Yeast Extract Stimulates Ginsenoside Production in Hairy Root Cultures of American Ginseng Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactors
Abstract
:1. Introduction
2. Results
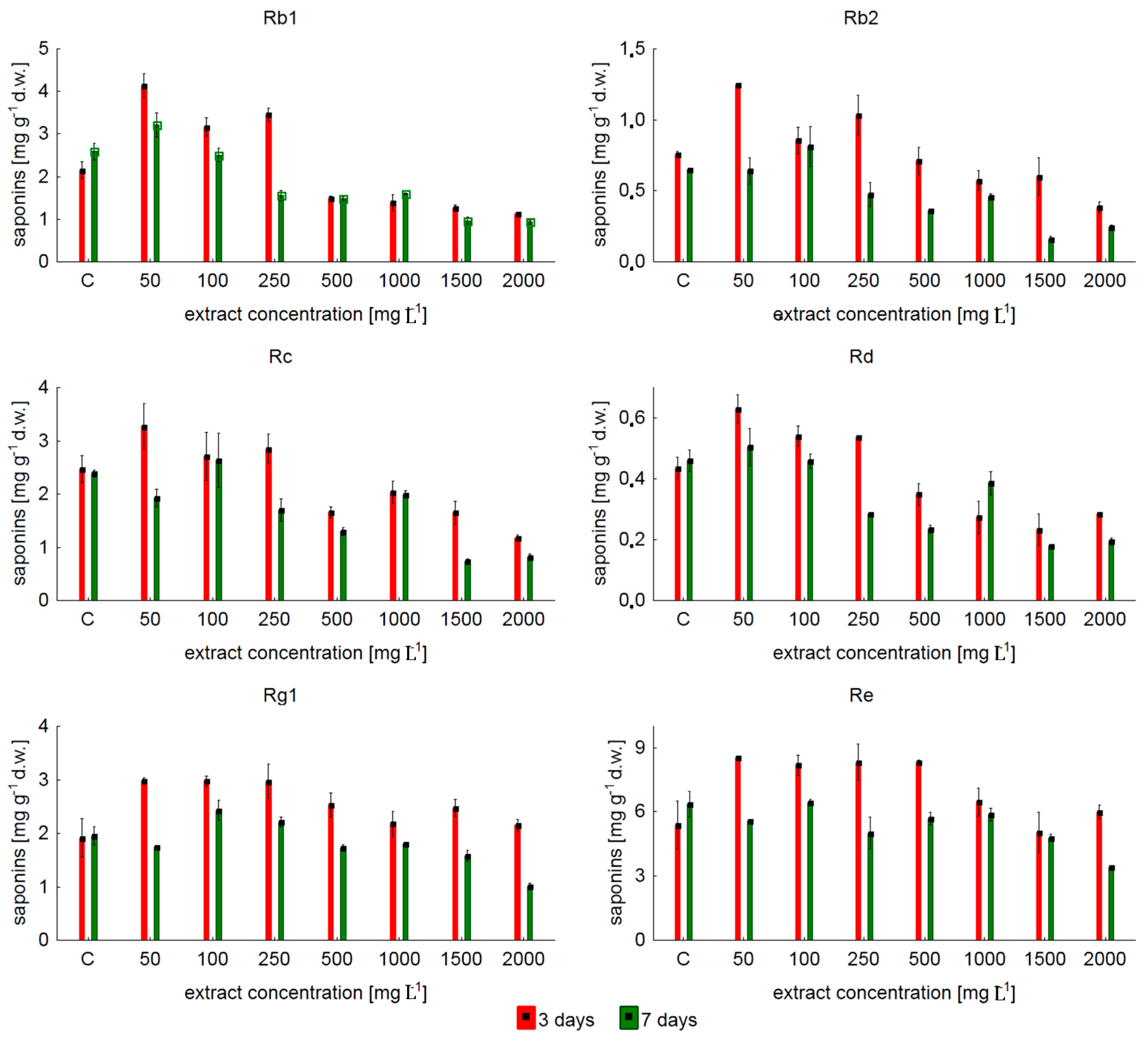
2.1. Effect of the Concentration of Yeast Extract on Ginsenoside Accumulation in Shake Flask Cultures
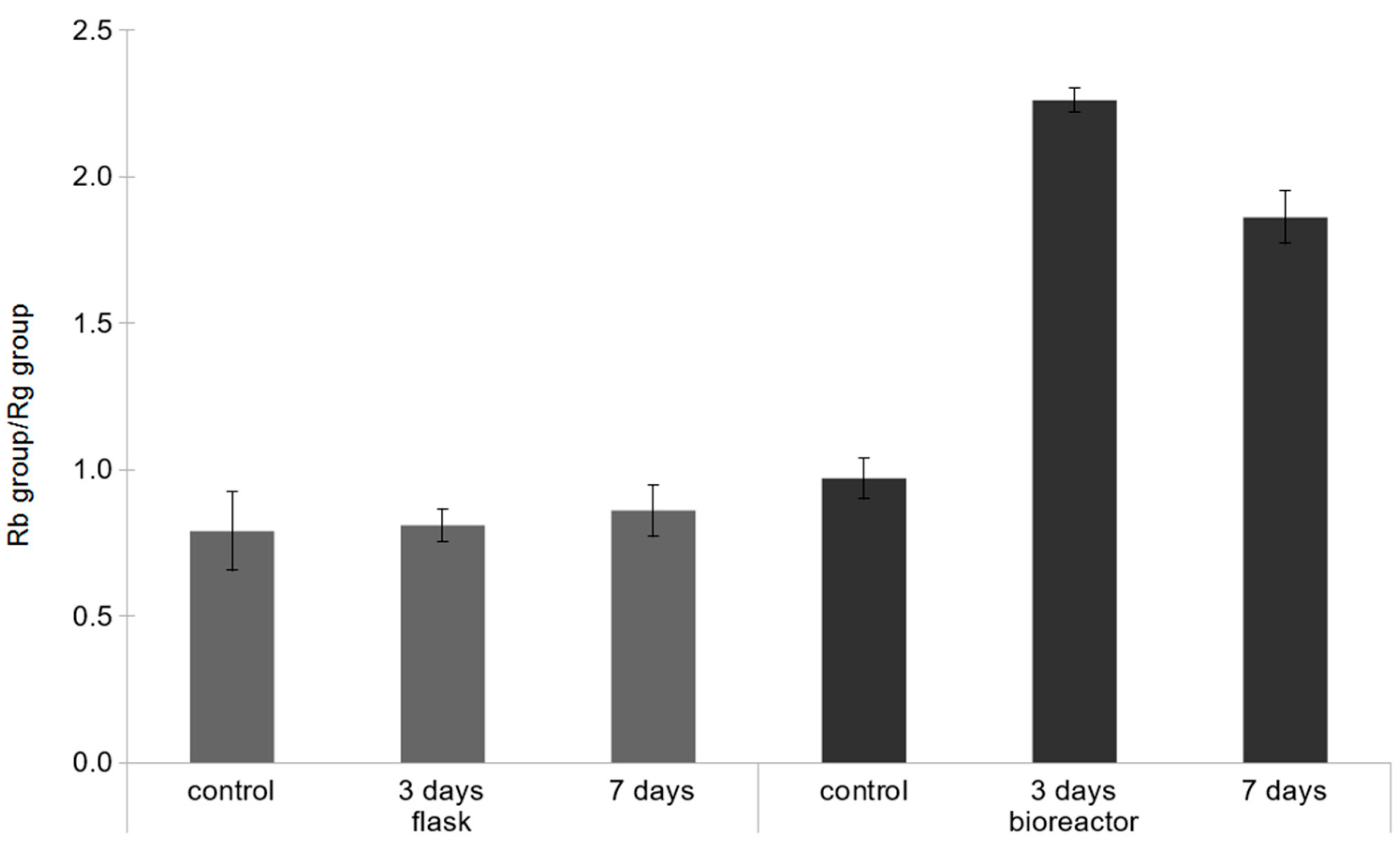
2.2. Effect of the Concentration of Yeast Extract on Ginsenoside Accumulation in Bioreactor Culture
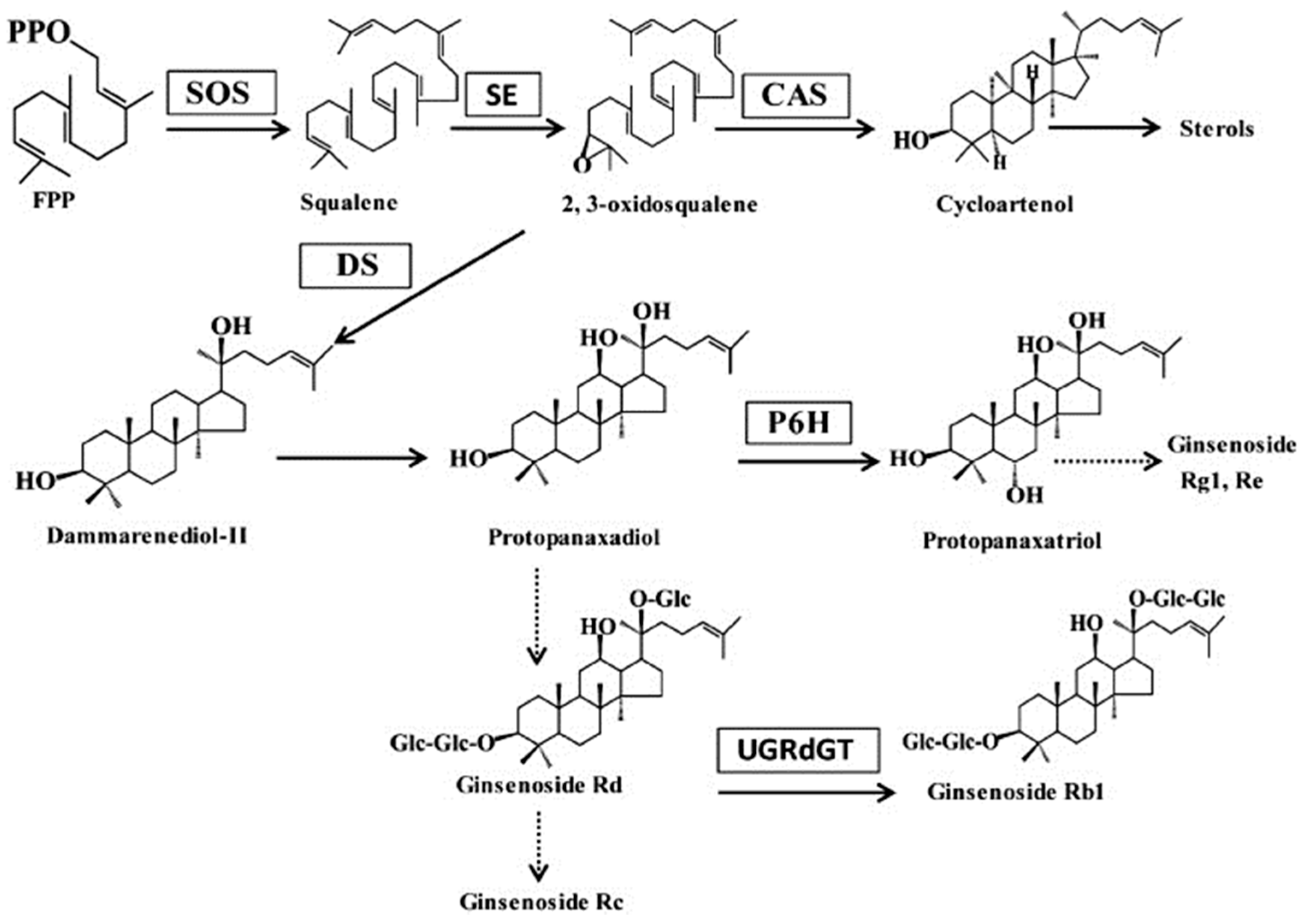
3. Discussion
4. Materials and Methods
4.1. Hairy Root Culture
4.2. Shake Flasks Cultures
4.3. Hairy Roots in the Nutrient Sprinkle Bioreactor
4.4. Elicitation Process
4.5. Ginsenoside Content Determination
4.5.1. Sample Preperation
4.5.2. Solid Phase Extraction (SPE) Steps
4.6. Quantitative Analysis of Ginsenosides
4.6.1. Standard Solution and Standard Curve Preparation
4.6.2. HPLC Analysis of Ginsenosides
4.6.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Pitta-Alvarez, S.I.; Spollansky, T.C.; Giulietti, A.M. The influence of different biotic and abviotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzym. Microb. Technol. 2000, 26, 252–258. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Chiu, F.C.K.; Lo, C.M.Y. The effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza. Enzym. Microb. Technol. 2001, 28, 100–105. [Google Scholar] [CrossRef]
- Ghorpade, R.P.; Chopra, A.; Nikam, T.D. Influence of biotic and abiotic elicitors on four major isomers of boswellic acid in callus culture of Boswellia serrata Roxb. Plant Omics 2011, 4, 169–176. [Google Scholar]
- Loc, N.H.; Anh, N.H.T.; Khuyen, L.T.M.; An, T.N.T. Effects of yeast extract and methyl jasmonate on the enhancement of solasodine biosynthesis in cell cultures of Solanum hainanense Hance. J. Biosci. Biotechnol. 2014, 3, 1–6. [Google Scholar]
