Gubenyiliu II Inhibits Breast Tumor Growth and Metastasis Associated with Decreased Heparanase Expression and Phosphorylation of ERK and AKT Pathways
Abstract
:1. Introduction
2. Results
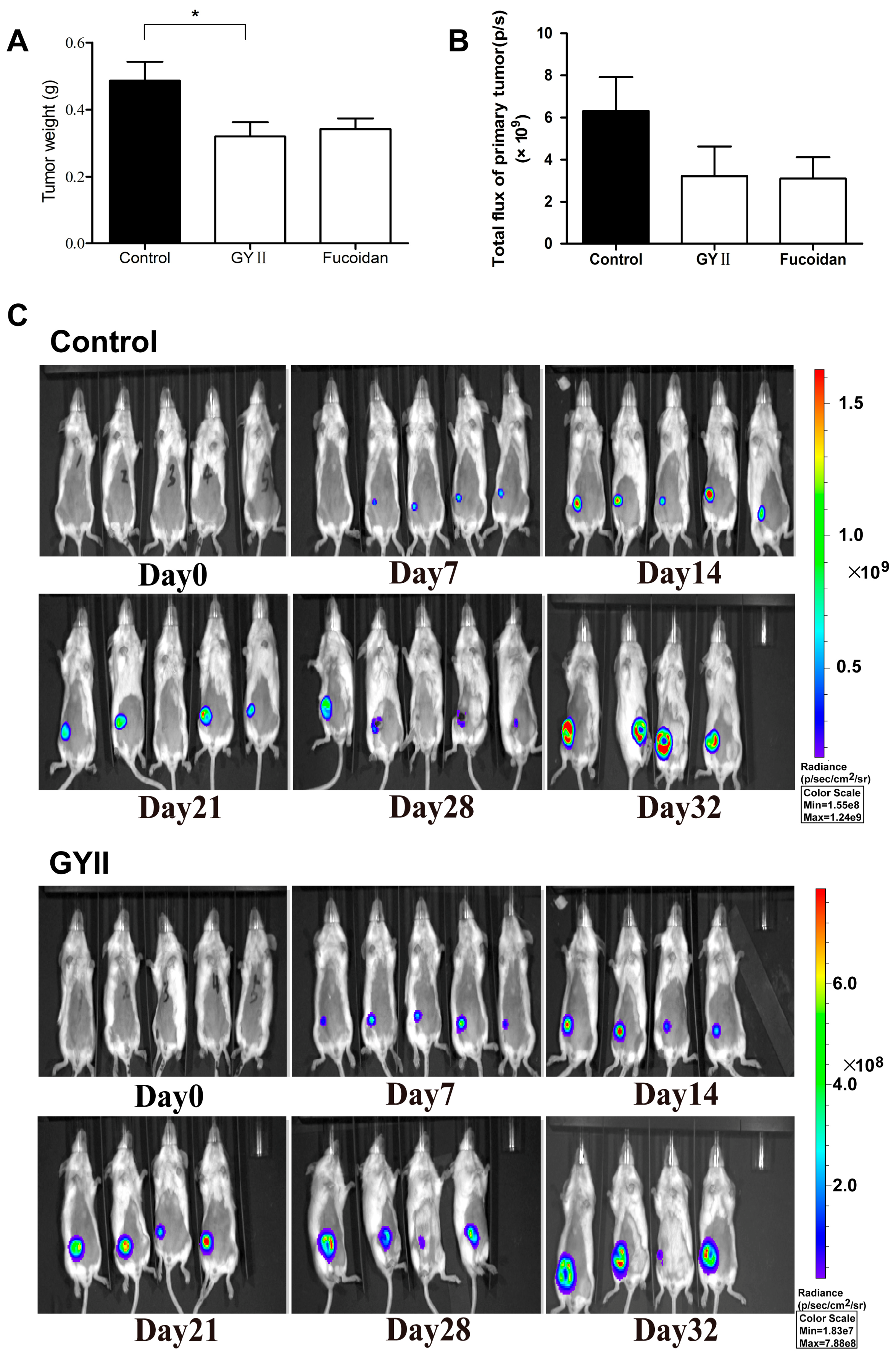
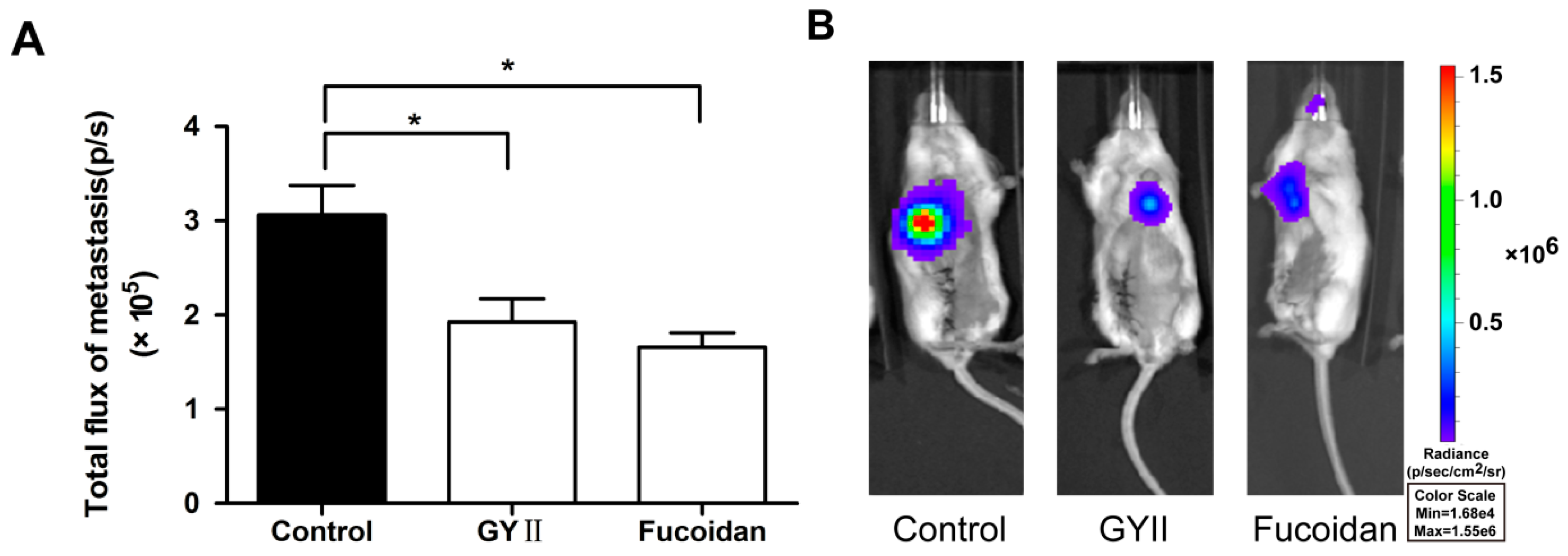
2.1. GYII Inhibited Primary Tumor Growth and Lung Metastasis in a Murine Mammary Carcinoma Model
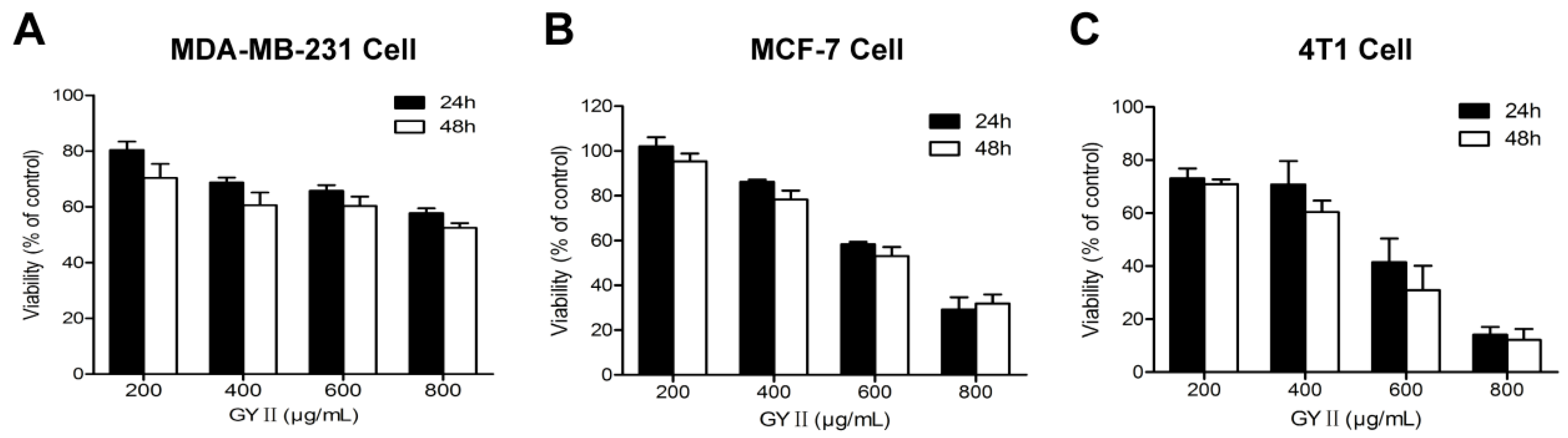
2.2. Selective Inhibition of Breast Cancer Cell Proliferation by GYII
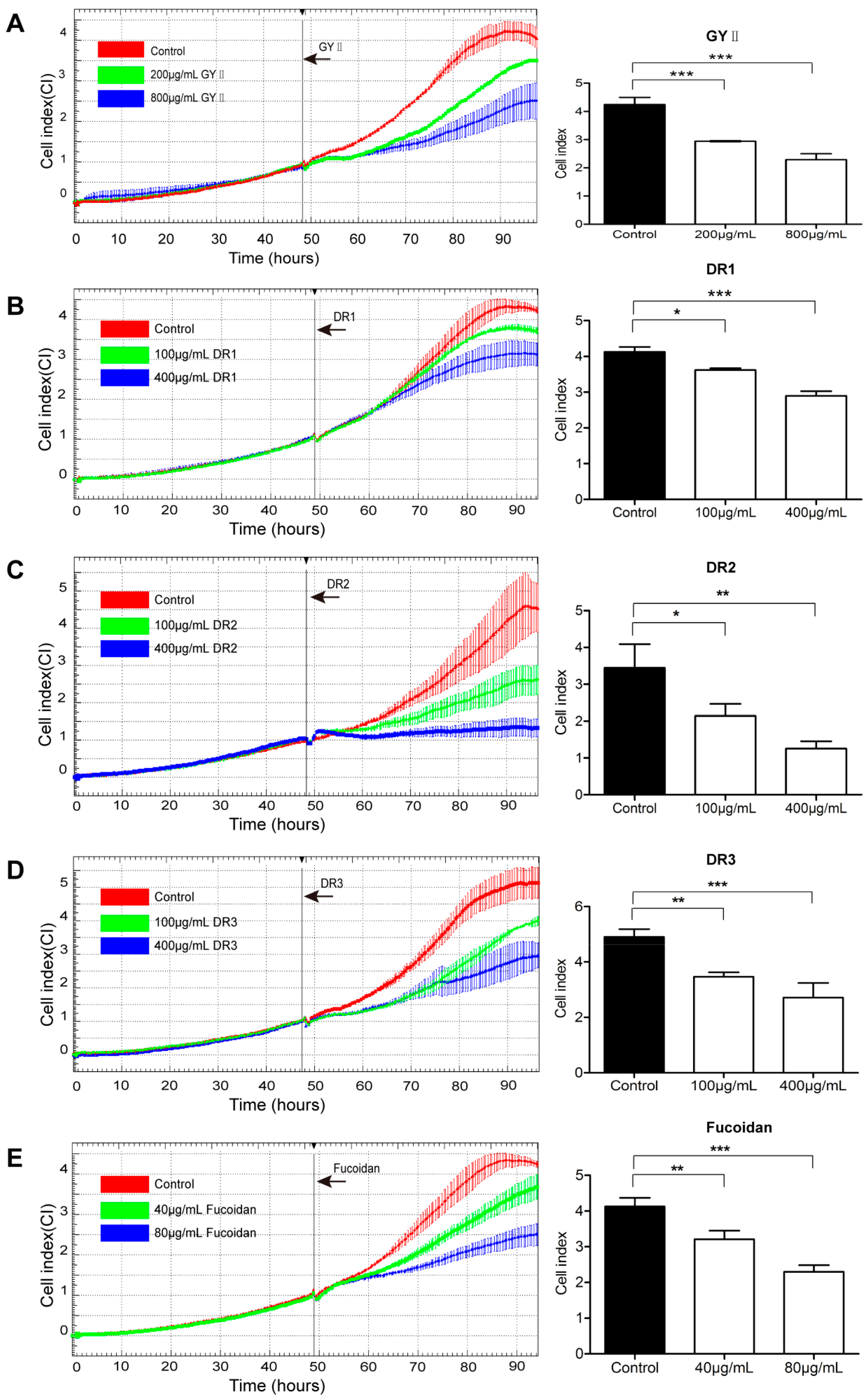
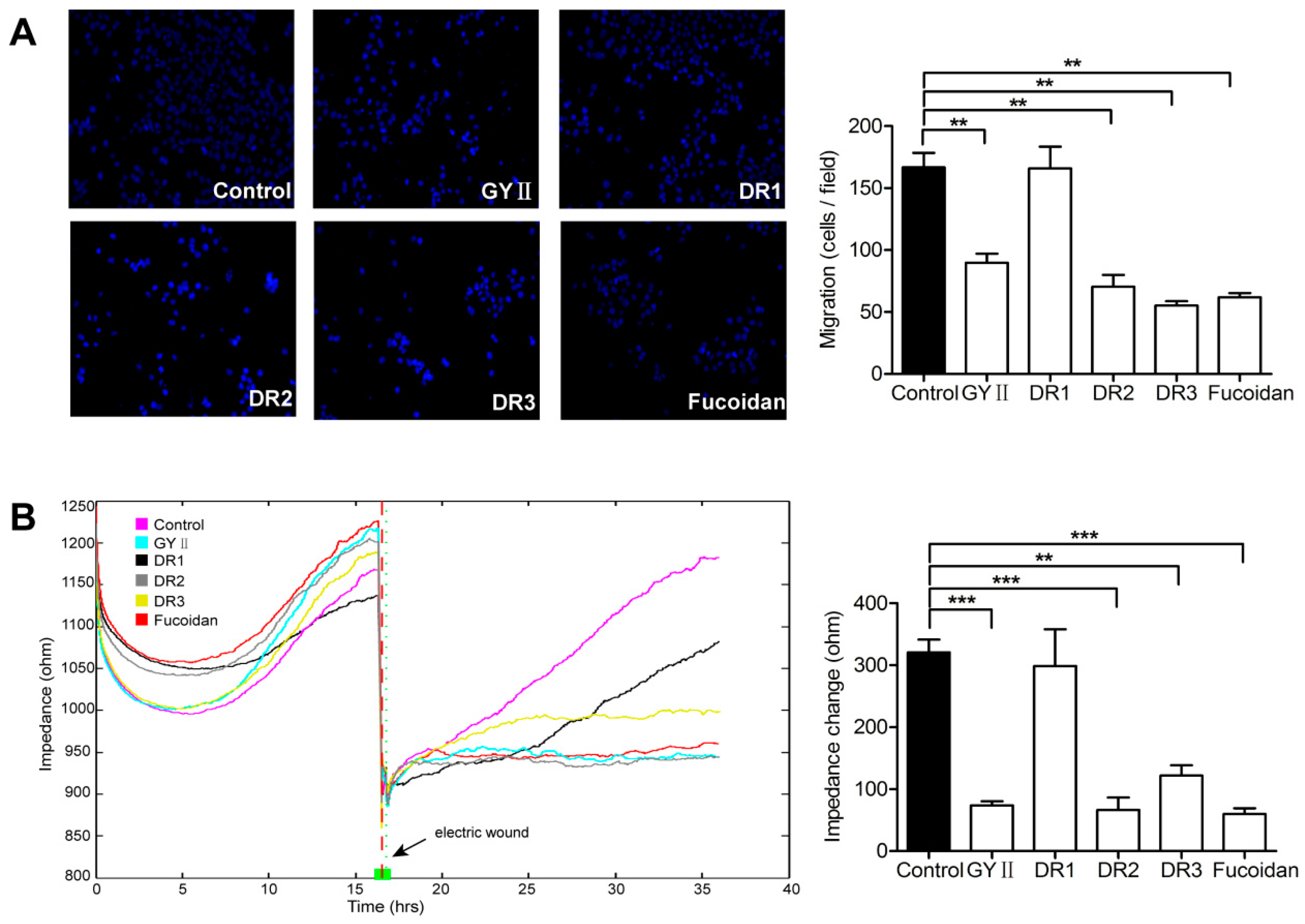
2.3. Differential Inhibitory Effects of GYII and Its Decomposed Recipes on 4T1 Cell Proliferation and Migration
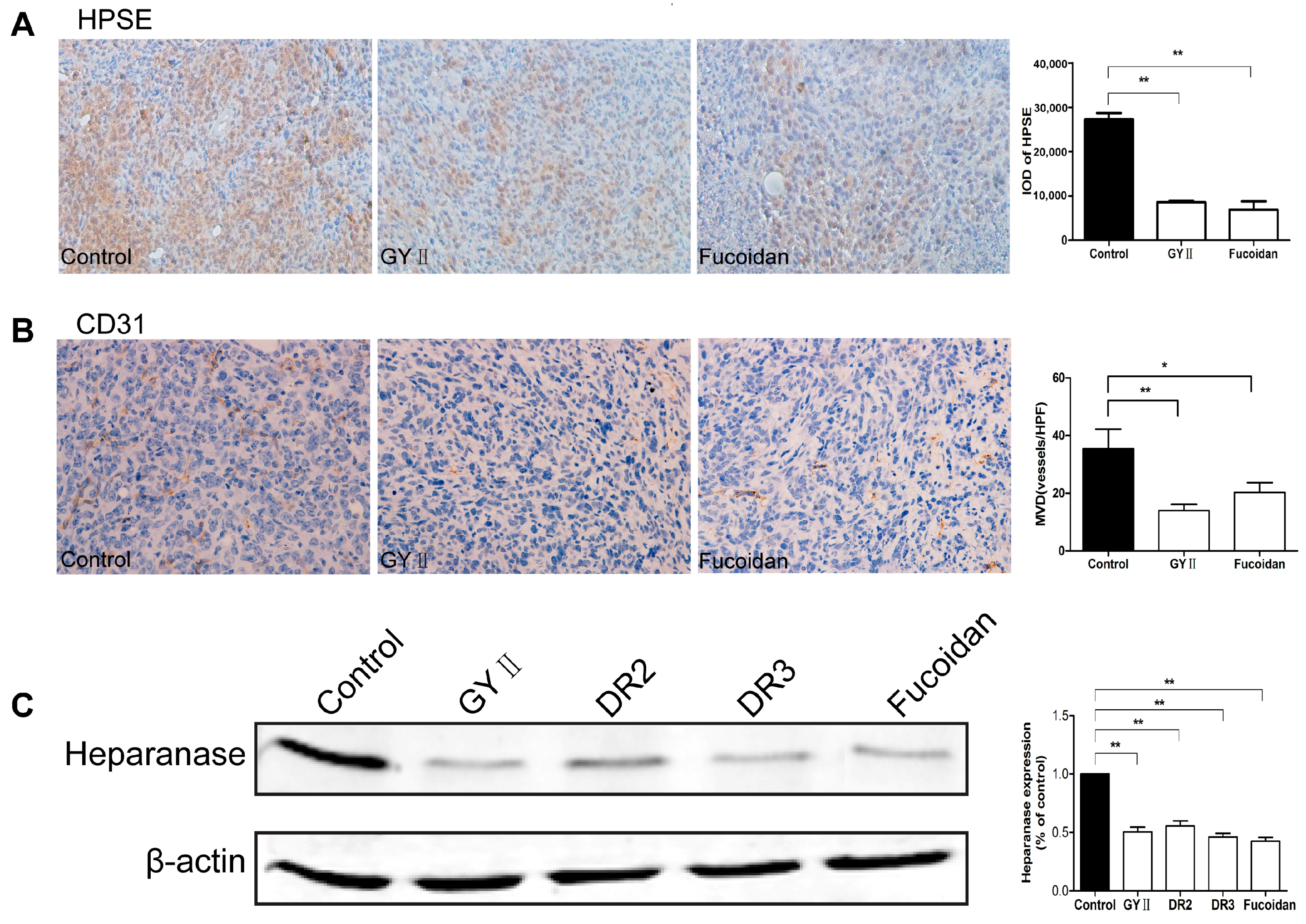
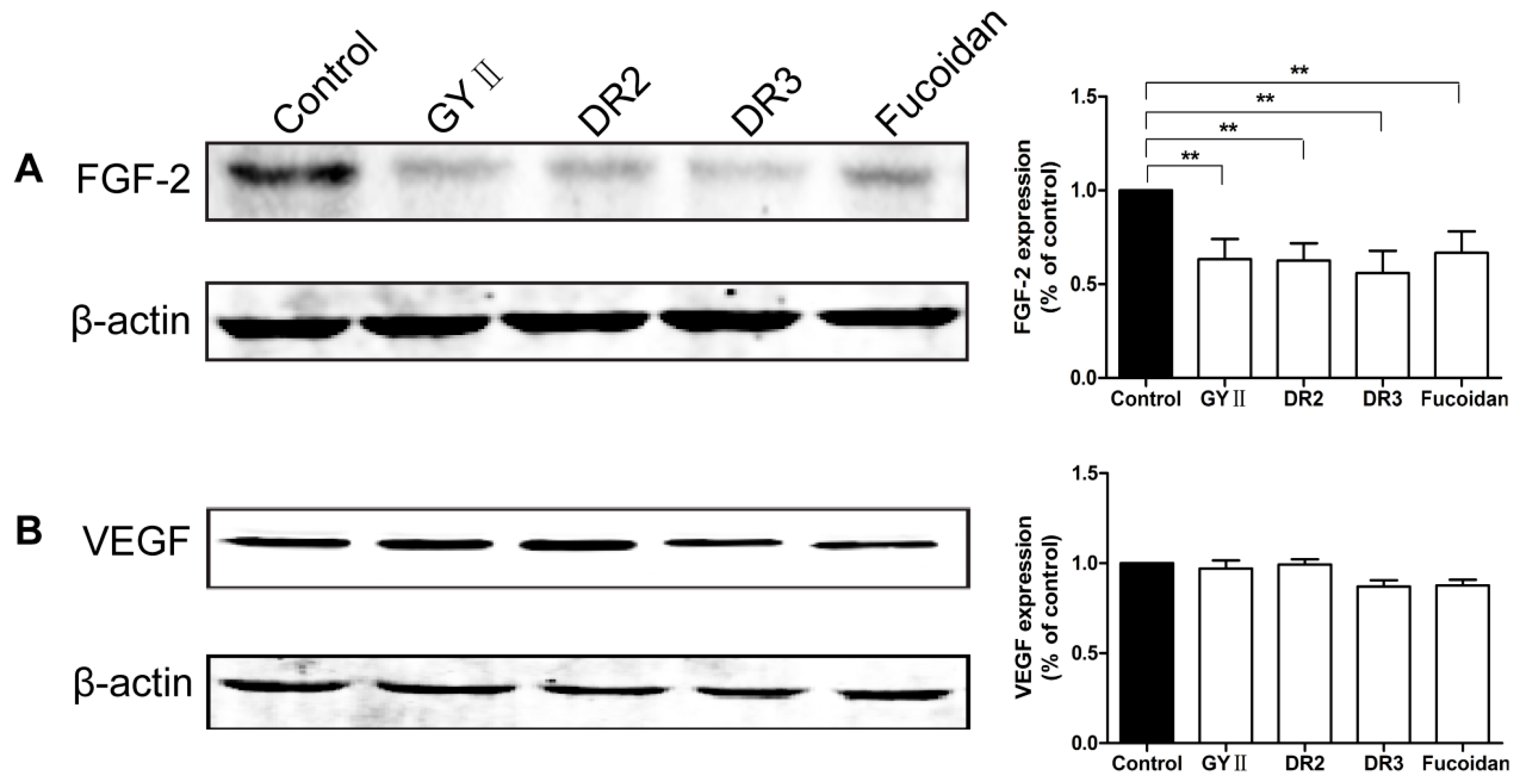
2.4. Molecular Mechanisms of the Inhibitory Effects of GYII on Anti-Tumor Activity
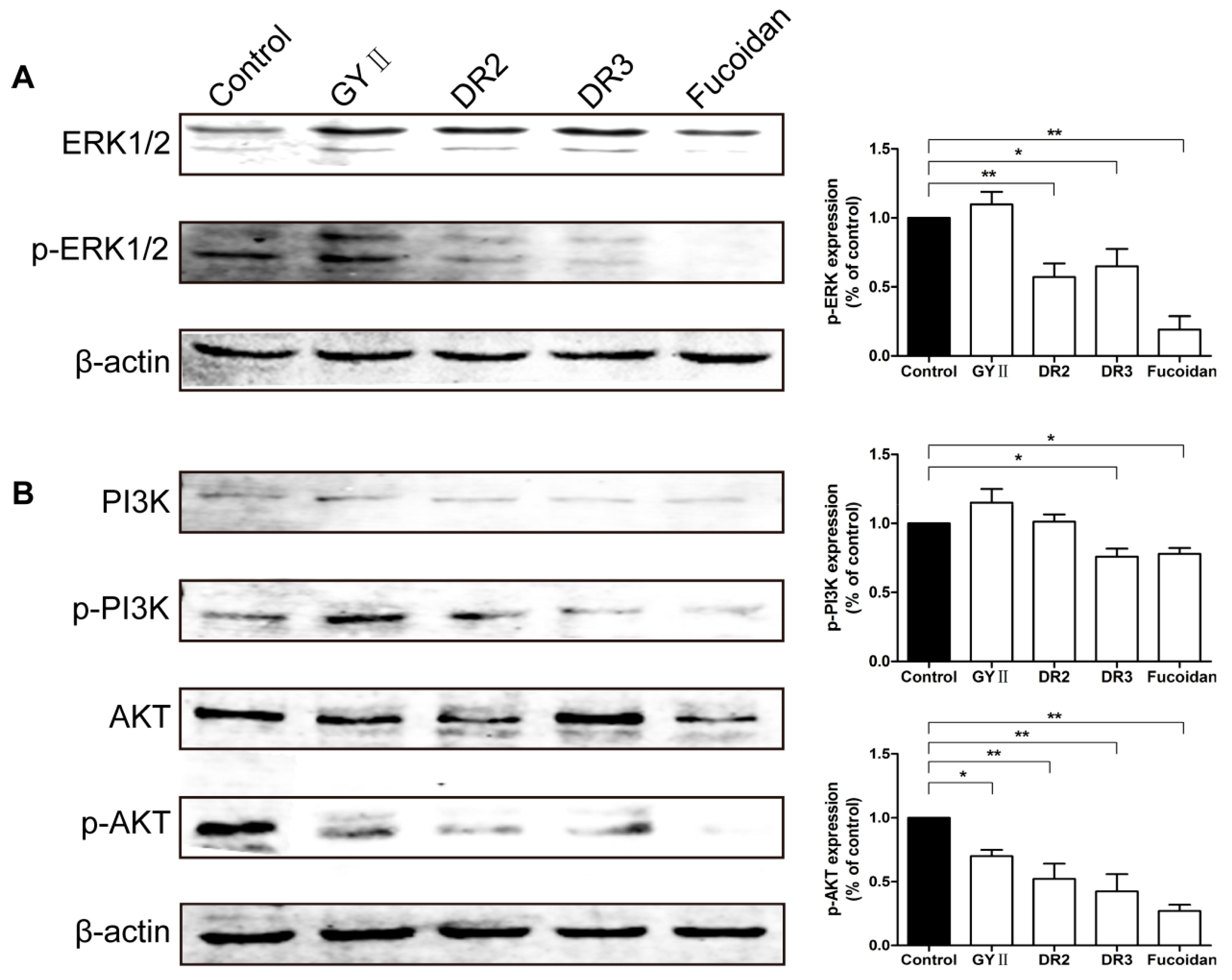
2.5. Effects of GYII and Its Decomposed Recipes on the ERK and PI3K/AKT Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of GYII and Its Decomposed Recipes
4.3. Cell Culture
4.4. Murine 4T1-luc2 Breast Carcinoma Model
4.5. MTT Assay
4.6. RTCA for Cell Proliferation
4.7. Transwell® Migration Assay
4.8. ECIS Wound Healing Assay
4.9. Western Blotting
4.10. Immunohistochemistry
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| AKT | Serine-threonine kinase |
| BAP | Blood activation prescription |
| CD31 | Cluster of differentiation 31 |
| CEA | Carcino-embryonic antigen |
| DMSO | Dimethyl sulphoxide |
| ECIS | Electric cell substrate impedance sensing |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinase |
| FGF-2 | Basic fibroblast growth factor |
| FBS | Fetal bovine serum |
| HS | Heparan sulfate |
| HPSE | Heparanase |
| IC50 | Half-maximal inhibitory concentrations |
| IHC | Immunohistochemistry |
| IR | Inhibitory rate |
| IVIS | In-vivo imaging system |
