Current Perspective on In Vivo Molecular Imaging of Immune Cells
Abstract
:1. Introduction
2. Imaging Modalities
2.1. MRI (T1, T2)
2.2. Optical Imaging
2.3. Miscellaneous: Upconversion Nanoparticles and Quantum Dots
3. Applications of Imaging Immune Cells
3.1. Macrophages/Monocytes
3.1.1. Tumor-Associated Macrophages and Immunotherapy
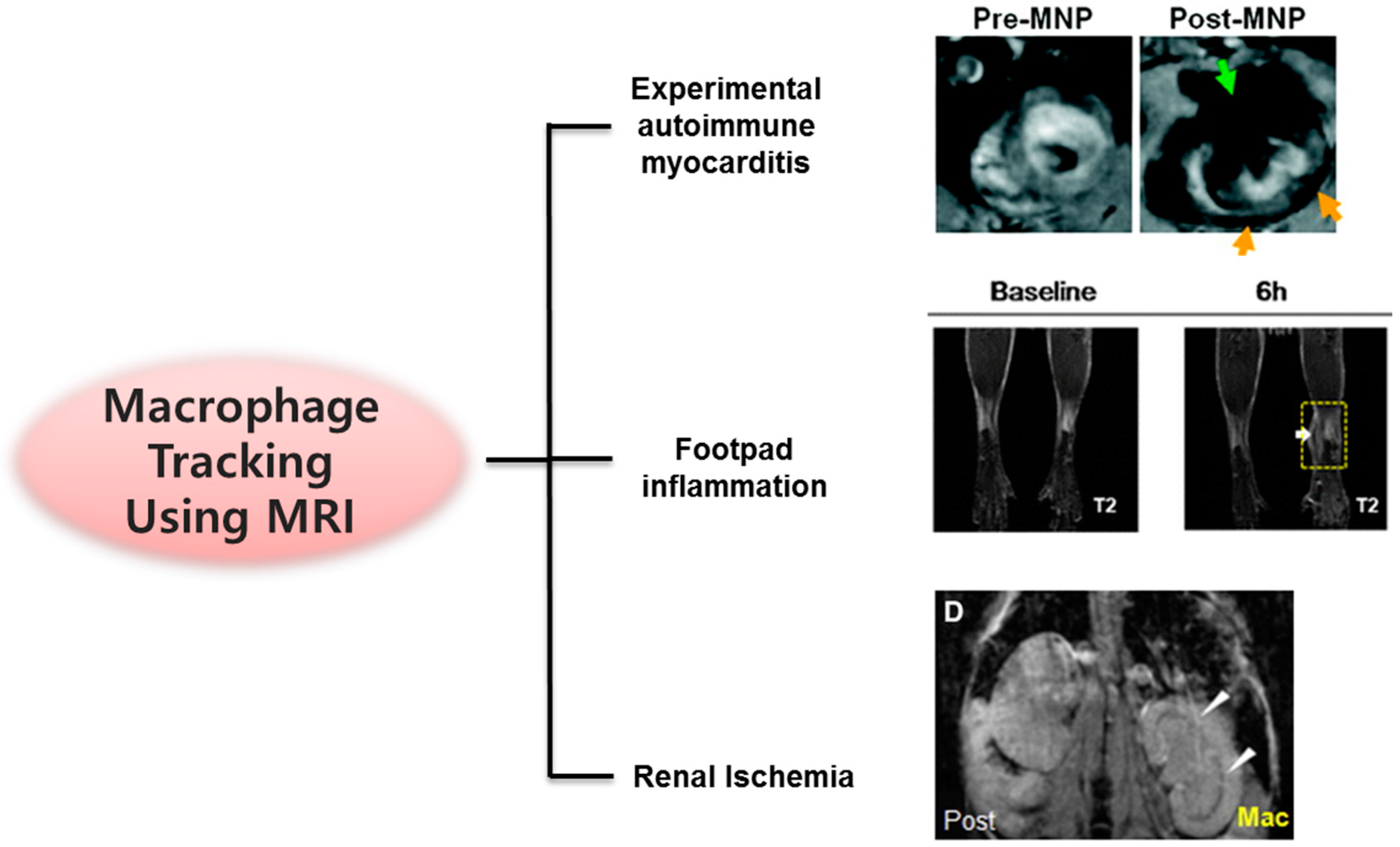
3.1.2. Cardiovascular Diseases: Myocarditis, Myocardial Infarction, and Aneurysm
3.1.3. Inflammation and Ischemia
3.2. T Cells
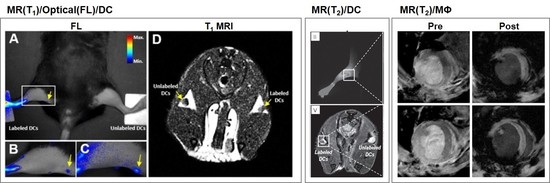
3.3. Dendritic Cells
3.4. Natural Killer Cells
4. Limitations of Existing Cell Tracking Approaches and Future Prospects
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, C.; Peng, H.; Xu, A.; Wang, S. Immune system and artificial immune system application. In World Congress on Medical Physics and Biomedical Engineering 2006; Magjarevic, R., Nagel, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 477–480. [Google Scholar]
- Cusick, M.F.; Libbey, J.E.; Fujinami, R.S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 2012, 42, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Elkord, E. Regulatory T cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kolosnjaj-Tabi, J.; Wilhelm, C.; Clément, O.; Gazeau, F. Cell labeling with magnetic nanoparticles: Opportunity for magnetic cell imaging and cell manipulation. J. Nanobiotechnol. 2013, 11 (Suppl. 1), S7. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Woo, J.; Lee, J.H.; Joo, H.J.; Choi, Y.; Kim, H.; Moon, W.K.; Kim, S.J. In vivo tracking of dendritic cell using MRI reporter gene, ferritin. PLoS ONE 2015, 10, e0125291. [Google Scholar] [CrossRef] [PubMed]
- Bhirde, A.; Xie, J.; Swierczewska, M.; Chen, X. Nanoparticles for cell labeling. Nanoscale 2011, 3, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.; Hong, K.-J. In vivo noninvasive molecular imaging for immune cell tracking in small animals. Immune Netw. 2012, 12, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, E.T.; Bulte, J.W.M. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 2013, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Bhuniya, S.; Lee, H.; Kim, H.M.; Shin, W.S.; Kim, J.S.; Hong, K.S. In vivo tracking of phagocytic immune cells using a dual imaging probe with gadolinium-enhanced MRI and near-infrared fluorescence. ACS Appl. Mater. Interfaces 2016, 8, 10266–10273. