Loss of the Phenolic Hydroxyl Group and Aromaticity from the Side Chain of Anti-Proliferative 10-Methyl-aplog-1, a Simplified Analog of Aplysiatoxin, Enhances Its Tumor-Promoting and Proinflammatory Activities
Abstract
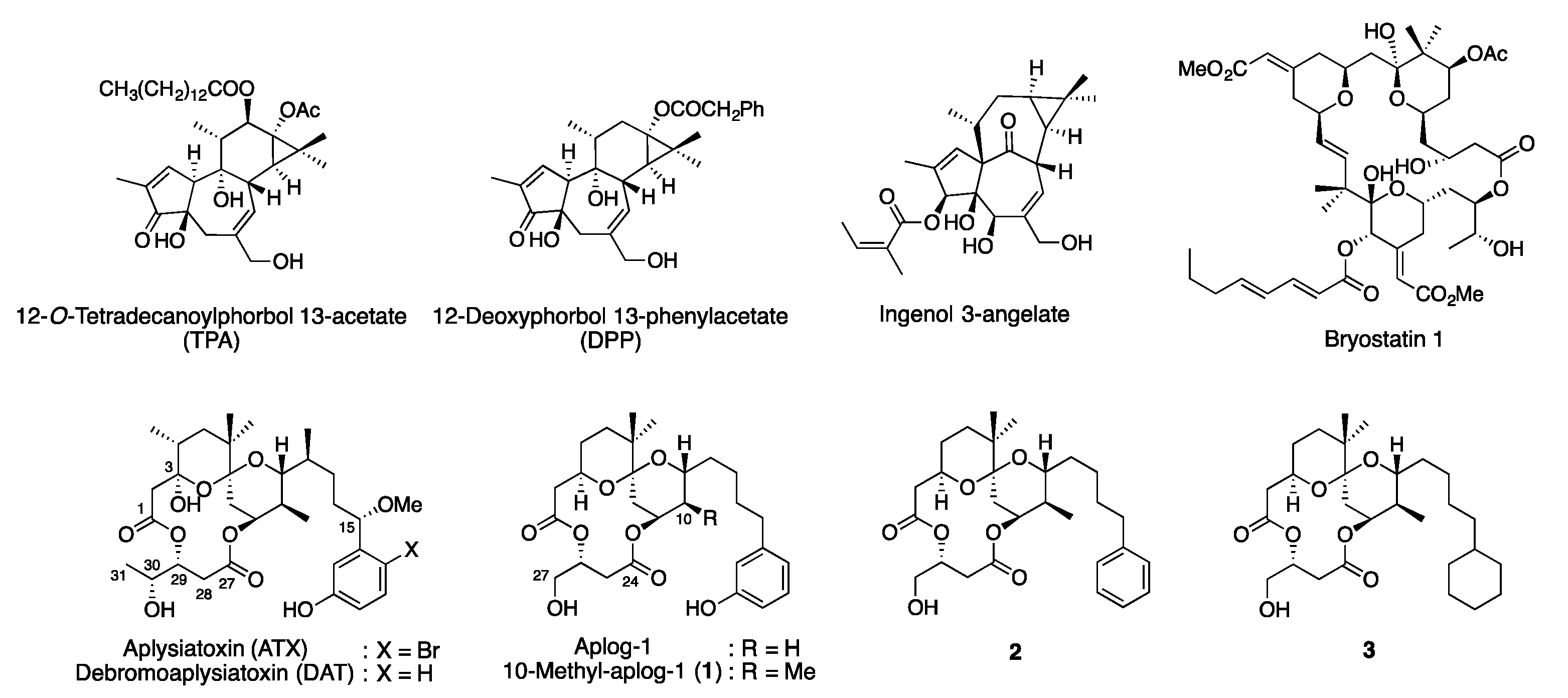
:1. Introduction
2. Results and Discussions
2.1. Anti-Proliferative Activity of 2 and 3 towards 39 Human Cancer Cell Lines
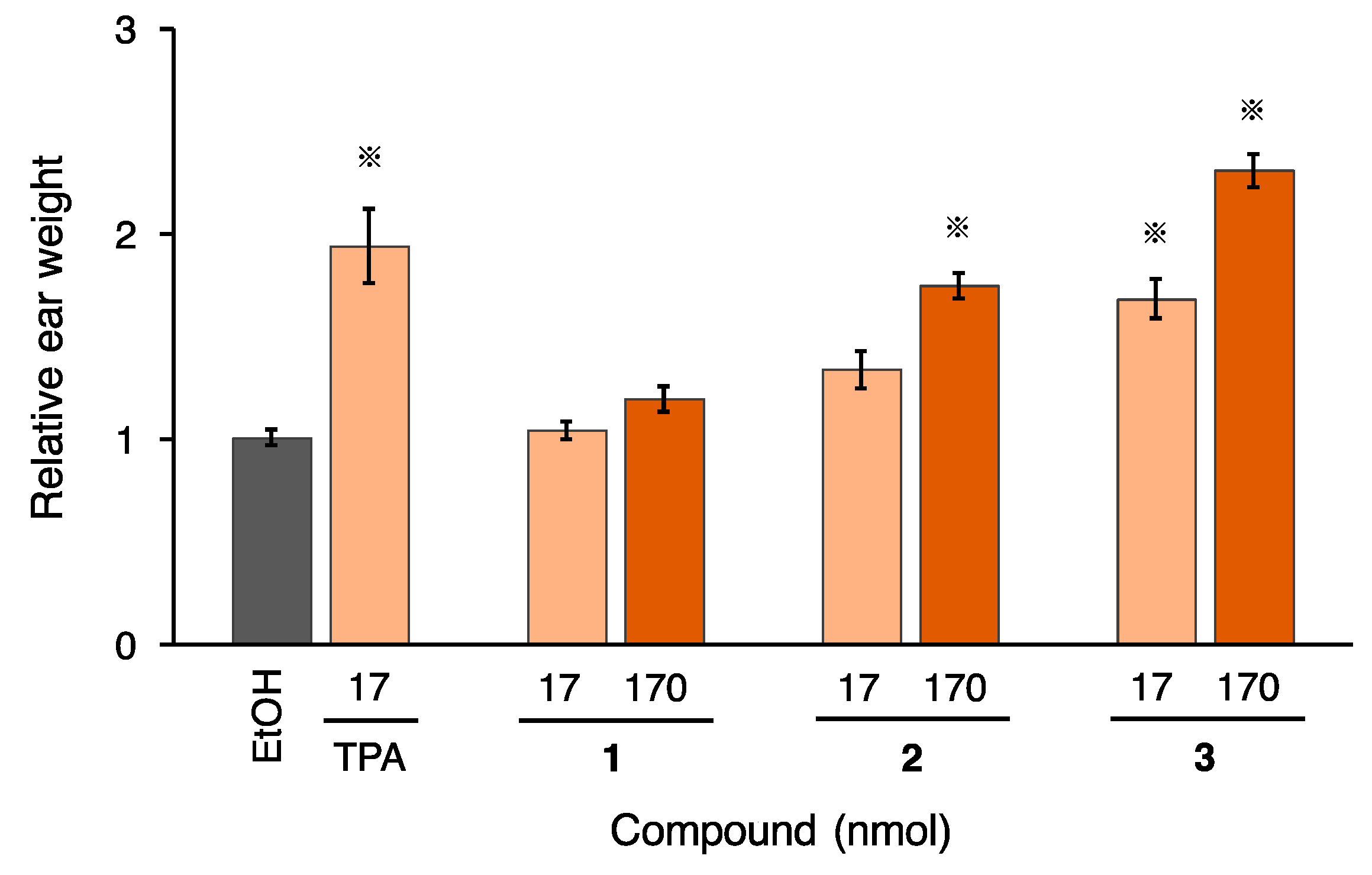
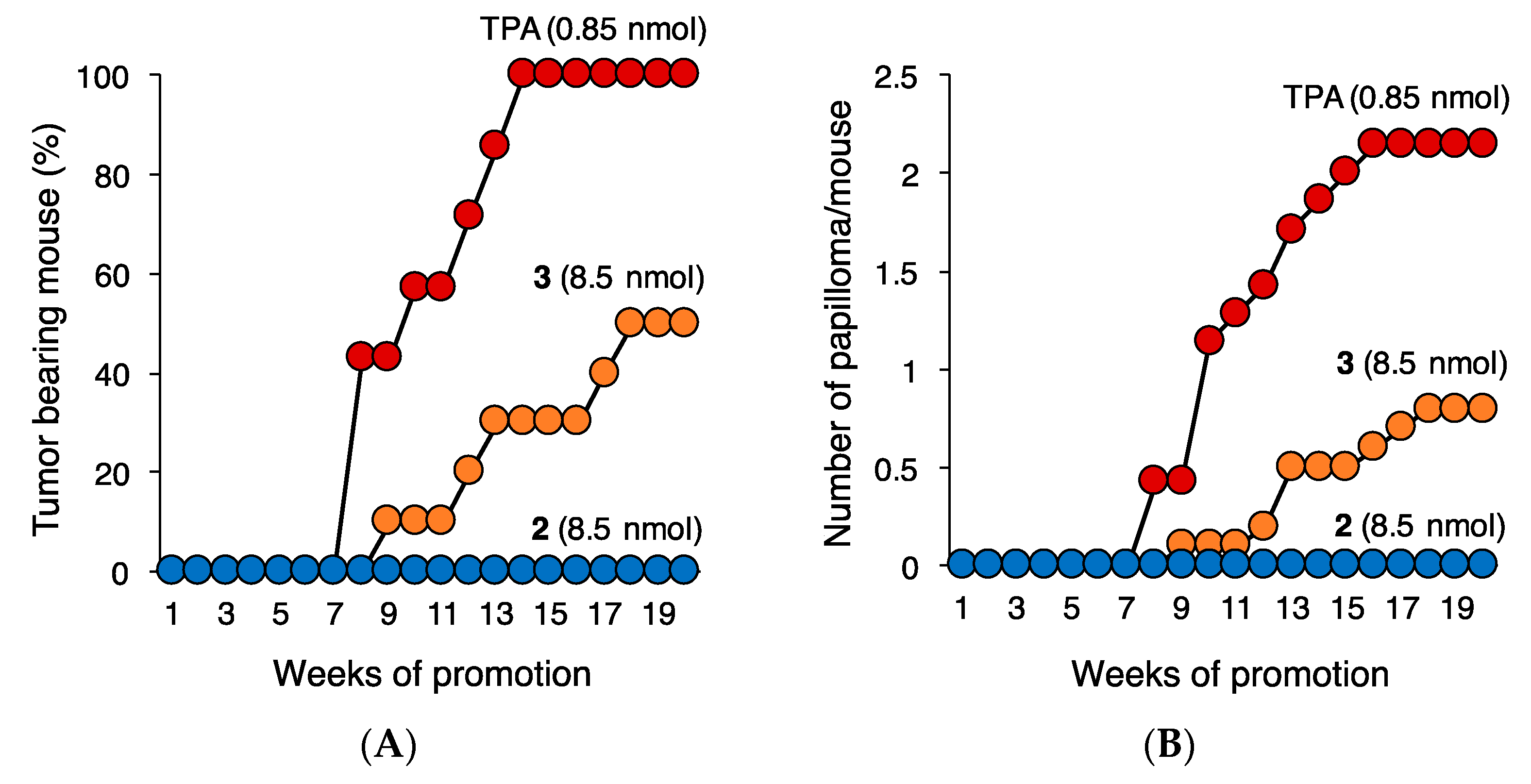
2.2. Proinflammatory and Tumor-Promoting Activity of 2 and 3
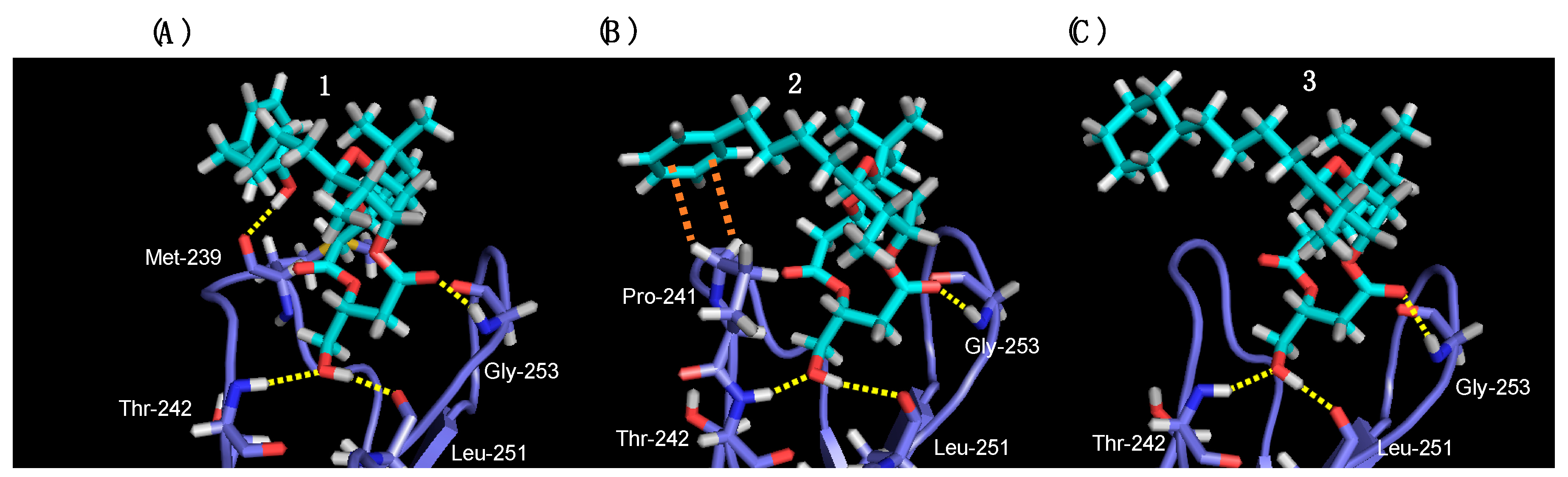
2.3. Binding Affinity of 2 and 3 for PKCδ-C1B Domain
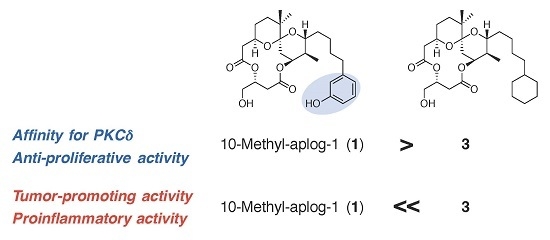
2.4. Structure–Activity Relationship in the Phenolic Moiety of 10-Methyl-aplog-1 (1)
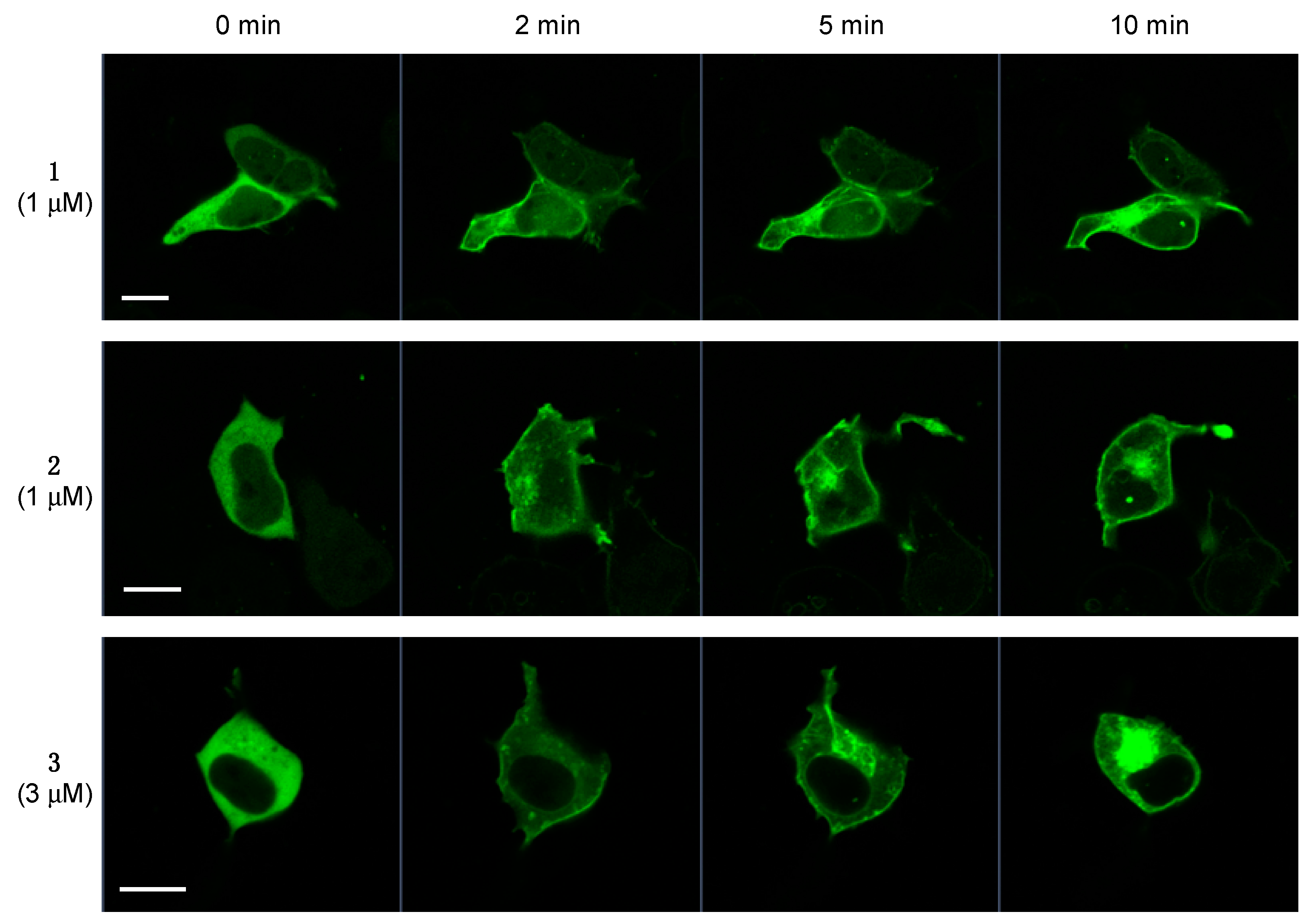
2.5. Translocation of PKCδ-GFP Induced by 1–3
3. Materials and Methods
3.1. General Remarks
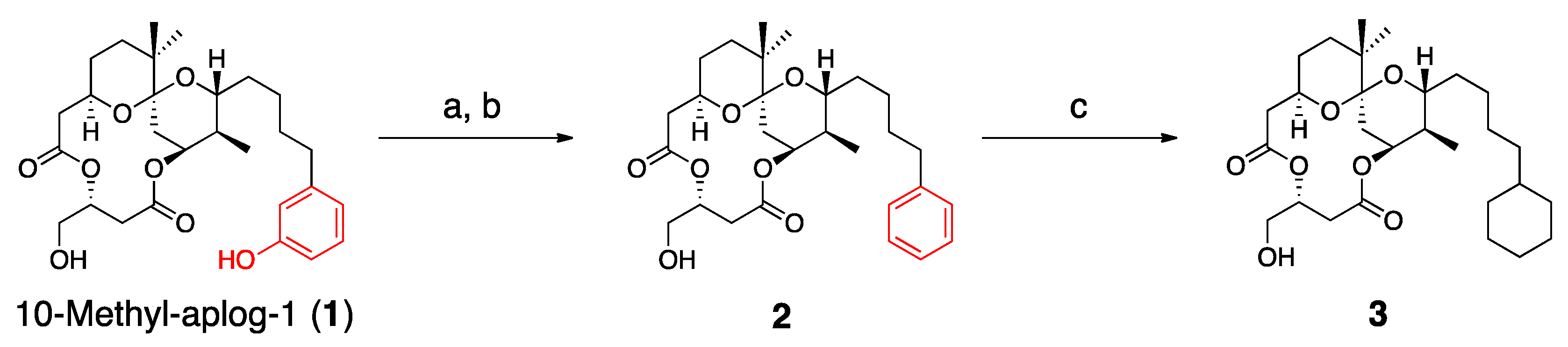
3.2. Synthesis of 2 and 3
3.3. Measurement of Cell Growth Inhibition
3.4. Mouse Ear Swelling Test
3.5. Two-Stage Carcinogenesis Experiment
3.6. Inhibition of the Specific Binding of [3H]PDBu to PKCδ-C1B Peptide
3.7. Molecular Docking Simulation
3.8. Translocation of PKCδ-GFP
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fujiki, H.; Sugimura, T. New classes of tumor promoters: Teleocidin, aplysiatoxin, and palytoxin. Adv. Cancer Res. 1987, 49, 223–264. [Google Scholar] [PubMed]
- Ramos, O.F.; Masucci, M.G.; Klein, E. Activation of cytotoxic activity of human blood lymphocytes by tumor-promoting compounds. Cancer Res. 1984, 44, 1857–1862. [Google Scholar] [PubMed]
- Blanco-Molina, M.; Tron, G.C.; Macho, A.; Lucena, C.; Calzado, M.A.; Muñoz, E.; Appendino, G. Ingenol esters induce apoptosis in Jurkat cells through an AP-1 and NF-κB independent pathway. Chem. Biol. 2001, 8, 767–778. [Google Scholar] [CrossRef]
- Nishizuka, Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 1984, 308, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995, 9, 484–496. [Google Scholar] [PubMed]