| MTT | 3-(4,5-dimethyl-thiazol-2-yl)-2,5-Diphenyl tetrazolium bromide |
| MVD | Micro-vessel density |
| OD | Optical density |
| PI3K | Phosphatidylinositol-3-kinase |
| PBS | Phosphate buffer solution |
| RTCA | Real-time cell analysis |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TCGA | The cancer genome atlas |
| VEGF | Vascular endothelial growth factor |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, G.; Li, X.; Zhang, Y.; Yang, J.; Chang, J.; Sun, X.; Zhou, X.; Guo, Y.; Xu, Y.; et al. Traditional Chinese medicine in cancer care: A review of controlled clinical studies published in Chinese. PLoS ONE 2013, 8, e60338. [Google Scholar]
- Qi, F.; Zhao, L.; Zhou, A.; Zhang, B.; Li, A.; Wang, Z.; Han, J. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci. Trends 2015, 9, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, R.; Tang, W.; Wang, X.; Rao, X.; Wang, Y.; Guan, T.; Zhao, W. The clinical research of Gubenyiliu II on tumors. Chin. J. Inf. TCM 2000, 7, 41–42. [Google Scholar]
- Li, S.G.; Chen, H.Y.; Ou-Yang, C.S.; Wang, X.X.; Yang, Z.J.; Tong, Y.; Cho, W.C. The efficacy of Chinese herbal medicine as an adjunctive therapy for advanced non-small cell lung cancer: A systematic review and meta-analysis. PLoS ONE 2013, 8, e57604. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Jiang, M.; Huang, Z.; Chen, M.; Chen, K.; Zhou, J.; Yin, L.; Tang, Y.; Wang, M.; Ye, L.; et al. A novel herbal formula induces cell cycle arrest and apoptosis in association with suppressing the PI3K/AKT pathway in human lung cancer A549 cells. Integr. Cancer Ther. 2014, 13, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, R.C.; Tang, W.J. Influence of combined therapy of guben yiliu III, moxibustion and chemotherapy on immune function and blood coagulation mechanism in patients with mid-late stage malignant tumor. Zhongguo Zhong Xi Yi Jie He Za Zhi 2002, 22, 104–106. [Google Scholar] [PubMed]