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Noh, Y.-W.; Park, H.S.; Cho, M.Y.; Hong, K.S.; Lee, H.; Shin, D.H.; Kang, J.; Dung, M.H.; Poo, H.; et al. Self-Fluorescence of Chemically Crosslinked MRI Nanoprobes to Enable Multimodal Imaging of Therapeutic Cells. Small 2012, 8, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Bhuniya, S.; Moon, H.; Lee, H.; Hong, K.S.; Lee, S.; Yu, D.-Y.; Kim, J.S. Uridine-based paramagnetic supramolecular nanoaggregate with high relaxivity capable of detecting primitive liver tumor lesions. Biomaterials 2011, 32, 6533–6540. [Google Scholar] [CrossRef] [PubMed]
- Legacz, M.; Roepke, K.; Giersig, M.; Pison, U. Contrast agents and cell labeling strategies for in vivo imaging. Adv. Nanopart. 2014, 3, 13. [Google Scholar] [CrossRef]
- Lee, H.W.; Gangadaran, P.; Kalimuthu, S.; Ahn, B.-C. Advances in molecular imaging strategies for in vivo tracking of immune cells. BioMed Res. Int. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Lucignani, G.; Ottobrini, L.; Martelli, C.; Rescigno, M.; Clerici, M. Molecular imaging of cell-mediated cancer immunotherapy. Trends Biotechnol. 2006, 24, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Park, H.E.; Kang, J.; Lee, H.; Cheong, C.; Lim, Y.T.; Ihm, S.H.; Seung, K.-B.; Jaffer, F.A.; Narula, J.; et al. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation 2012, 125, 2603–2612. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Xu, Y.; Yang, L.; Wu, J.; Zhu, W.; Li, D.; Cheng, Z.; Xia, C.; Guo, Y.; Gong, Q.; et al. Negatively charged magnetite nanoparticle clusters as efficient MRI probes for dendritic cell labeling and in vivo tracking. Adv. Funct. Mater. 2015, 25, 3581–3591. [Google Scholar] [CrossRef]
- Williams, J.B.; Ye, Q.; Hitchens, T.K.; Kaufman, C.L.; Ho, C. MRI detection of macrophages labeled using micrometer-sized iron oxide particles. J. Magn. Reson. Imaging 2007, 25, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [PubMed]