- Newton, A.C. Protein kinase C: Structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 2001, 101, 2353–2364. [Google Scholar] [CrossRef] [PubMed]
- Antal, C.E.; Hudson, A.M.; Kang, E.; Zanca, C.; Wirth, C.; Stephenson, N.L.; Trotter, E.W.; Gallegos, L.L.; Miller, C.J.; Furnari, F.B.; et al. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell 2015, 160, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Herald, C.L.; Doubek, D.L.; Herald, D.L.; Arnold, E.; Clardy, J. Isolation and structure of bryostatin 1. J. Am. Chem. Soc. 1982, 104, 6846–6848. [Google Scholar] [CrossRef]
- Lorenzo, P.S.; Dennis, P.A. Modulating protein kinase C (PKC) to increase the efficacy of chemotherapy: Stepping into darkness. Drug Resist. Update 2003, 6, 329–339. [Google Scholar] [CrossRef]
- Kollár, P.; Rajchard, J.; Balounová, Z.; Pazourek, J. Marine natural products: Bryostatins in preclinical and clinical studies. Pharm. Biol. 2014, 52, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Szállási, Z.; Krsmanovic, L.; Blumberg, P.M. Nonpromoting 12-deoxyphorbol 13-esters inhibit phorbol 12-myristate 13-acetate induced tumor promotion in CD-1 mouse skin. Cancer Res. 1993, 53, 2507–2512. [Google Scholar] [PubMed]
- Lebwohl, M.; Swanson, N.; Anderson, L.L.; Melgaard, A.; Xu, Z.; Berman, B. Ingenol mebutate gel for actinic keratosis. N. Engl. J. Med. 2012, 366, 1010–1019. [Google Scholar] [PubMed]
- Trost, B.M.; Dong, G. Total synthesis of bryostatin 16 using atom-economical and chemoselective approaches. Nature 2008, 456, 485–488. [Google Scholar] [PubMed]
- Keck, G.E.; Poudel, Y.B.; Cummins, T.J.; Rudra, A.; Covel, J.A. Total synthesis of bryostatin 1. J. Am. Chem. Soc. 2010, 133, 744–747. [Google Scholar] [PubMed]
- Lu, Y.; Woo, S.K.; Krische, M.J. Total synthesis of bryostatin 7 via C–C bond-forming hydrogenation. J. Am. Chem. Soc. 2011, 133, 13876–13879. [Google Scholar] [PubMed]