- Yang, G.; Xu, Y.; Fu, Q.; Han, D.; Yu, J.; Yang, Z.; Tang, W. Clinical Observation on Guben Yiliu II Combined with Chemotherapy in the Treatment of 28 Cases of Advanced Breast Cancer. J. Tradit. Chin. Med. 2008, 49, 1081–1083. [Google Scholar]
- Zhang, G.; Wang, X.; Li, P.; Yang, G.; Tang, Y.; Liu, X.; Sheng, X. Inhibitory effect of Gubenyiliu Formula II and its combinative effect with chemotherapeutants on mouse Lewis lung carcinoma. J. Beijing Univ. Tradit. Chin. Med. 2007, 30, 108–111. [Google Scholar]
- Ma, C.; Wang, X.M.; Yu, M.W.; Zhang, G.L.; Nan, N.; Zhang, Y.; Cao, K.X.; Li, J.P. Inhibitory effects of Guben Yiliu Formula II (II) and its blood activation prescriptions on the growth of MCF-7 human breast cancer xenografts in nude mice. Chin. J. Integr. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited—The role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Sui, H.; Wang, Q.; He, N.; Duan, C.; Han, L.; Li, Q.; Lu, M.; Lv, S. A Chinese herbal formula, Yi-Qi-Fu-Sheng, inhibits migration/invasion of colorectal cancer by down-regulating MMP-2/9 via inhibiting the activation of ERK/MAPK signaling pathways. BMC Complement. Altern. Med. 2013, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, Y.; Cheung, F.; Wang, X.; Zhang, Z.; Feng, Y. A Chinese medicine formula Gegen Qinlian decoction suppresses expansion of human renal carcinoma with inhibition of matrix metalloproteinase-2. Integr. Cancer Ther. 2015, 14, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhuang, Q.; Zheng, L.; Cao, Z.; Shen, A.; Li, Q.; Fu, C.; Feng, J.; Peng, J. Pien Tze Huang inhibits liver metastasis by targeting TGF-beta signaling in an orthotopic model of colorectal cancer. Oncol. Rep. 2015, 33, 1922–1928. [Google Scholar] [PubMed]