- Mou, Y.; Hou, Y.; Chen, B.; Hua, Z.; Zhang, Y.; Xie, H.; Xia, G.; Wang, Z.; Huang, X.; Han, W.; et al. In vivo migration of dendritic cells labeled with synthetic superparamagnetic iron oxide. Int. J. Nanomed. 2011, 6, 2633–2640. [Google Scholar]
- Meier, R.; Golovko, D.; Tavri, S.; Henning, T.D.; Knopp, C.; Piontek, G.; Rudelius, M.; Heinrich, P.; Wels, W.S.; Daldrup-Link, H. Depicting adoptive immunotherapy for prostate cancer in an animal model with magnetic resonance imaging. Magn. Reson. Med. 2011, 65, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Histed, S.N.; Aras, O. NK cell imaging by in vitro and in vivo labelling approaches. Q. J. Nucl. Med. Mol. Imaging 2014, 58, 276–283. [Google Scholar] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Yamada, S.; Tsuboi, S.; Nakane, Y.; Tsukasaki, Y.; Komatsuzaki, A.; Jin, T. Near-infrared emitting PbS quantum dots for in vivo fluorescence imaging of the thrombotic state in septic mouse Brain. Molecules 2016, 21, 1080. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Lv, F.; Cao, B.; He, X.; Liu, T. Saccharide substituted zinc phthalocyanines: Optical properties, interaction with bovine serum albumin and near infrared fluorescence imaging for sentinel lymph nodes. Molecules 2014, 19, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Tavri, S.; Jha, P.; Meier, R.; Henning, T.D.; Müller, T.; Hostetter, D.; Knopp, C.; Johansson, M.; Reinhart, V.; Boddington, S.; et al. Optical imaging of cellular immunotherapy against prostate cancer. Mol. Imaging 2009, 8, 15–26. [Google Scholar] [PubMed]
- Selt, M.; Tennstaedt, A.; Beyrau, A.; Nelles, M.; Schneider, G.; Löwik, C.; Hoehn, M. In vivo non-invasive tracking of macrophage recruitment to experimental stroke. PLoS ONE 2016, 11, e0156626. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kalimuthu, S.; Ahn, B.C. In vivo cell tracking with bioluminescence imaging. Nucl. Med. Mol. Imaging 2015, 49, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, M.R.; Cariati, M.; Kothari, A.; Tuch, D.S.; Purushotham, A. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clin. Transl. Imaging 2016, 4, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Xu, L.; Gong, H.; Zhu, W.; Wang, C.; Xu, J.; Feng, L.; Cheng, L.; Peng, R.; Liu, Z. Antigen-loaded upconversion nanoparticles for dendritic cell stimulation, tracking, and vaccination in dendritic cell-based immunotherapy. ACS Nano 2015, 9, 6401–6411. