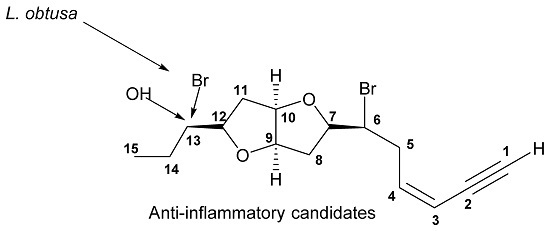
Isolaurenidificin and Bromlaurenidificin, Two New C15-Acetogenins from the Red Alga Laurencia obtusa
Abstract
:1. Introduction
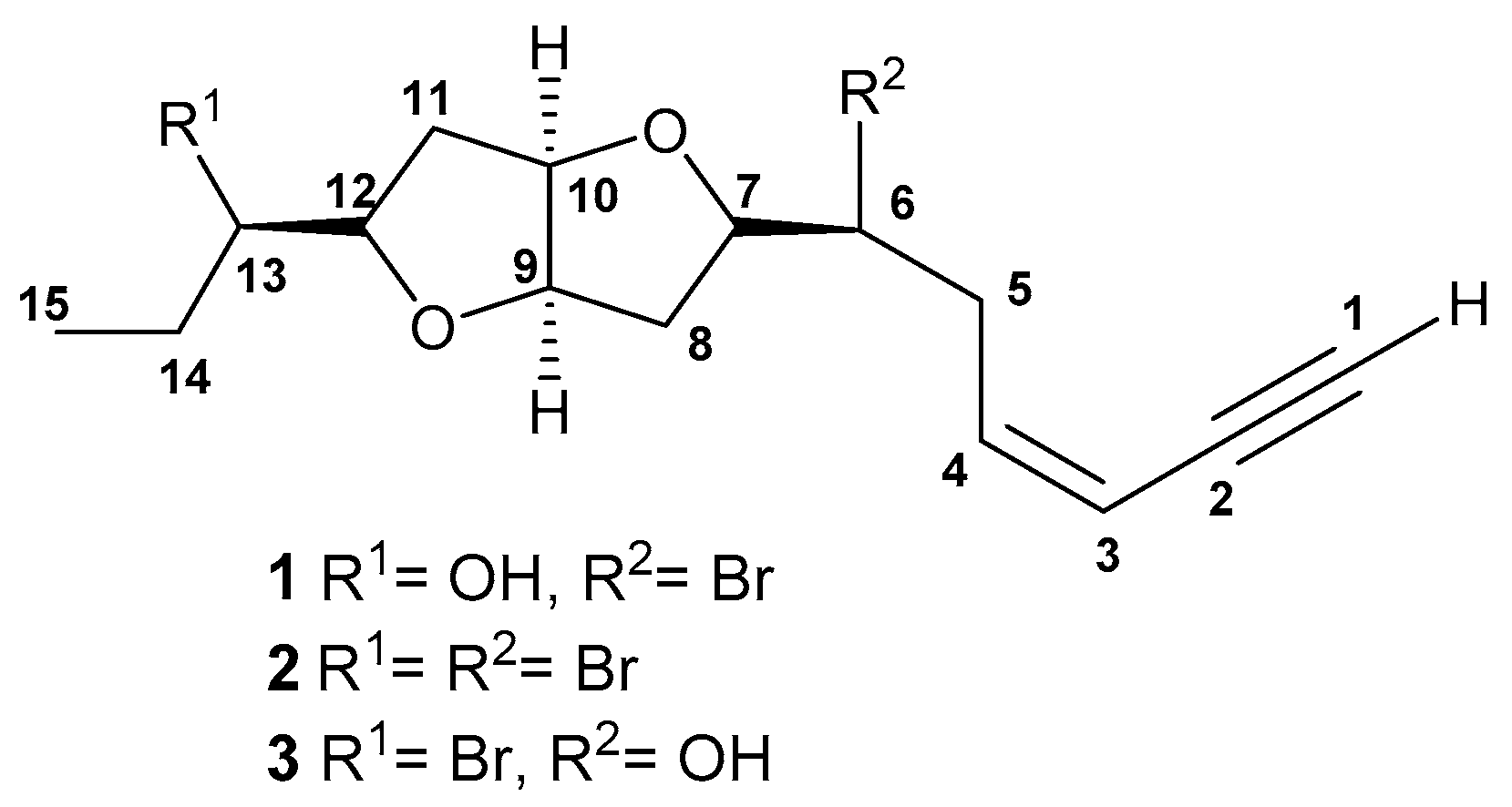
2. Results and Discussion
3. Experimental
3.1. General
3.2. Extraction and Isolation
3.3. Spectral Data
3.4. Toxicity of the Isolated Compounds
3.4.1. Brine Shrimp Lethality (Artemia salina) Assay
3.4.2. Cytotoxicity Bioassay
Cell Culture
Cytotoxicity Assay
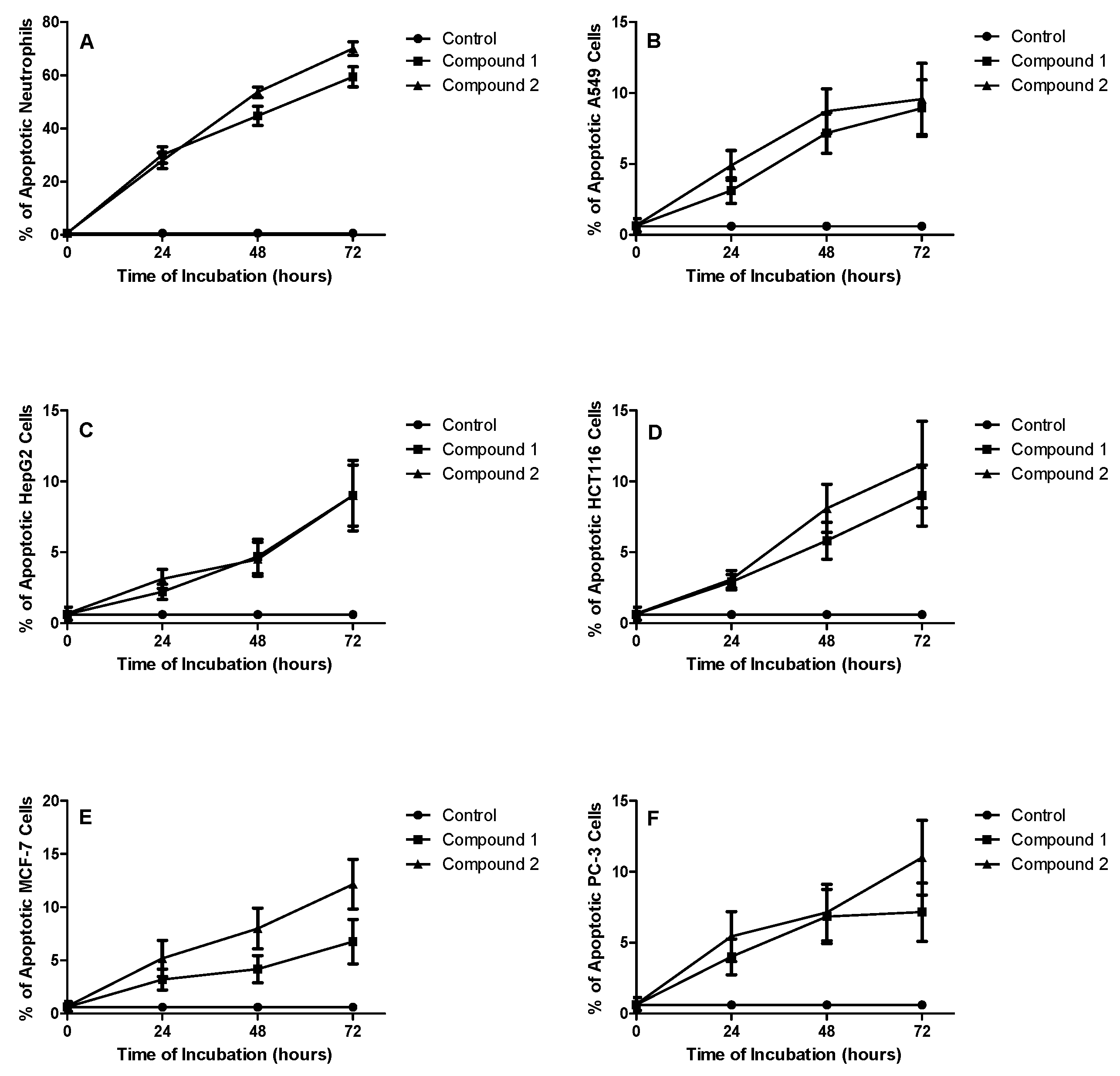
Apoptotic Effect on Neutrophils
Culture of Neutrophils
Assessment of Cell Viability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suzuki, M.; Takahashi, Y.; Nakano, S.; Abe, T.; Masuda, M.; Ohnishi, T.; Noya, Y.; Seki, K. An experimental approach to study the biosynthesis of brominated metabolites by the red algal genus Laurencia. Phytochemistry 2009, 70, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Masudai, M.; Abe, T.; Suzuki, T.; Suzuki, M. Morphological and chemotaxonomic studies on Laurencia composita and L. okamurae (Ceramiales, Rhodophyta). Phycologia 1996, 35, 550–562. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. Algae Base. World-Wide Electronic Publication, National University of Ireland: Galway, 2015. Available online: http://www.algaebase.org (accessed on 29 November 2015).