- Vlodavsky, I.; Friedmann, Y.; Elkin, M.; Aingorn, H.; Atzmon, R.; Ishai-Michaeli, R.; Bitan, M.; Pappo, O.; Peretz, T.; Michal, I.; et al. Mammalian heparanase: Gene cloning, expression and function in tumor progression and metastasis. Nat. Med. 1999, 5, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P. Heparin, heparan sulfate and heparanase in cancer: Remedy for metastasis? Anticancer Agents Med. Chem. 2008, 8, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, G.; Nian, J.; Yu, M.; Chen, S.; Zhang, Y.; Yang, G.; Yang, L.; Cheng, P.; Yan, C.; et al. Elevated heparanase expression is associated with poor prognosis in breast cancer: a study based on systematic review and TCGA data. Oncotarget 2017. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.Y. Fucoidan as a marine anticancer agent in preclinical development. Mar. Drugs 2014, 12, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Pappo, O.; Elkin, M.; San, T.; Bar-Shavit, R.; Hazan, R.; Peretz, T.; Vlodavsky, I.; Abramovitch, R. Heparanase promotes growth, angiogenesis and survival of primary breast tumors. Int. J. Cancer 2006, 118, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Ilan, N.; Elkin, M.; Vlodavsky, I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol. 2006, 38, 2018–2039. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Elkin, M.; Ilan, N. Impact of heparanase and the tumor microenvironment on cancer metastasis and angiogenesis: Basic aspects and clinical applications. Rambam Maimonides Med. J. 2011, 2, e0019. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Butt, M.S.; Qayyum, M.M.; Suleria, H.A. Immunity: Plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, J.; Lin, H. Effect and Molecular Mechanisms of Traditional Chinese Medicine on Regulating Tumor Immunosuppressive Microenvironment. BioMed Res. Int. 2015, 2015, 261620. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, D.; Fu, Y.L.; Hu, W. Screening of QHF formula for effective ingredients from Chinese herbs and its anti-hepatic cell cancer effect in combination with chemotherapy. Chin. Med. J. 2008, 121, 363–368. [Google Scholar] [PubMed]
