Photoreactions of Endohedral Metallofullerene with Siliranes: Electronic Properties of Carbosilylated Lu3N@Ih-C80 †
Abstract
:1. Introduction
2. Results and Discussion
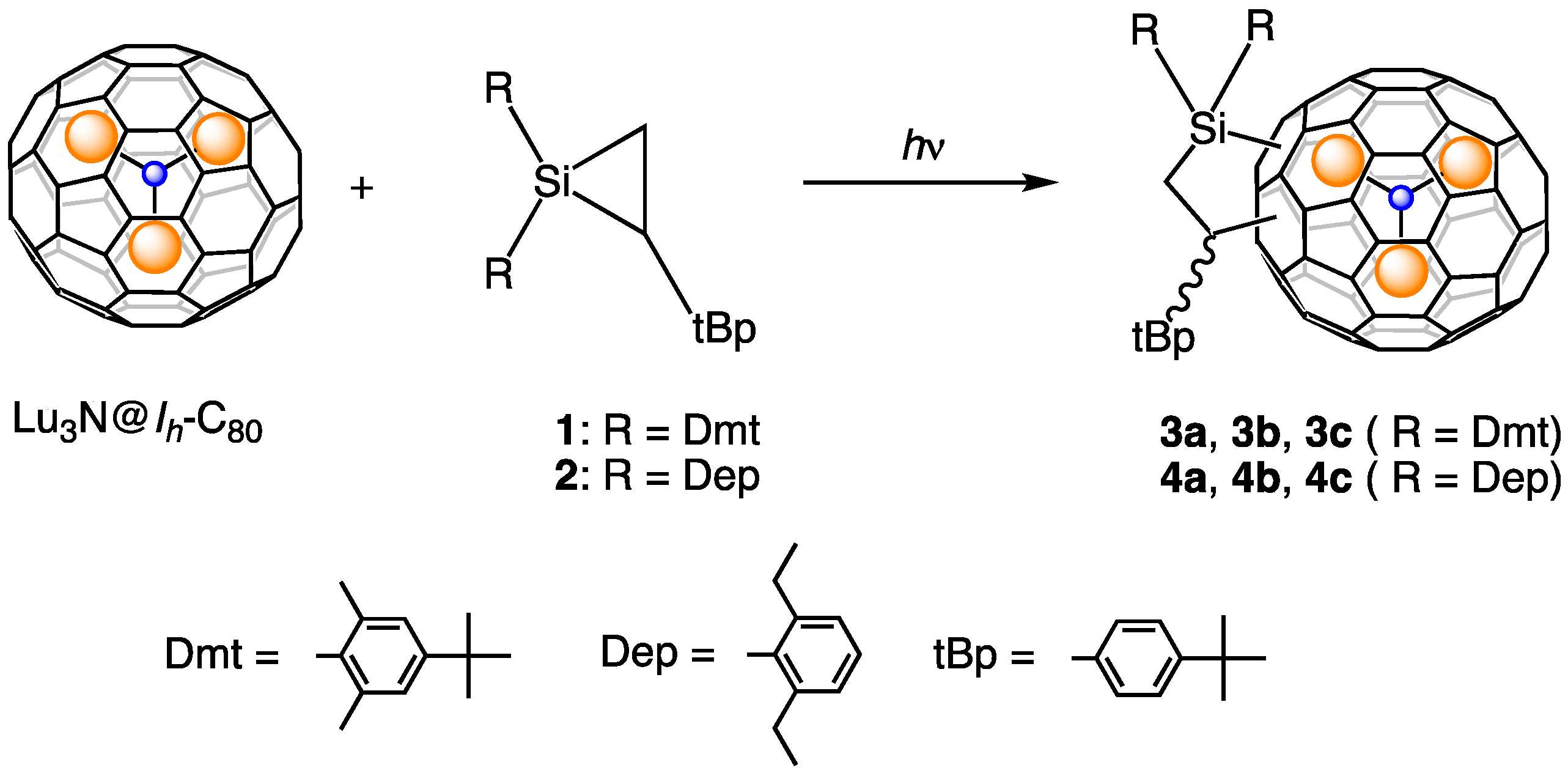
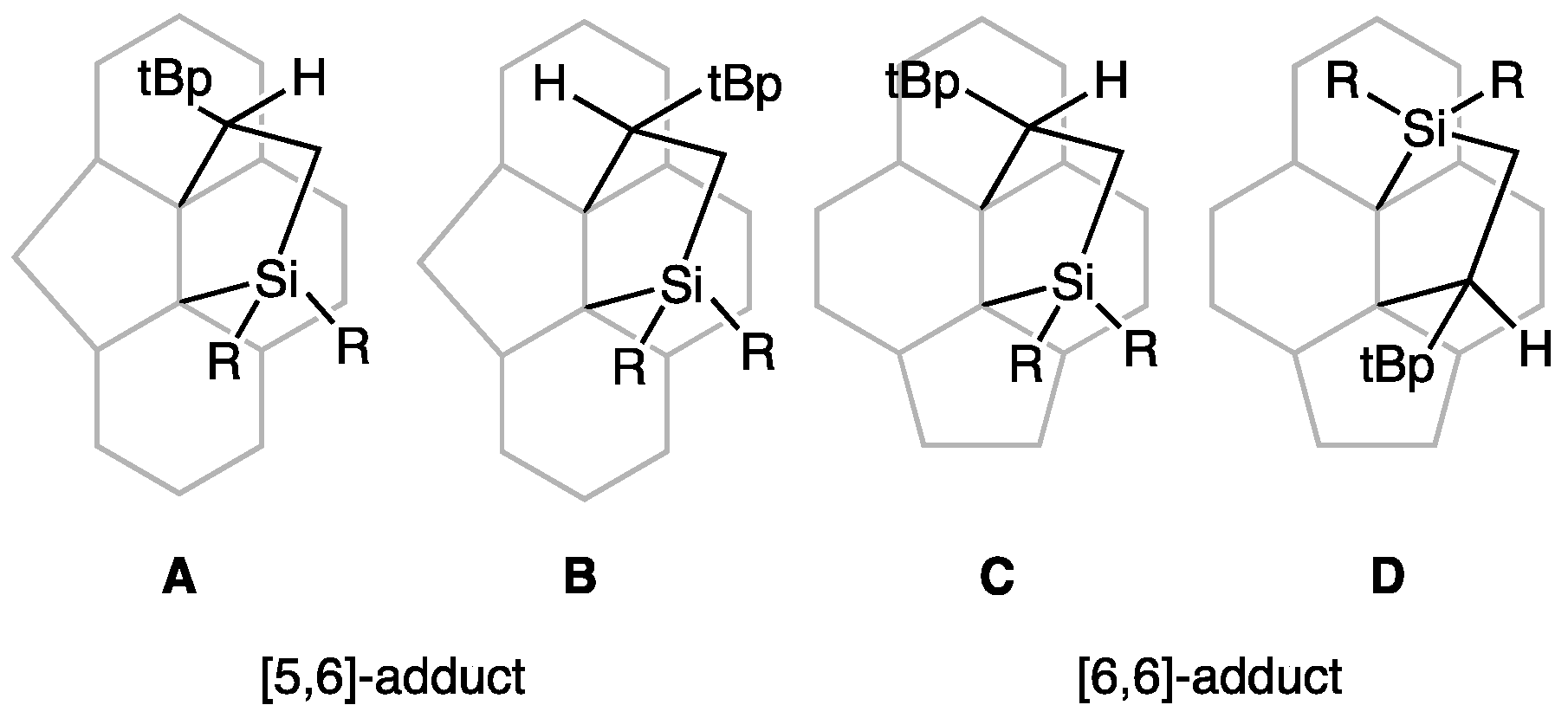
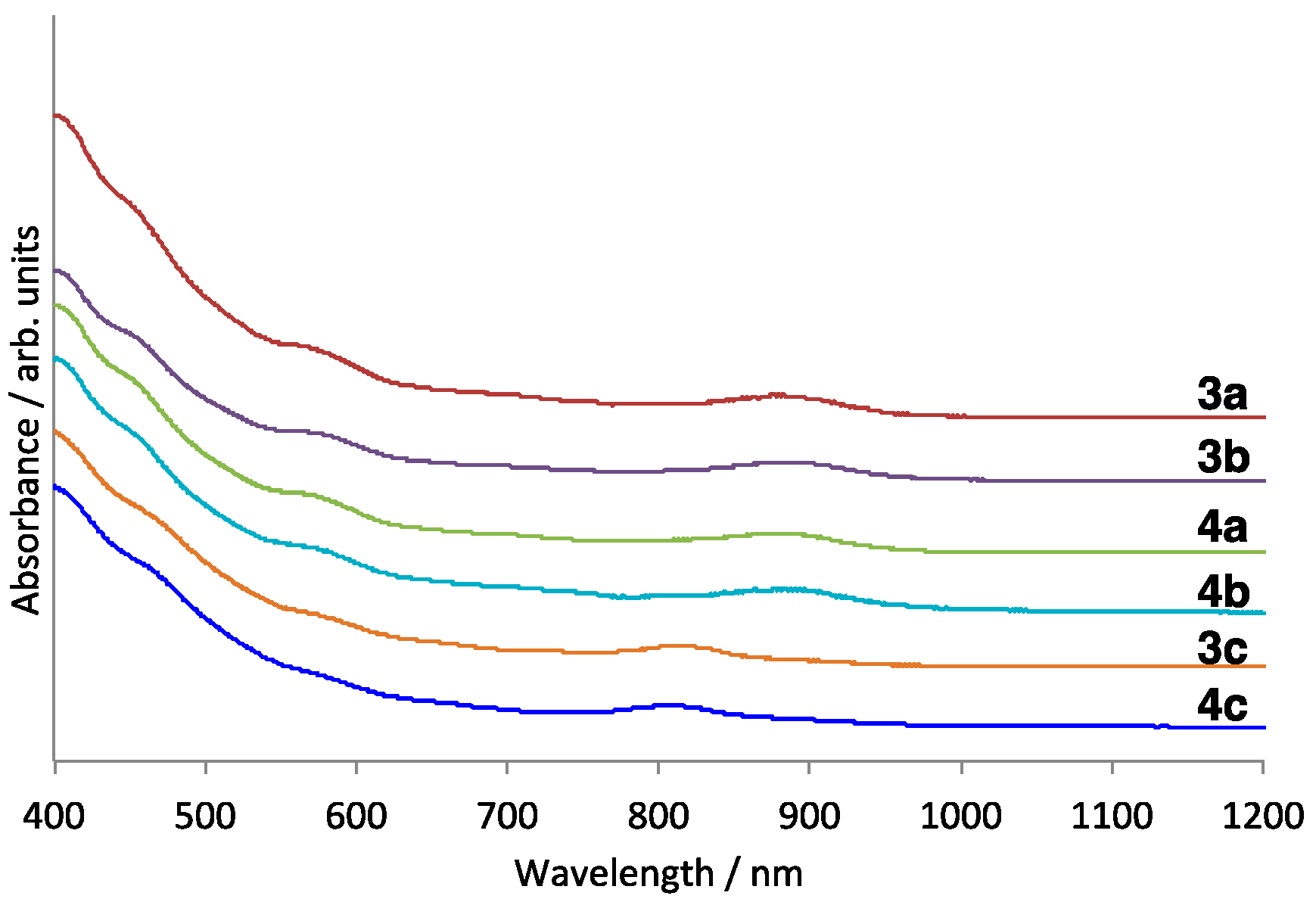
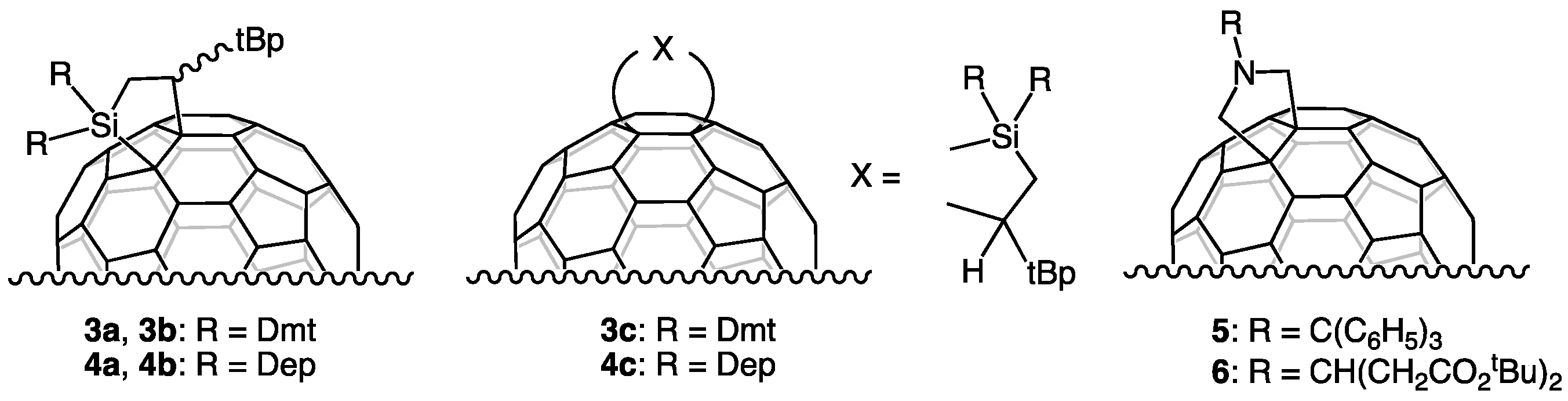
2.1. Preparation and Structural Analysis of the Carbosilylated Derivatives of Lu3N@Ih-C80
2.2. Electrochemical Studies
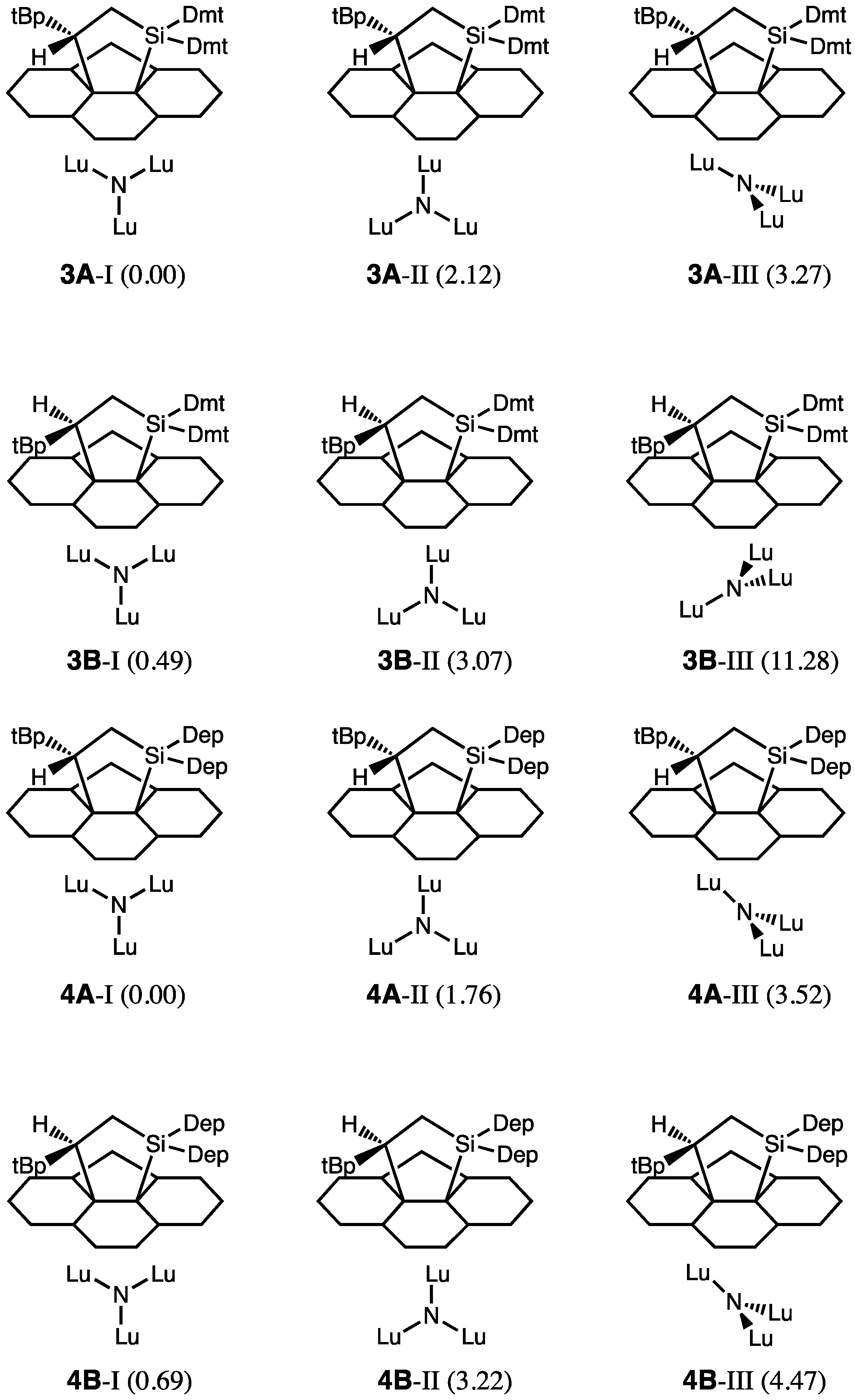
2.3. Theoretical Calculations
3. Materials and Methods
3.1. General
3.2. Photoreaction of Lu3N@Ih-C80 with 1
3.3. Photoreaction of Lu3N@Ih-C80 with 2
3.4. Computational Method
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akasaka, T.; Nagase, S. (Eds.) Endofullerenes: A New Family of Carbon Clusters; Kluwer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Dunsch, L.; Yang, S. Metal nitride cluster fullerenes: Their current state and future prospects. Small 2007, 8, 1298–1320. [Google Scholar] [CrossRef] [PubMed]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, electrochemical, and structural properties of endohedral metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Akasaka, T.; Nagase, S. Endohedral metal atoms in pristine and functionalized fullerene cages. Acc. Chem. Res. 2010, 43, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Wudl, F.; Nagase, S. (Eds.) Chemistry of Nanocarbons; Wiley: Chichester, UK, 2010. [Google Scholar]
- Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 2012, 41, 7723–7760. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Nazario, D.M.; Pinzón, J.R.; Stevenson, S.; Echegoyen, L.A. Buckyball maracas: Exploring the inside and outside properties of endohedral fullerenes. J. Phys. Org. Chem. 2013, 26, 194–205. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T.; Nagase, S. Carbene additions to fullerenes. Chem. Rev. 2013, 113, 7209–7264. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Akasaka, T.; Nagase, S. Carbide cluster metallofullerenes: Structure, properties, and possible origin. Acc. Chem. Res. 2013, 46, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef] [PubMed]
- Nagase, S. Theory and calculations of molecules containing heavier main group elements and fullerenes encaging transition metals: Interplay with experiment. Bull. Chem. Soc. Jpn. 2014, 87, 167–195. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T. Emergence of highly elaborated π-space and extending its functionality based on nanocarbons: New vistas in the fullerene world. Bull. Chem. Soc. Jpn. 2014, 87, 1289–1314. [Google Scholar] [CrossRef]
- Stevenson, S.; Rice, G.; Glass, T.; Harich, K.; Cromer, F.; Jordan, M.R.; Craft, J.; Bible, R.; Olmstead, M.M.; Maitra., K.; et al. Small-bandgap endohedral metallofullerenes in high yield and purity. Nature 1999, 401, 55–57. [Google Scholar]
- Zhang, J.; Stevenson, S.; Dorn, H.C. Trimetallic nitride template endohedral metallofullerenes: Discovery, structural characterization, reactivity, and applications. Acc. Chem. Res. 2013, 46, 1458–1557. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.B.; Cardona, C.M.; Guldi, D.M.; Sankaranarayanan, S.G.; Reese, M.O.; Kopidakis, N.; Peet, J.; Walker, B.; Bazan, G.C.; Van Keuren, E.; et al. Endohedral fullerenes for organic photovoltaic devices. Nat. Mater. 2009, 8, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.B.; Cardona, C.M.; Swain, F.B.; Guldi, D.M.; Sankaranarayanan, S.G.; Van Keuren, E.; Holloway, B.C.; Drees, M. Tuning conversion efficiency in metallo endohedral fullerene-based organic photovoltaic devices. Adv. Funct. Mater. 2009, 19, 2332–2337. [Google Scholar] [CrossRef]
- Liedtke, M.; Sperlich, A.; Kraus, H.; Baumann, A.; Deibel, C.; Wirix, M.J.; Loos, J.; Cardona, C.M.; Dyakonov, V. Triplet exciton generation in bulk-heterojunction solar cells based on endohedral fullerenes. J. Am. Chem. Soc. 2011, 133, 9088–9094. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kako, M.; Suzuki, M.; Mizorogi, N.; Tsuchiya, T.; Olmstead, M.M.; Balch, A.L.; Akasaka, T.; Nagase, S. Synthesis of silylene-bridged endohedral metallofullerene Lu3N@Ih-C80. J. Am. Chem. Soc. 2012, 134, 16033–16039. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kako, M.; Mizorogi, N.; Tsuchiya, T.; Akasaka, T.; Nagase, S. Bis-silylation of Lu3N@Ih-C80: Considerable variation in the electronic structures. Org. Lett. 2012, 14, 5908–5911. [Google Scholar] [CrossRef] [PubMed]
- Kako, M.; Miyabe, M.; Sato, K.; Suzuki, M.; Mizorogi, N.; Wang, W.-W.; Yamada, M.; Maeda, Y.; Olmstead, M.M.; Balch, A.L.; et al. Preparation, structural determination, and characterization of electronic properties of bis-silylated and bis-germylated Lu3N@Ih-C80. Chem. Eur. J. 2015, 21, 16411–16420. [Google Scholar] [CrossRef] [PubMed]
- Kako, M.; Sugiura, T.; Akasaka, T. Photochemical addition of silirane to endohedral metallofullerene: Electronic properties of carbosilylated Sc3N@Ih-C80. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 201–206. [Google Scholar] [CrossRef]
- Nagatsuka, J.; Sugitani, S.; Kako, M.; Nakahodo, T.; Mizorogi, N.; Ishitsuka, M.O.; Maeda, Y.; Tsuchiya, T.; Akasaka, T.; Gao, X.; et al. Photochemical addition of C60 with siliranes: Synthesis and characterization of carbosilylated and hydrosilylated C60 derivatives. J. Am. Chem. Soc. 2010, 132, 12106–12120. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Pinzón, J.R.; Echegoyen, L. Influence of the encapsulated clusters on the electrochemical behaviour of endohedral fullerene derivatives: Comparative study of N-tritylpyrrolidino derivatives of Sc3N@Ih-C80 and Lu3N@Ih-C80. ChemPhysChem 2011, 12, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Aroua, S.; Yamakoshi, Y. Prato reaction of M3N@Ih-C80 (M = Sc, Lu, Y, Gd) with reversible isomerization. J. Am. Chem. Soc. 2012, 134, 20242–20245. [Google Scholar] [CrossRef] [PubMed]
- Aroua, S.; Garcia-Borras, M.; Osuna, S.; Yamakoshi, Y. Rearrangement of M3N@Ih-C80 fulleropyrrolidines: Exohedral functional groups versus endohedral metal clusters. Chem. Eur. J. 2014, 20, 14032–14039. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Densityfunctional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01, Gaussian, Inc.: Wallingford, CT, USA, 2013.
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Cao, X.Y.; Dolg, M. Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J. Mol. Struct. 2002, 581, 139–147. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| compound | Eox1 | Ered1 | Ered2 |
|---|---|---|---|
| Lu3N@Ih-C80 b | +0.61 | –1.39 | –1.83 |
| 3a | +0.23 c | –1.38 | –1.73 |