- Kurata, K.; Taniguchi, K.; Agatsuma, Y.; Suzuki, M. Diterpenoid feeding-deterrents from Laurencia saitoi. Phytochemistry 1998, 47, 363–369. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R.; Sharp, K. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2011, 28, 345–387. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S. Phytochemical studies of the southern Australian marine alga, Laurenci aelata. Phytochemistry 2011, 72, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D. Laurencia rigida: Chemical investigations of its antifouling dichloromethane extract. J. Nat. Prod. 1997, 60, 967–970. [Google Scholar] [CrossRef]
- Davyt, D.; Fernandez, R.; Suescun, L.; Mombrú, A.W.; Saldaña, J.; Domínguez, L.; Coll, J.; Fujii, M.T.; Manta, E. New sesquiterpene derivatives from the red alga Laurencia scoparia. Isolation, structure determination, and anthelmintic activity. J. Nat. Prod. 2001, 64, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Wanke, T.; Philippus, A.C.; Zatelli, G.A.; Vieira, L.F.O.; Lhullier, C.; Falkenberg, M. C15 acetogenins from the Laurencia complex: 50 years of research—An overview. Rev. Bras. Farmacogn. 2015, 25, 569–587. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.; Northcote, P.T.; Prinset, M.P. Marine natural products. Nat. Prod. Rep. 2010, 27, 165–237. [Google Scholar] [CrossRef] [PubMed]
- Alarif, W.M.; Al-Lihaibi, S.S.; Ayyad, S.E.; Abdel-Rhman, M.H.; Badria, F.A. Laurene-type sesquiterpenes from the Red Sea red alga Laurencia obtusa as potential antitumor-antimicrobial agents. Eur. J. Med. Chem. 2012, 55, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Alarif, W.M.; Al-Footy, K.O.; Zubair, M.S.; Halid, P.H.M.; Ghandourah, M.H.; Basaif, S.A.; Al-Lihaibi, S.S.; Ayyad, S.E.; Badria, F.A. The role of new eudesmane-type sesquiterpenoid and known eudesmane derivatives from the red alga Laurencia obtusa as potential antifungal-antitumour agents. Nat. Prod. Res. 2015, 30, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Angawi, R.F.; Alarif, W.M.; Hamza, R.I.; Badria, F.A.; Ayyad, S.E. New cytotoxic laurene, cuparene and laurokamurene type-sesquiterpenes from the red alga Laurencia obtusa. Helv. Chem. Acta 2014, 97, 1388–1395. [Google Scholar] [CrossRef]
- Ayyad, S.E.; Al-Footy, K.O.; Alarif, W.M.; Sobahi, T.R.; Bassaif, S.A.; Makki, M.S.; Asiri, A.M.; Al Halwani, A.Y.; Badria, A.F.; Badria, F.A. Bioactive C-15 Acetogenins from the red alga Laurencia obtusa. Chem. Pharm. Bull. 2011, 59, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.M.; Li, C.S.; Ji, N.Y.; Wang, B.G. Laurenidificin, a new brominated C15-acetogenin from the marine red alga Laurencia nidifica. Chin. Chem. Lett. 2010, 21, 1213–1215. [Google Scholar] [CrossRef]
- Meyer, B.; Ferrigni, N.; Putnam, J.; Jacobsen, L.; Nichols, D.; McLaughlin, J. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Haslett, C.; Guthrie, L.A.; Kopaniak, M.M.; Johnston, R.B., Jr.; Henson, P.M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 1985, 119, 101–110. [Google Scholar] [PubMed]
Sample Availability: Not available. |
 ) and HMBC (
) and HMBC (  ) correlations of 1 and 2.