- Liang, F.; Li, L.; Wang, M.; Niu, X.; Zhan, J.; He, X.; Yu, C.; Jiang, M.; Lu, A. Molecular network and chemical fragment-based characteristics of medicinal herbs with cold and hot properties from Chinese medicine. J. Ethnopharmacol. 2013, 148, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.H.; Shi, W.D.; Zhu, X.Y.; Chen, Z.; Liu, L.M. Qingyihuaji formula inhibits progress of liver metastases from advanced pancreatic cancer xenograft by targeting to decrease expression of Cyr61 and VEGF. Integr. Cancer Ther. 2012, 11, 37–47. [Google Scholar] [CrossRef] [PubMed]
- El-Assal, O.N.; Yamanoi, A.; Ono, T.; Kohno, H.; Nagasue, N. The clinicopathological significance of heparanase and basic fibroblast growth factor expressions in hepatocellular carcinoma. Clin. Cancer Res. 2001, 7, 1299–1305. [Google Scholar] [PubMed]
- Doviner, V.; Maly, B.; Kaplan, V.; Gingis-Velitski, S.; Ilan, N.; Vlodavsky, I.; Sherman, Y. Spatial and temporal heparanase expression in colon mucosa throughout the adenoma-carcinoma sequence. Mod. Pathol. 2006, 19, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Vlodavsky, I.; Friedmann, Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J. Clin. Investig. 2001, 108, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Zhang, Q.; Zhao, S.; Wang, J.; Lu, K.; Song, Y.; Zhao, L.; Kang, X.; Wang, J.; Xu, S.; et al. The expression and clinical significance of microRNA-1258 and heparanase in human breast cancer. Clin. Biochem. 2013, 46, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Hu, J.; Luo, Y.; Liu, Q.; Li, T.; Parish, C.R.; Freeman, C.; Zhu, X.; Ma, W.; Hu, X.; et al. Upregulation of heparanase in high-glucose-treated endothelial cells promotes endothelial cell migration and proliferation and correlates with Akt and extracellular-signal-regulated kinase phosphorylation. Mol. Vis. 2012, 18, 1684–1695. [Google Scholar] [PubMed]