- Shinde, A.M.; Malpathak, N.; Fulzele, D.P. Enhanced production of phytoestrogenic isoflavones from hairy root cultures of Psoralea corylifolia L. using elicitation and precursor feeding. Biotechnol. Bioprocess Eng. 2009, 14, 288–294. [Google Scholar] [CrossRef]
- Udomsuk, L.; Jarukamjorn, K.; Tanaka, H.; Putalun, W. Improved isoflavonoid production in Pueraria candollei hairy root cultures using elicitation. Biotechnol. Lett. 2011, 33, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Pirian, K.; Piri, K. Influence of yeast extract as a biotic elicitor on noradrenaline production in hairy root culture of Portulaca oleracea L. Int. J. Agron. Plant Prod. 2013, 4, 2960–2964. [Google Scholar]
- Hasanloo, T.; Rahnama, H.; Sepehrifar, R.; Shams, M.R. The Influence of yeast extract on the production of flavonolignans in hairy root cultures of Silybum marianum L. Gaertn. Biomedica 2008, 21, 358–361. [Google Scholar]
- Wawrosch, C.; Schwaiger, S.; Stuppner, H.; Kopp, B. Lignan formation in hairy root cultures of Edelweiss (Leontopodium nivale ssp. Alpinum (Cass.) Greuter). Fitoterapia 2014, 97, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Bauer, N.; Kiseljak, D.; Jelaska, S. The effect of yeast extract and methyl jasmonate on rosmarinic acid accumulation in Coleus blumei hairy roots. Biol. Plant 2009, 53, 650–656. [Google Scholar] [CrossRef]
- Kang, S.; Min, H. Ginseng, the ‘immunity boost’: The effects of Panax ginseng on immune system. J. Ginseng Res. 2012, 36, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Nah, S.Y. Ginseng ginsenoside pharmacology in the nervous system: Involvement in the regulation of ion channels and receptors. Front. Physiol. 2014, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Fernandez-Moriano, C.; Gómez-Serranillos, M.P. Potential neuroprotective activity of Ginseng in Parkinson’s disease: A review. J. Neuroimmune Pharmacol. 2015, 10, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Li, T.; Fong, C.M.V.; Chen, X.; Chen, X.; Wang, Y.T.; Huang, M.Q.; Lu, J.J. Saponins from Chinese medicines as anticancer agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.B.; Wong, H.L.; Teng, W.L. Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep. 2001, 20, 647–677. [Google Scholar]