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, L.; Xu, H.; Liu, Z. Towards whole-body imaging at the single cell level using ultra-sensitive stem cell labeling with oligo-arginine modified upconversion nanoparticles. Biomaterials 2012, 33, 4872–4881. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kutikov, A.; Shen, J.; Duan, C.; Song, J.; Han, G. Stem cell labeling using polyethylenimine conjugated (α-NaYbF(4):Tm(3+))/CaF(2) upconversion nanoparticles. Theranostics 2013, 3, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I.; Lee, K.T.; Suh, Y.D.; Hyeon, T. Upconverting nanoparticles: A versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev. 2015, 44, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Bu, W.; Zhang, S.; Liu, J.; Fan, W.; Zhou, L.; Peng, W.; Shi, J. Gd3+-ion-doped upconversion nanoprobes: Relaxivity mechanism probing and sensitivity optimization. Adv. Funct. Mater. 2013, 23, 298–307. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, X.; Peng, J.; Li, F. Core-shell lanthanide upconversion nanophosphors as four-modal probes for tumor angiogenesis imaging. ACS Nano 2013, 7, 11290–11300. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; Allport, J.R.; Graves, E.E.; Love, V.; Josephson, L.; Lichtman, A.H.; Weissleder, R. High resolution three-dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors. Cancer Res. 2003, 63, 6838–6846. [Google Scholar] [PubMed]
- Mallett, C.L.; McFadden, C.; Chen, Y.; Foster, P.J. Migration of iron-labeled KHYG-1 natural killer cells to subcutaneous tumors in nude mice, as detected by magnetic resonance imaging. Cytotherapy 2012, 14, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.T.; Cho, M.Y.; Noh, Y.W.; Chung, J.W.; Chung, B.H. Near-infrared emitting fluorescent nanocrystals-labeled natural killer cells as a platform technology for the optical imaging of immunotherapeutic cells-based cancer therapy. Nanotechnology 2009, 20, 475102. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.W.; Lin, Y.C.; Yao, P.L.; Yuan, A.; Chen, H.Y.; Shun, C.T.; Tsai, M.F.; Chen, C.H.; Yang, P.C. Tumor-associated macrophages: The double-edged sword in cancer progression. J. Clin. Oncol. 