- Wender, P.A.; Baryza, J.L.; Bennett, C.E.; Bi, F.C.; Brenner, S.E.; Clarke, M.O.; Horan, J.C.; Kan, C.; Lacôte, E.; Lippa, B.; et al. The practical synthesis of a novel and highly potent analogue of bryostatin. J. Am. Chem. Soc. 2002, 124, 13648–13649. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Baryza, J.L.; Brenner, S.E.; DeChristopher, B.A.; Loy, B.A.; Schrier, A.J.; Verma, V.A. Design, synthesis, and evaluation of potent bryostatin analogs that modulate PKC translocation selectivity. Proc. Natl. Acad. Sci. USA 2011, 108, 6721–6726. [Google Scholar] [PubMed]
- Jørgensen, L.; McKerrall, S.J.; Kuttruff, C.A.; Ungeheuer, F.; Felding, J.; Baran, P.S. 14-step synthesis of (+)-ingenol from (+)-3-carene. Science 2013, 341, 878–882. [Google Scholar] [PubMed]
- Kawamura, S.; Chu, H.; Felding, J.; Baran, P.S. Nineteen-step total synthesis of (+)-phorbol. Nature 2016, 532, 90–93. [Google Scholar] [PubMed]
- Kato, Y.; Scheuer, P.J. Aplysiatoxin and debromoaplysiatoxin, constituents of the marine mollusk Stylocheilus longicauda. J. Am. Chem. Soc. 1974, 96, 2245–2246. [Google Scholar] [PubMed]
- Nakagawa, Y.; Yanagita, R.C.; Hamada, N.; Murakami, A.; Takahashi, H.; Saito, N.; Nagai, H.; Irie, K. A simple analogue of tumor-promoting aplysiatoxin is an antineoplastic agent rather than a tumor promoter: Development of a synthetically accessible protein kinase C activator with bryostatin-like activity. J. Am. Chem. Soc. 2009, 131, 7573–7579. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Kikumori, M.; Yanagita, R.C.; Murakami, A.; Tokuda, H.; Nagai, H.; Irie, K. Synthesis and biological evaluation of the 12, 12-dimethyl derivative of Aplog-1, an anti-proliferative analog of tumor-promoting aplysiatoxin. Biosci. Biotechnol. Biochem. 2011, 75, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Kikumori, M.; Yanagita, R.C.; Tokuda, H.; Suzuki, N.; Nagai, H.; Suenaga, K.; Irie, K. Structure-activity studies on the spiroketal moiety of a simplified analogue of debromoaplysiatoxin with antiproliferative activity. J. Med. Chem. 2012, 55, 5614–5626. [Google Scholar] [CrossRef] [PubMed]