- Hu, X.; Neill, S.; Cai, W.; Tang, Z. Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid-induced saponin accumulation in suspension-cultured cells of Panax ginseng. Physiol. Plant. 2003, 118, 414–421. [Google Scholar] [CrossRef]
- Ali, M.B.; Yu, K.W.; Hahn, E.J.; Paek, K.Y. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006, 25, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, X.; Neill, S.J.; Fang, J.; Cai, W. Fungal elicitor induces singlet oxygen generation, ethylene release and saponin synthesis in cultured cells of Panax ginseng CA Meyer. Plant Cell Physiol. 2005, 46, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Wong, K.; Ho, K.P.; Zhou, L.G. Enhancement of saponin production in Panax ginseng cell culture by osmotic stress and nutrient feeding. Enzym. Microb. Technol. 2005, 36, 133–138. [Google Scholar] [CrossRef]
- Huang, C.; Zhong, J.J. Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate. Process. Biochem. 2013, 48, 1227–1234. [Google Scholar] [CrossRef]
- Yu, K.W.; Gao, W.; Hahn, E.J.; Paek, K.Y. Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng CA Meyer. Biochem. Eng. J. 2002, 11, 211–215. [Google Scholar] [CrossRef]
- Yu, K.W.; Murthy, H.N.; Hahn, E.J.; Paek, K.Y. Ginsenoside production by hairy root cultures of Panax ginseng: Influence of temperature and light quality. Biochem. Eng. J. 2005, 23, 53–56. [Google Scholar] [CrossRef]
- Palazón, J.; Cusidó, R.M.; Bonfill, M.; Mallol, A.; Moyano, E.; Morales, C.; Piñol, M.T. Elicitation of different Panax ginseng transformed root phenotypes for an improved ginsenoside production. Plant Physiol. Biochnol. 2003, 41, 1019–1025. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. Copper-induced changes in the growth, oxidative metabolism, and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep. 2006, 25, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Choi, Y.E.; Shin, C.G.; Kim, Y.Y.; Kim, Y.S. Enhanced ginsenoside productivity by combination of ethephon and methyl jasmoante in ginseng (Panax ginseng CA Meyer) adventitious root cultures. Biotechnol. Lett. 2006, 28, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cao, X.; Zhang, R.; Peng, Y.; Zhao, S.; Wu, J. Stimulation of saponin production in Panax ginseng hairy roots by two oligosaccharides from Paris polyphylla var. yunnanensis. Biotechnol. Lett. 2007, 29, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cult. 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Wu, C.H.; Popova, E.V.; Hahn, E.J.; Paek, K.Y. Linoleic and α-linolenic fatty acids affect biomass and secondary metabolite production and nutritive properties of Panax ginseng adventitious roots cultured in bioreactors. Biochem. Eng. J. 2009, 47, 109–115. [Google Scholar] [CrossRef]
- Wang, J.; Gao, W.; Zuo, B.; Zhang, L.; Huang, L. Effect of methyl jasmonate on the ginsenoside content of Panax ginseng adventitious root cultures and on the genes involved in triterpene biosynthesis. Res. Chem. Intermed. 2013, 39, 1973–1980. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Li, Y.; Li, J.; Ouyang, Y.; He, Z.; Zhao, S. Enhancement of ginsenoside biosynthesis and secretion by Tween 80 in Panax ginseng hairy roots. Biotechnol. Appl. Biochem. 2014, 62, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.J.; Wang, D.J. Improvement of cell growth and production of ginseng saponin and polysaccharide in suspension cultures of Panax notoginseng: Cu2+ effect. J. Biotechnol. 1996, 46, 69–72. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, J.J. Manipulation of ginsenoside heterogeneity in cell cultures of Panax notoginseng by addition of jasmonates. J. Biosci. Bioeng. 2002, 93, 48–53. [Google Scholar] [CrossRef]