- Suzuki, T.; Yasuda, H.; Funaishi, K.; Arai, D.; Ishioka, K.; Ohgino, K.; Tani, T.; Hamamoto, J.; Ohashi, A.; Naoki, K.; et al. Multiple roles of extracellular fibroblast growth factors in lung cancer cells. Int. J. Oncol. 2015, 46, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.; Brandt, R.; Dredge, K. PG545, a heparan sulfate mimetic, reduces heparanase expression in vivo, blocks spontaneous metastases and enhances overall survival in the 4T1 breast carcinoma model. PLoS ONE 2012, 7, e52175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Roy, S.; Cochran, E.; Zouaoui, R.; Chu, C.L.; Duffner, J.; Zhao, G.; Smith, S.; Galcheva-Gargova, Z.; Karlgren, J.; et al. M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PLoS ONE 2011, 6, e21106. [Google Scholar] [CrossRef] [PubMed]
- Ferro, V.; Dredge, K.; Liu, L.; Hammond, E.; Bytheway, I.; Li, C.; Johnstone, K.; Karoli, T.; Davis, K.; Copeman, E.; et al. PI-88 and novel heparan sulfate mimetics inhibit angiogenesis. Semin. Thromb. Hemost. 2007, 33, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.Q.; Elkin, M.; Aingorn, E.; Ishai-Michaeli, R.; Stein, C.A.; Vlodavsky, I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int. J. Cancer 1999, 83, 424–431. [Google Scholar] [CrossRef]
- Lou, C.; Zhu, Z.; Zhao, Y.; Zhu, R.; Zhao, H. Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol. Rep. 2017, 37, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Fang, H.; Yang, Z.G.; Wang, X.Y.; Ruan, L.M.; Fang, D.R.; Ding, Y.G.; Wang, Y.N.; Zhang, Y.; Jiang, X.L.; et al. Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int. J. Dermatol. 