- Kikumori, M.; Yanagita, R.C.; Tokuda, H.; Suenaga, K.; Nagai, H.; Irie, K. Structural optimization of 10-methyl-aplog-1, a simplified analog of debromoaplysiatoxin, as an anticancer lead. Biosci. Biotechnol. Biochem. 2016, 80, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, H.; Tanaka, K.; Yanagita, R.C.; Murakami, A.; Murakami, K.; Tokuda, H.; Suzuki, N.; Nakagawa, Y.; Irie, K. Structure–activity studies on the side chain of a simplified analog of aplysiatoxin (aplog-1) with anti-proliferative activity. Bioorg. Med. Chem. 2013, 21, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, T.; Akashi, A.; Yaguchi, K.; Iwasaki, Y.; Shigetsura, M.; Monguchi, Y.; Sajiki, H. Efficient and practical arene hydrogenation by heterogeneous catalysts under mild conditions. Chem. Eur. J. 2009, 15, 6953–6963. [Google Scholar] [CrossRef] [PubMed]
- Yamori, T.; Matsunaga, A.; Sato, S.; Yamazaki, K.; Komi, A.; Ishizu, K.; Mita, I.; Edatsugi, H.; Matsuba, Y.; Takezawa, K.; et al. Potent antitumor activity of MS-247, a novel DNA minor groove binder, evaluated by an in vitro and in vivo human cancer cell line panel. Cancer Res. 1999, 59, 4042–4049. [Google Scholar] [PubMed]
- Kikumori, M.; Yanagita, R.C.; Irie, K. Improved and large-scale synthesis of 10-methyl-aplog-1, a potential lead for an anticancer drug. Tetrahedron 2014, 70, 9776–9782. [Google Scholar] [CrossRef]
- Yanagita, R.C.; Kamachi, H.; Tanaka, K.; Murakami, A.; Nakagawa, Y.; Tokuda, H.; Nagai, H.; Irie, K. Role of the phenolic hydroxyl group in the biological activities of simplified analogue of aplysiatoxin with antiproliferative activity. Bioorg. Med. Chem. Lett. 2010, 20, 6064–6066. [Google Scholar] [CrossRef] [PubMed]
- Baird, W.M.; Boutwell, R.K. Tumor-promoting activity of phorbol and four diesters of phorbol in mouse skin. Cancer Res. 1971, 31, 1074–1079. [Google Scholar] [PubMed]
