2007, 282, 10585–10593. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Cai, T.; Tian, J.; Qu, W.; Xie, Z.J. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J. Biol. Chem. 2006, 281, 19709–19719. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Nakade, S.; Miyawaki, A.; Mikoshiba, K.; Ogawa, K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J. Cell Biol. 1992, 119, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, M.P.; Scherer, P.E.; Vidugiriene, J.; Tang, Z.; Hermanowski-Vosatka, A.; Tu, Y.H.; Cook, R.F.; Sargiacomo, M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: Implications for human disease. J. Cell Biol. 1994, 126, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Ye, Q.; Tian, J.; Jing, R.; Xie, Z. Regulation of α1 Na/K-ATPase expression by cholesterol. J. Biol. Chem. 2011, 286, 15517–15524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, T.; Yang, C.; Turner, D.A.; Giovannucci, D.R.; Xie, Z. Regulation of inositol 1,4,5-trisphosphate receptor-mediated calcium release by the Na/K-ATPase in cultured renal epithelial cells. J. Biol. Chem. 2008, 283, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Xie, Z. Protein Interaction and Na/K-ATPase-Mediated Signal Transduction. Molecules 2017, 22, 990. https://doi.org/10.3390/molecules22060990
Cui X, Xie Z. Protein Interaction and Na/K-ATPase-Mediated Signal Transduction. Molecules. 2017; 22(6):990. https://doi.org/10.3390/molecules22060990
Chicago/Turabian StyleCui, Xiaoyu, and Zijian Xie. 2017. "Protein Interaction and Na/K-ATPase-Mediated Signal Transduction" Molecules 22, no. 6: 990. https://doi.org/10.3390/molecules22060990