2008, 47, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, E.; Damiani, E.; Orlando, F.; Lucarini, G.; Bartozzi, B.; Lombardi, P.; Salvatore, C.; Geroni, C.; Donati, A.; Provinciali, M. Antiangiogenic and antitumor activities of berberine derivative NAX014 compound in a transgenic murine model of HER2/neu-positive mammary carcinoma. Carcinogenesis 2015, 36, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010. [Google Scholar]
- Zhao, X.N.; Liang, J.L.; Chen, H.B.; Liang, Y.E.; Guo, H.Z.; Su, Z.R.; Li, Y.C.; Zeng, H.F.; Zhang, X.J. Anti-Fatigue and Antioxidant Activity of the Polysaccharides Isolated from Millettiae speciosae Champ. Leguminosae. Nutrients 2015, 7, 8657–8669. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, W.; Dong, S.; Song, L.; Zhao, S.; Wu, C.; Wang, X.; Liu, F.; Xie, J.; Wang, J.; et al. Protective effects of sea buckthorn polysaccharide extracts against LPS/d-GalN-induced acute liver failure in mice via suppressing TLR4-NF-kappaB signaling. J. Ethnopharmacol. 2015, 176, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.E.; Oei, Y.; Hornig, Y.S.; Yu, S.F.; Dusich, J.; Purchio, T.; Contag, P.R. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin. Exp. Metastasis 2003, 20, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Limame, R.; Wouters, A.; Pauwels, B.; Fransen, E.; Peeters, M.; Lardon, F.; De Wever, O.; Pauwels, P. Comparative analysis of dynamic cell viability, migration and invasion assessments by novel real-time technology and classic endpoint assays. PLoS ONE 2012, 7, e46536. [Google Scholar] [CrossRef] [PubMed]
- Szulcek, R.; Bogaard, H.J.; van Nieuw, A.G. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor angiogenesis and metastasis—Correlation in invasive breast carcinoma. N. Engl. J. Med. 1991, 324, 1–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Whole Prescription | Decomposed Recipes | Components | Ratio in GYII (%) | Ratio in Decomposed Recipes (%) |