- Wang, Q.J.; Fang, T.-W.; Fenick, D.; Garfield, S.; Bienfait, B.; Marquez, V.E.; Blumberg, P.M. The lipophilicity of phorbol esters as a critical factor in determining the pattern of translocation of protein kinase C δ fused to green fluorescent protein. J. Biol. Chem. 2000, 275, 12136–12146. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a tumor promoter in cancer induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.K.; Pandey, P.; Sun, X.; Cheng, K.; Datta, R.; Saxena, S.; Kharbanda, S.; Kufe, D. Mitochondrial translocation of protein kinase Cδ in phorbol ester-induced cytochrome c release and apoptosis. J. Biol. Chem. 2000, 275, 21793–21796. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Oliva, J.L.; Kothapalli, D.; Fournier, A.; Assoian, R.K.; Kazanietz, M.G. Phorbol ester-induced G1 phase arrest selectively mediated by protein kinase Cδ-dependent induction of p21. J. Biol. Chem. 2005, 280, 33926–33934. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Guerrico, A.M.; Kazanietz, M.G. Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade: A key role for protein kinase Cδ. J. Biol. Chem. 2005, 280, 38982–38991. [Google Scholar] [CrossRef] [PubMed]
- Szállási, Z.; Denning, M.F.; Smith, C.B.; Dlugosz, A.A.; Yuspa, S.H.; Pettit, G.R.; Blumberg, P.M. Bryostatin 1 protects protein kinase C-delta from down-regulation in mouse keratinocytes in parallel with its inhibition of phorbol ester-induced differentiation. Mol. Pharmacol. 1994, 46, 840–850. [Google Scholar] [PubMed]
- Serova, M.; Ghoul, A.; Benhadji, K.A.; Faivre, S.; le Tourneau, C.; Cvitkovic, E.; Lokiec, F.; Lord, J.; Ogbourne, S.M.; Calvo, F.; et al. Effects of protein kinase C modulation by PEP005, a novel ingenol angelate, on mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling in cancer cells. Mol. Cancer Ther. 2008, 7, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, Y.; Kikumori, M.; Ueno, S.; Tokuda, H.; Suzuki, N.; Irie, K. Structure-activity studies at position 27 of aplog-1, a simplified analog of debromoaplysiatoxin with anti-proliferative activity. Tetrahedron 2013, 69, 7636–7645. [Google Scholar] [CrossRef]
- Sharkey, N.A.; Blumberg, P.M. Highly lipophilic phorbol esters as inhibitors of specific [3H] phorbol 12, 13-dibutyrate binding. Cancer Res. 1985, 45, 19–24. [Google Scholar] [PubMed]