- Yue, C.; Zhong, J.J. Impact of external calcium and calcium sensors on ginsenoside Rb1 biosynthesis by Panax notoginseng cells. Biotechnol. Bioeng. 2004, 89, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Steingroewer, J.; Bley, T.; Georgiev, V.; Ivanov, I.; Lenk, F.; Marchev, A.; Pavlov, A. Bioprocessing of differentiated plant in vitro systems. Eng. Life Sci. 2013, 13, 26–38. [Google Scholar] [CrossRef]
- Simonetti, G.; Tocci, N.; Valletta, A.; Brasili, E.; D’Auria, F.D.; Idoux, A.; Pasqua, G. In vitro antifungal activity of extracts obtained from Hypericum perforatum adventitious roots cultured in a mist bioreactor against planktonic cells and biofilm of Malassezia furfur. Nat. Prod. Res. 2016, 30, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Marsik, P.; Langhansova, L.; Dvorakova, M.; Cigler, P.; Hruby, M.; Vanek, T. Increased ginsenosides production by elicutation of in vitro cultivated Panax ginseng adventitious roots. Med. Aromat. Plants 2014, 3, 1–5. [Google Scholar]
- Kochan, E.; Królicka, A.; Chmiel, A. Growth and ginsenoside production in Panax quinquefolium hairy roots cultivated in flasks and nutrient sprinkle bioreactor. Acta Physiol. Plant. 2012, 34, 1513–1518. [Google Scholar] [CrossRef]
- Hong, M.L.K.; Bhatt, A.; Ping, N.S.; Keng, C.L. Detection of elicitation effect on Hyoscyamus niger L. root cultures for the root growth and production of tropane alkaloids. Romanian Biotechnol. Lett. 2012, 17, 7340–7351. [Google Scholar]
- Wilczańska-Barska, A.; Królicka, A.; Głód, D.; Majdan, M.; Kawiak, A.; Krauze-Baranowska, M. Enhanced accumulation of secondary metabolites in hairy root cultures of Scutellaria lateriflora following elicitation. Biotechnol. Lett. 2012, 34, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.K. Effect of elicitors and precursors on azadirachtin production in hairy root culture of Azadirachta indica. Appl. Biochem. Biotechnol. 2014, 172, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.J.; Zhou, X.; Zhong, J.J. Protopanaxadiol 6-hydroxylase and its role in regulating the ginsenoside heterogeneity in Panax notoginseng cells. Biotechnol. Bioeng. 