|---|---|---|---|---|
| GYII | DR1 | Codonopsis pilosula (Franch.) Nannf. | 7.6 | 14.3 |
| Poria cocos (Schw.). Wolf. | 5.1 | 9.5 | ||
| Atractylodis macrocephala Koidz. | 5.1 | 9.5 | ||
| Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao | 15.2 | 28.6 | ||
| Ligustrum lucidum Ait. | 7.6 | 14.3 | ||
| Lycium barbarum L. | 7.6 | 14.3 | ||
| Epimedium brevicornum Maxim | 5.1 | 9.5 | ||
| DR2 | Ligusticum chuanxiong Hort. | 5.1 | 20.0 | |
| Spatholobus suberectus Dunn | 15.2 | 60.0 | ||
| Curcuma kwangsiensis S. G. Lee et C. F. Liang | 5.1 | 20.0 | ||
| DR3 | Sarcandra glabra (Thunb.) Nakai | 7.6 | 35.7 | |
| Fritillaria thunbergii Miq. | 7.6 | 35.7 | ||
| Sophora flavescens Ait. | 6.1 | 28.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, G.-L.; Sun, X.; Cao, K.-X.; Shang, Y.-W.; Gong, M.-X.; Ma, C.; Nan, N.; Li, J.-P.; Yu, M.-W.; et al. Gubenyiliu II Inhibits Breast Tumor Growth and Metastasis Associated with Decreased Heparanase Expression and Phosphorylation of ERK and AKT Pathways. Molecules 2017, 22, 787. https://doi.org/10.3390/molecules22050787
Zhang Y, Zhang G-L, Sun X, Cao K-X, Shang Y-W, Gong M-X, Ma C, Nan N, Li J-P, Yu M-W, et al. Gubenyiliu II Inhibits Breast Tumor Growth and Metastasis Associated with Decreased Heparanase Expression and Phosphorylation of ERK and AKT Pathways. Molecules. 2017; 22(5):787. https://doi.org/10.3390/molecules22050787
Chicago/Turabian StyleZhang, Yi, Gan-Lin Zhang, Xu Sun, Ke-Xin Cao, Ya-Wen Shang, Mu-Xin Gong, Cong Ma, Nan Nan, Jin-Ping Li, Ming-Wei Yu, and et al. 2017. "Gubenyiliu II Inhibits Breast Tumor Growth and Metastasis Associated with Decreased Heparanase Expression and Phosphorylation of ERK and AKT Pathways" Molecules 22, no. 5: 787. https://doi.org/10.3390/molecules22050787