) correlations of 1 and 2.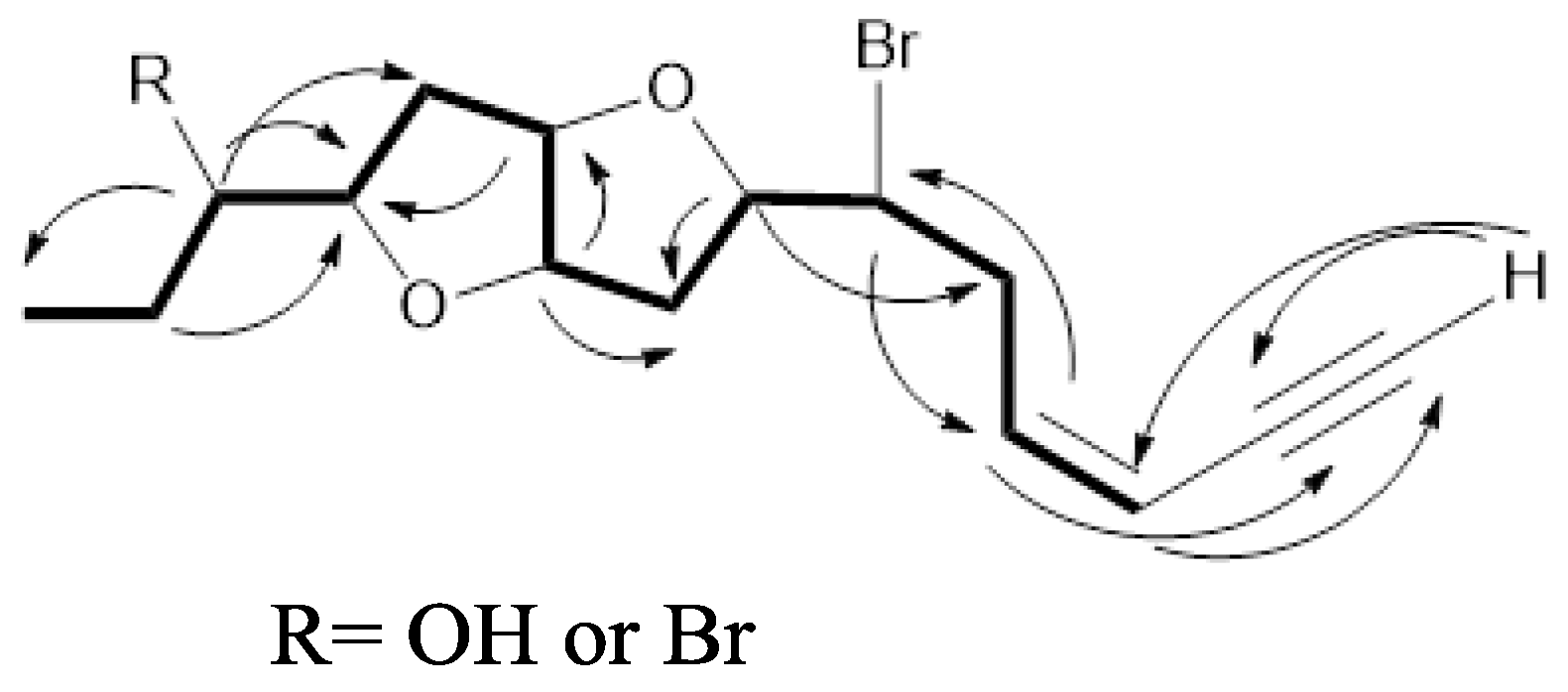
| Compound | IC50 (mM) | ||||
|---|---|---|---|---|---|
| A 549 | HepG2 | HCT116 | MCF-7 | PC-3 | |
| 1 | 15.6 | 15.1 | >20 | 15.5 | 17.9 |
| 2 | 15.9 | 15.3 | 17.9 | 17.9 | >20 |
| Compound | % Apoptotic Neutrophils (Mean ± S.D.) | |||
|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | |
| (Control) | 0.41 ± 0.01 | 0.41 ± 0.01 | 0.41 ± 0.01 | 0.41 ± 0.01 |
| 1 | 0.63 ± 0.55 | 30.10 ± 3.11 | 44.86 ± 3.59 | 59.45 ± 3.76 |
| 2 | 0.80 ± 0.56 | 27.91 ± 2.93 | 53.62 ± 2.01 | 70.13 ± 2.51 |
| Position | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δH | Mult. J in Hz | δC | δH | Mult. J in Hz | δC | |
| 1 | 3.15 | d, J = 1.7 | 82.8 | 2.76 | d, J = 1.7 | 82.8 |
| 2 | - | 79.9 | - | 79.8 | ||
| 3 | 5.63 | ddd, J = 11.1, 3.4,1.7 | 111 | 5.35 | ddd, J = 11.1, 2.6, 1.7 | 110.6 |
| 4 | 6.18 | ddd, J = 11.1, 10.2, 7.7 | 141.1 | 5.85 | ddd, J = 11.9, 11.1, 6.8 | 141.4 |
| 5 | 2.9 | m | 35.5 | 2.83 | m | 35.4 |
| 2.96 | dddd, J = 15.3, 8.5, 7.0, 1.7 | 2.87 | m | |||
| 6 | 4.15 | ddd, J = 10.2, 8.5, 4.3 | 55.7 | 3.83 | m | 55 |
| 7 | 4.08 | dd, J = 14.5, 7.7 | 83.6 | 3.56 | ddd, J = 12.8, 7.7,5.1 | 82.7 |
| 8 | 2.02 | m | 36.8 | 1.66 | ddd, J = 13.6, 6.8, 1.7 | 36.9 |
| 2.32 | ddd, J = 13.6, 7.7, 6.8 | 1.85 | ddd, J = 13.6, 6.0, 1.7 | |||
| 9 | 4.55 | ddd, J = 6.0, 4.3, 1.7 | 84.3 | 3.99 | ddd, J = 6.0, 3.4,1.7 | 84.9 |
| 10 | 4.53 | ddd, J = 6.0, 4.3, 1.7 | 85 | 3.82 | m | 84.1 |