2005, 23, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Golovko, D.; Ruffell, B.; DeNardo, D.G.; Castaneda, R.; Ansari, C.; Rao, J.; Tikhomirov, G.A.; Wendland, M.F.; Corot, C.; et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin. Cancer Res. 2011, 17, 5695–5704. [Google Scholar] [CrossRef] [PubMed]
- Makela, A.V.; Gaudet, J.M.; Foster, P.J. Quantifying tumor associated macrophages in breast cancer: A comparison of iron and fluorine-based MRI cell tracking. Sci. Rep. 2017, 7, 42109. [Google Scholar] [CrossRef] [PubMed]
- Leimgruber, A.; Berger, C.; Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.P.; Waterman, P.; Figueiredo, J.L.; Kohler, R.H.; Elpek, N.; Mempel, T.R.; et al. Behavior of endogenous tumor-associated macrophages assessed in vivo using a functionalized nanoparticle. Neoplasia 2009, 11, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Naresh, N.K.; Xu, Y.; Klibanov, A.L.; Vandsburger, M.H.; Meyer, C.H.; Leor, J.; Kramer, C.M.; French, B.A.; Epstein, F.H. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology 2012, 264, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Dengler, M.A.; van der Kuip, H.; Yildiz, H.; Rösch, S.; Klumpp, S.; Klingel, K.; Kandolf, R.; Helluy, X.; Hiller, K.-H.; et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: A human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur. Heart J. 2013, 34, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.-Y.; Lee, H.; Kim, E.-J.; Moon, H.; Chang, K.; Rho, J.; Hong, K.S. Magnetic resonance imaging of superparamagnetic iron oxide-labeled macrophage infiltrates in acute-phase renal ischemia-reperfusion mouse model. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, H.W.; Jeon, Y.H.; Singh, T.D.; Choi, Y.J.; Park, J.Y.; Kim, J.S.; Lee, H.; Hong, K.S.; Lee, I.; et al. Combined fluorescence and magnetic resonance imaging of primary macrophage migration to sites of acute inflammation using near-infrared fluorescent magnetic nanoparticles. Mol. Imaging Biol. 2015, 17, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-J.; Min, R.; Tricot, G.; Barlogie, B.; Yi, Q. Tumor lysate–specific cytotoxic T lymphocytes in multiple myeloma: Promising effector cells for immunotherapy. Blood 2002, 99, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- Manzo, T.; Heslop, H.E.; Rooney, C.M. Antigen-specific T cell therapies for cancer. Hum. Mol. Genet. 