2008, 1, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qian, Z.G.; Zhong, J.J. Enhancement of ginsenoside biosynthesis in cell cultures of Panax ginseng by N,N′-dicyclohexylcarbodiimide elicitation. J. Biotechnol. 2013, 165, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Palazon, J.; Mallol, A.; Eibl, R.; Lettenbauer, C.; Cusido, R.M.; Pinol, M.T. Growth and ginsenoside production in hairy root cultures of Panax ginseng using a novel bioreactor. Planta Med. 2003, 69, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Scragg, A.H. The problems associated with high biomass levels in plant cell suspensions. Plant Cell Tissue Organ Cult. 1995, 43, 163–170. [Google Scholar] [CrossRef]
- Eibl, R.; Eibl, D. Design and use of the wave bioreactor for plant cell culture. In Plant Tissue Culture Engineering Focus on Biotechnology; Gupta, S.D., Ibaraki, Y., Eds.; Springer: Berlin, Germany, 2006; Volume 6, pp. 203–327. [Google Scholar]
- Kimball, S.R.; Jefferson, L.S. New functions for amino acids: effects on gene transcription and translation. Am. J. Clin. Nutr. 2006, 83, 500S–507S. [Google Scholar] [PubMed]
- Rahimi, S.; Devi, B.S.R.; Khorolragchaa, A.; Kim, Y.J.; Kim, J.H.; Jung, S.K.; Yang, D.C. Effect of salicylic acid and yeast extract on the accumulation of jasmonic acid and sesquiterpenoids in Panax ginseng adventitious roots. Russ. J. Plant Physiol. 2014, 61, 811–817. [Google Scholar] [CrossRef]
- Biswas, T.; Mathur, A.K.; Mathur, A. A literature update elucidating production of panax ginsenosides with a special focus on strategies enriching the anti-neoplastic minor ginsenosides in ginseng preparations. Appl. Microbiol. Biotechnol. 2017, 101, 4009–4032. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of sojabean root cells. Exp. Cell. Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Rb1, Rb2, Erc, Rd, Rg1 and Re are available from the authors. |