| 11 | 2.02 | m | 34.9 | 1.91 | ddd, J = 13.6, 7.7, 6.0 | 37.5 |
| 2.22 | ddd, J = 13.6, 7.7, 6.8 | 2.28 | dd, J = 13.6, 5.1 | |||
| 12 | 3.88 | ddd, J = 14.7, 8.5, 6.8 | 84.3 | 3.93 | ddd, J = 9.4, 8.5, 6.0 | 83.6 |
| 13 | 3.54 | ddd, J = 11.1, 6.8, 4.3 | 75.2 | 4.24 | ddd, J = 11.9, 9.4, 2.6 | 61.4 |
| 14 | 1.51 | m | 26.5 | 1.76 | ddq, J = 14.5, 9.4, 7.7 | 28.4 |
| 1.45 | m | 2.15 | dddd, J = 14.5, 10.2, 7.7, 2.6 | |||
| 15 | 1 | t, J = 6.8 | 10.1 | 1.04 | t, J = 7.7 | 11.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bawakid, N.O.; Alarif, W.M.; Alburae, N.A.; Alorfi, H.S.; Al-Footy, K.O.; Al-Lihaibi, S.S.; Ghandourah, M.A. Isolaurenidificin and Bromlaurenidificin, Two New C15-Acetogenins from the Red Alga Laurencia obtusa. Molecules 2017, 22, 807. https://doi.org/10.3390/molecules22050807
Bawakid NO, Alarif WM, Alburae NA, Alorfi HS, Al-Footy KO, Al-Lihaibi SS, Ghandourah MA. Isolaurenidificin and Bromlaurenidificin, Two New C15-Acetogenins from the Red Alga Laurencia obtusa. Molecules. 2017; 22(5):807. https://doi.org/10.3390/molecules22050807
Chicago/Turabian StyleBawakid, Nahed O., Walied M. Alarif, Najla A. Alburae, Hajer S. Alorfi, Khalid O. Al-Footy, Sultan S. Al-Lihaibi, and Mohamed A. Ghandourah. 2017. "Isolaurenidificin and Bromlaurenidificin, Two New C15-Acetogenins from the Red Alga Laurencia obtusa" Molecules 22, no. 5: 807. https://doi.org/10.3390/molecules22050807