2015, 24, R67–R73. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Edinger, M.; Bachmann, M.H.; Negrin, R.S.; Fathman, C.G.; Contag, C.H. Bioluminescence imaging of lymphocyte trafficking in vivo. Exp. Hematol. 2001, 29, 1353–1360. [Google Scholar] [CrossRef]
- Gonzales, C.; Yoshihara, H.A.I.; Dilek, N.; Leignadier, J.; Irving, M.; Mieville, P.; Helm, L.; Michielin, O.; Schwitter, J. In vivo detection and tracking of T cells in various organs in a melanoma tumor model by 19F-fluorine MRS/MRI. PLoS ONE 2016, 11, e0164557. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.T.; Noh, Y.W.; Han, J.H.; Cai, Q.Y.; Yoon, K.H.; Chung, B.H. Biocompatible polymer-nanoparticle-based bimodal imaging contrast agents for the labeling and tracking of dendritic cells. Small 2008, 4, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Nam, S.H.; Kim, J.; Shin, H.S.; Suh, Y.D.; Hong, K.S. Clear-cut observation of clearance of sustainable upconverting nanoparticles from lymphatic system of small living mice. Sci. Rep. 2016, 6, 27407. [Google Scholar] [CrossRef] [PubMed]
- Bouchlaka, M.N.; Ludwig, K.D.; Gordon, J.W.; Kutz, M.P.; Bednarz, B.P.; Fain, S.B.; Capitini, C.M. 19F-MRI for monitoring human NK cells in vivo. OncoImmunology 2016, 5, e1143996. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Meier, R.; Rudelius, M.; Piontek, G.; Piert, M.; Metz, S.; Settles, M.; Uherek, C.; Wels, W.; Schlegel, J.; et al. In vivo tracking of genetically engineered, anti-HER2/neu directed natural killer cells to HER2/neu positive mammary tumors with magnetic resonance imaging. Eur. Radiol. 2005, 15, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Sheu, A.Y.; Zhang, Z.; Omary, R.A.; Larson, A.C. MRI-monitored transcatheter intra-arterial delivery of SPIO-labeled natural killer cells to hepatocellular carcinoma: Preclinical studies in a rodent model. Investig. Radiol. 2013, 48, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.S.; Shin, J.H.; Ren, G.; Park, M.J.; Cheng, K.; Chen, X.; Wu, J.C.; Sunwoo, J.B.; Cheng, Z. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials 2012, 33, 5584–5592. [Google Scholar] [CrossRef] [PubMed]