- Shindo, M.; Irie, K.; Nakahara, A.; Ohigashi, H.; Konishi, H.; Kikkawa, U.; Fukuda, H.; Wender, P.A. Toward the identification of selective modulators of protein kinase C (PKC) isozymes: Establishment of a binding assay for PKC isozymes using synthetic C1 peptide receptors and identification of the critical residues involved in the phorbol ester binding. Bioorg. Med. Chem. 2001, 9, 2073–2081. [Google Scholar] [CrossRef]
- Irie, K.; Oie, K.; Nakahara, A.; Yanai, Y.; Ohigashi, H.; Wender, P.A.; Fukuda, H.; Konishi, H.; Kikkawa, U. Molecular basis for protein kinase C isozyme-selective binding: the synthesis, folding, and phorbol ester binding of the cysteine-rich domains of all protein kinase C isozymes. J. Am. Chem. Soc. 1998, 120, 9159–9167. [Google Scholar] [CrossRef]
- Szállási, Z.; Bogi, K.; Gohari, S.; Biro, T.; Acs, P.; Blumberg, P.M. Non-equivalent Roles for the First and Second Zinc Fingers of Protein Kinase Cδ Effect of their mutation on phorbol ester-induced translocation in NIH 3T3 cells. J. Biol. Chem. 1996, 271, 18299–18301. [Google Scholar] [CrossRef]
- Masuda, A.; Irie, K.; Nakagawa, Y.; Ohigashi, H. Binding selectivity of conformationally restricted analogues of (−)-indolactam-V to the C1 domains of protein kinase C isozymes. Biosci. Biotechnol. Biochem. 2002, 66, 1615–1617. [Google Scholar] [CrossRef]
- Ashida, Y.; Yanagita, R.C.; Takahashi, C.; Kawanami, Y.; Irie, K. Binding mode prediction of aplysiatoxin, a potent agonist of protein kinase C, through molecular simulation and structure-activity study on simplified analogs of the receptor-recognition domain. Bioorg. Med. Chem. 2016, 24, 4218–4227. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.H.; Kishi, Y.; Perez-Sala, D.; Rando, R.R. The pharmacophore of debromoaplysiatoxin responsible for protein kinase C activation. Proc. Natl. Acad. Sci. USA 1991, 88, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Rando, R.R. Structural basis of protein kinase C activation by tumor promoters. Acc. Chem. Res. 1998, 31, 163–172. [Google Scholar] [CrossRef]