| Ginsenosides [mg g−1 d.w.] ± SE | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rb1 | Rc | Rb2 | Rd | Rg1 | Re | Rb group | Rg Group | Total | |
| C | 2.66 ± 0.03 a | 1.96 ± 0.05 a | 0.83 ± 0.06 a | 1.01 ± 0.04 a | 1.88 ± 0.03 a | 4.79 ± 0.09 a | 6.46 ± 0.03 a | 6.67 ± 0.12 a | 13.13 ± 0.14 a |
| 3d | 10.29 ± 0.27 b | 5.79 ± 0.07 b | 2.99 ± 0.07 b | 3.38 ± 0.13 b | 2.90 ± 0.14 b | 6.89 ± 0.11 b | 22.46 ± 0.82 b | 9.79 ± 0.25 b | 32.25 ± 0.44 b |
| 7d | 3.02 ± 0.14 a | 2.80 ± 0.12c | 0.58 ± 0.04a | 0.97 ± 0.04 a | 1.16 ± 0.01 c | 2.74 ± 0.04 c | 7.37 ± 0.03 a | 3.91 ± 0.05 c | 11.28 ± 0.38 a |
| Ginsenosides | Regresion Equation | r2 | LOD (µg mL−1) | LOQ (µg mL−1) |
|---|---|---|---|---|
| Rb1 | y = 136.13x − 1.55 | 0.9991 | 2.01 | 6.63 |
| Rb2 | y = 124.39x − 3.57 | 0.9984 | 2.88 | 9.50 |
| Rc | y = 117.58x − 1.99 | 0.9997 | 1.35 | 4.46 |
| Rd | y = 166.19x − 0.37 | 0.9987 | 2.00 | 6.60 |
| Rg1 | y = 156.21x − 2.26 | 0.9998 | 0.60 | 1.98 |
| Re | y = 143.98x − 0.64 | 0.9994 | 1.37 | 4.52 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochan, E.; Szymczyk, P.; Kuźma, Ł.; Lipert, A.; Szymańska, G. Yeast Extract Stimulates Ginsenoside Production in Hairy Root Cultures of American Ginseng Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactors. Molecules 2017, 22, 880. https://doi.org/10.3390/molecules22060880
Kochan E, Szymczyk P, Kuźma Ł, Lipert A, Szymańska G. Yeast Extract Stimulates Ginsenoside Production in Hairy Root Cultures of American Ginseng Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactors. Molecules. 2017; 22(6):880. https://doi.org/10.3390/molecules22060880
Chicago/Turabian StyleKochan, Ewa, Piotr Szymczyk, Łukasz Kuźma, Anna Lipert, and Grażyna Szymańska. 2017. "Yeast Extract Stimulates Ginsenoside Production in Hairy Root Cultures of American Ginseng Cultivated in Shake Flasks and Nutrient Sprinkle Bioreactors" Molecules 22, no. 6: 880. https://doi.org/10.3390/molecules22060880