| 3b | +0.25 c | –1.35 | –1.71 |
| 4a | +0.22 c | –1.37 | –1.75 |
| 4b | +0.21 c | –1.41 | –1.77 |
| 5d | –1.13 | –1.64 | |
| 6e | –1.14 |
| compound | HOMO | LUMO |
|---|---|---|
| Lu3N@Ih-C80 | –5.47 | –2.90 |
| 3A-I | –4.98 | –2.90 |
| 3B-I | –4.99 | –2.91 |
| 4A-I | –5.01 | –2.92 |
| 4B-I | –5.02 | –2.95 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kako, M.; Minami, K.; Kuroiwa, T.; Fukazawa, S.; Arikawa, Y.; Yamada, M.; Maeda, Y.; Li, Q.-Z.; Nagase, S.; Akasaka, T. Photoreactions of Endohedral Metallofullerene with Siliranes: Electronic Properties of Carbosilylated Lu3N@Ih-C80. Molecules 2017, 22, 850. https://doi.org/10.3390/molecules22050850
Kako M, Minami K, Kuroiwa T, Fukazawa S, Arikawa Y, Yamada M, Maeda Y, Li Q-Z, Nagase S, Akasaka T. Photoreactions of Endohedral Metallofullerene with Siliranes: Electronic Properties of Carbosilylated Lu3N@Ih-C80. Molecules. 2017; 22(5):850. https://doi.org/10.3390/molecules22050850
Chicago/Turabian StyleKako, Masahiro, Kazuya Minami, Taiki Kuroiwa, Shinpei Fukazawa, Yuki Arikawa, Michio Yamada, Yutaka Maeda, Qiao-Zhi Li, Shigeru Nagase, and Takeshi Akasaka. 2017. "Photoreactions of Endohedral Metallofullerene with Siliranes: Electronic Properties of Carbosilylated Lu3N@Ih-C80" Molecules 22, no. 5: 850. https://doi.org/10.3390/molecules22050850