- Nakamura, H.; Kishi, Y.; Pajares, M.A.; Rando, R.R. Structural basis of protein kinase C activation by tumor promoters. Proc. Natl. Acad. Sci. USA 1989, 86, 9672–9676. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, R.C.; Kamachi, H.; Kikumori, M.; Tokuda, H.; Suzuki, N.; Suenaga, K.; Suenaga, K.; Irie, K. Effects of the methoxy group in the side chain of debromoaplysiatoxin on its tumor-promoting and anti-proliferative activities. Bioorg. Med. Chem. Lett. 2013, 23, 4319–4323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.J.; Bhattacharyya, D.; Garfield, S.; Nacro, K.; Marquez, V.E.; Blumberg, P.M. Differential localization of protein kinase Cδ by phorbol esters and related compounds using a fusion protein with green fluorescent protein. J. Biol. Chem. 1999, 274, 37233–37239. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Reither, G.; Kaestner, L.; Lipp, P. Targeted activation of conventional and novel protein kinases C through differential translocation patterns. Mol. Cell. Biol. 2014, 34, 2370–2381. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Walker, J.W. Protein kinase Cδ and Σ mediate positive inotropy in adult ventricular myocytes. J. Mol. Cell. Cardiol. 2005, 38, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Von Burstin, V.A.; Xiao, L.; Kazanietz, M.G. Bryostatin 1 inhibits phorbol ester-induced apoptosis in prostate cancer cells by differentially modulating protein kinase C (PKC) δ translocation and preventing PKCδ-mediated release of tumor necrosis factor-α. Mol. Pharmacol. 2010, 78, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Keck, G.E.; Poudel, Y.B.; Rudra, A.; Stephens, J.C.; Kedei, N.; Lewin, N.E.; Blumberg, P.M. Role of the C8 gem-dimethyl group of bryostatin 1 on its unique pattern of biological activity. Bioorg. Med. Chem. Lett. 2012, 22, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, T.; Shirai, Y.; Sakai, N.; Yamamoto, T.; Matsuzaki, H.; Kikkawa, U.; Saito, N. Ceramide-induced apoptosis by translocation, phosphorylation, and activation of protein kinase Cδ in the Golgi complex. J. Biol. Chem. 2004, 279, 12668–12676. [Google Scholar] [CrossRef] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Van der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are not available. |