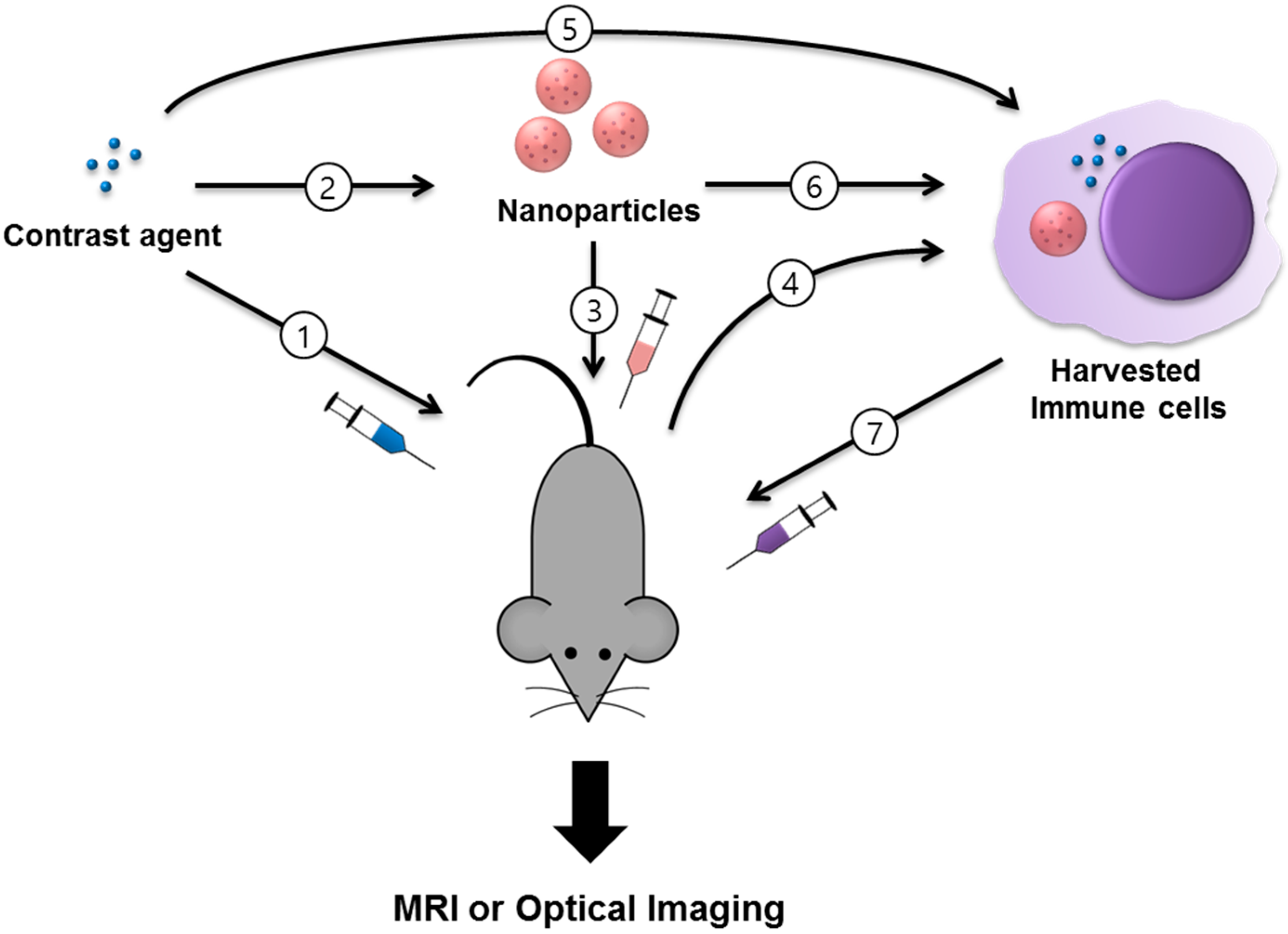
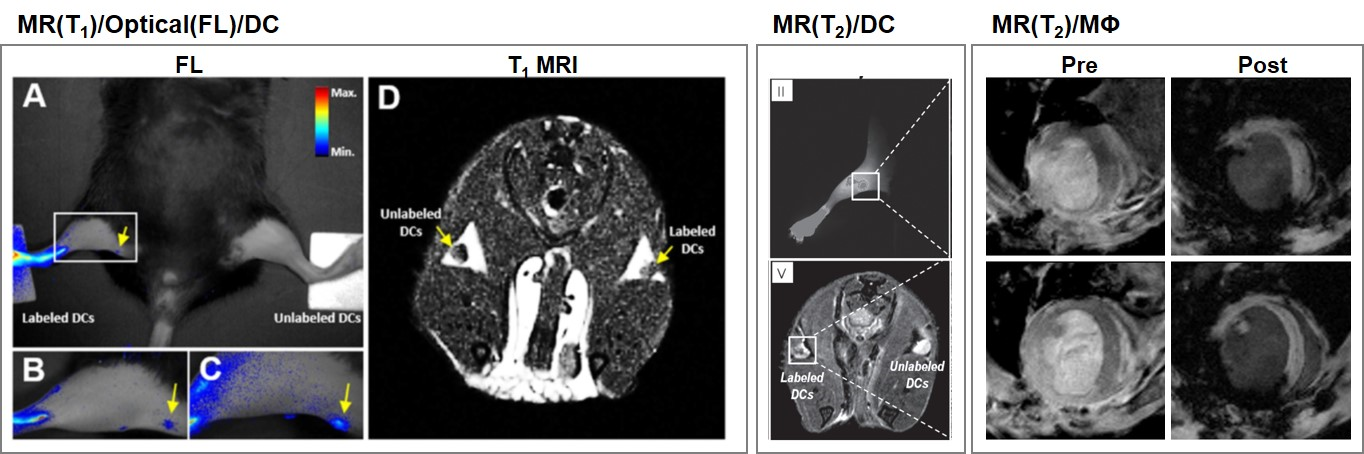
| Imaging Modality | Type | Labeled Cell Type | Contrast Agent | Animal Model | Applications (Target) | Tracking Time | Administration | Ref. |
|---|---|---|---|---|---|---|---|---|
| MR | T2 | T cell | SPIO | Tumor | B16 melanoma cell | 36 h | i.p. | [39] |
| MR | T2 | Dendritic cell | SPIO | Immunized | Lymph node mapping | 72 h | footpad | [18] |
| MR | T2 | NK-92-scFv(MOC31)-zeta cells | SPIO | Tumor | EpCAM-positive DU145 prostate cancer cell | 24 h | i.v. | [24] |
| MR | T2 | Novel NK cell line (KHYG-1) | USPIO | Tumor | PC-3M human prostate cancer cell | 4 days | i.v., i.p., s.c. | [40] |
| MR/Optical | T2/BLI | Macrophage/monocyte | SPIO | Stroke | Brain imaging | 72 h | i.v. | [29] |
| MR/Optical | T1/FL | Dendritic cell | Gd | Normal | Lymph node mapping | 24 h | footpad | [11] |
| Optical | FL | NK-92-scFv(MOC31)-zeta cells | DiD | Tumor | EpCAM-positive DU145 prostate cancer cell | 24 h | i.v. | [28] |
| Optical | FL | NK92MI | QD | tumor | MeWo human melanoma cell | 24 h | i.t. | [41] |
| Optical | PL | Mouse mesenchymal stem cell | UCNP | Normal | Biodistribution | 24 h | s.c. | [34] |
| Optical | PL | Dendritic cell | UCNP | Immunized | Lymph node mapping | 48 h | footpad | [33] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seth, A.; Park, H.S.; Hong, K.S. Current Perspective on In Vivo Molecular Imaging of Immune Cells. Molecules 2017, 22, 881. https://doi.org/10.3390/molecules22060881
Seth A, Park HS, Hong KS. Current Perspective on In Vivo Molecular Imaging of Immune Cells. Molecules. 2017; 22(6):881. https://doi.org/10.3390/molecules22060881
Chicago/Turabian StyleSeth, Anushree, Hye Sun Park, and Kwan Soo Hong. 2017. "Current Perspective on In Vivo Molecular Imaging of Immune Cells" Molecules 22, no. 6: 881. https://doi.org/10.3390/molecules22060881
APA StyleSeth, A., Park, H. S., & Hong, K. S. (2017). Current Perspective on In Vivo Molecular Imaging of Immune Cells. Molecules, 22(6), 881. https://doi.org/10.3390/molecules22060881