| Cancer Type | Cell Line | GI50 (log M) | ||
|---|---|---|---|---|
| 1 a | 2 | 3 | ||
| Breast | HBC-4 | −7.48 | −7.76 | −7.20 |
| MDA-MB-231 | −6.90 | −5.63 | −5.68 | |
| Colon | HCC2998 | −6.47 | −6.21 | −6.08 |
| Lung | NCI-H460 | −7.07 | −7.09 | −6.85 |
| A549 | −6.01 | −6.12 | −5.78 | |
| Stomach | St-4 | −6.24 | −5.93 | −5.89 |
| MKN45 | −4.97 | −6.51 | −6.13 | |
| Average for these seven cell lines | −6.45 | −6.46 | −6.23 | |
| Ki (nM) for PKCδ C1B | ||
|---|---|---|
| 1 a | 2 | 3 |
| 0.46 | 0.87 (0.18) b | 3.8 (0.67) b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanaki, Y.; Kikumori, M.; Tokuda, H.; Okamura, M.; Dan, S.; Adachi, N.; Saito, N.; Yanagita, R.C.; Irie, K. Loss of the Phenolic Hydroxyl Group and Aromaticity from the Side Chain of Anti-Proliferative 10-Methyl-aplog-1, a Simplified Analog of Aplysiatoxin, Enhances Its Tumor-Promoting and Proinflammatory Activities. Molecules 2017, 22, 631. https://doi.org/10.3390/molecules22040631
Hanaki Y, Kikumori M, Tokuda H, Okamura M, Dan S, Adachi N, Saito N, Yanagita RC, Irie K. Loss of the Phenolic Hydroxyl Group and Aromaticity from the Side Chain of Anti-Proliferative 10-Methyl-aplog-1, a Simplified Analog of Aplysiatoxin, Enhances Its Tumor-Promoting and Proinflammatory Activities. Molecules. 2017; 22(4):631. https://doi.org/10.3390/molecules22040631
Chicago/Turabian StyleHanaki, Yusuke, Masayuki Kikumori, Harukuni Tokuda, Mutsumi Okamura, Shingo Dan, Naoko Adachi, Naoaki Saito, Ryo C. Yanagita, and Kazuhiro Irie. 2017. "Loss of the Phenolic Hydroxyl Group and Aromaticity from the Side Chain of Anti-Proliferative 10-Methyl-aplog-1, a Simplified Analog of Aplysiatoxin, Enhances Its Tumor-Promoting and Proinflammatory Activities" Molecules 22, no. 4: 631. https://doi.org/10.3390/molecules22040631