New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation
Abstract
:1. Introduction
2. Results and Discussion
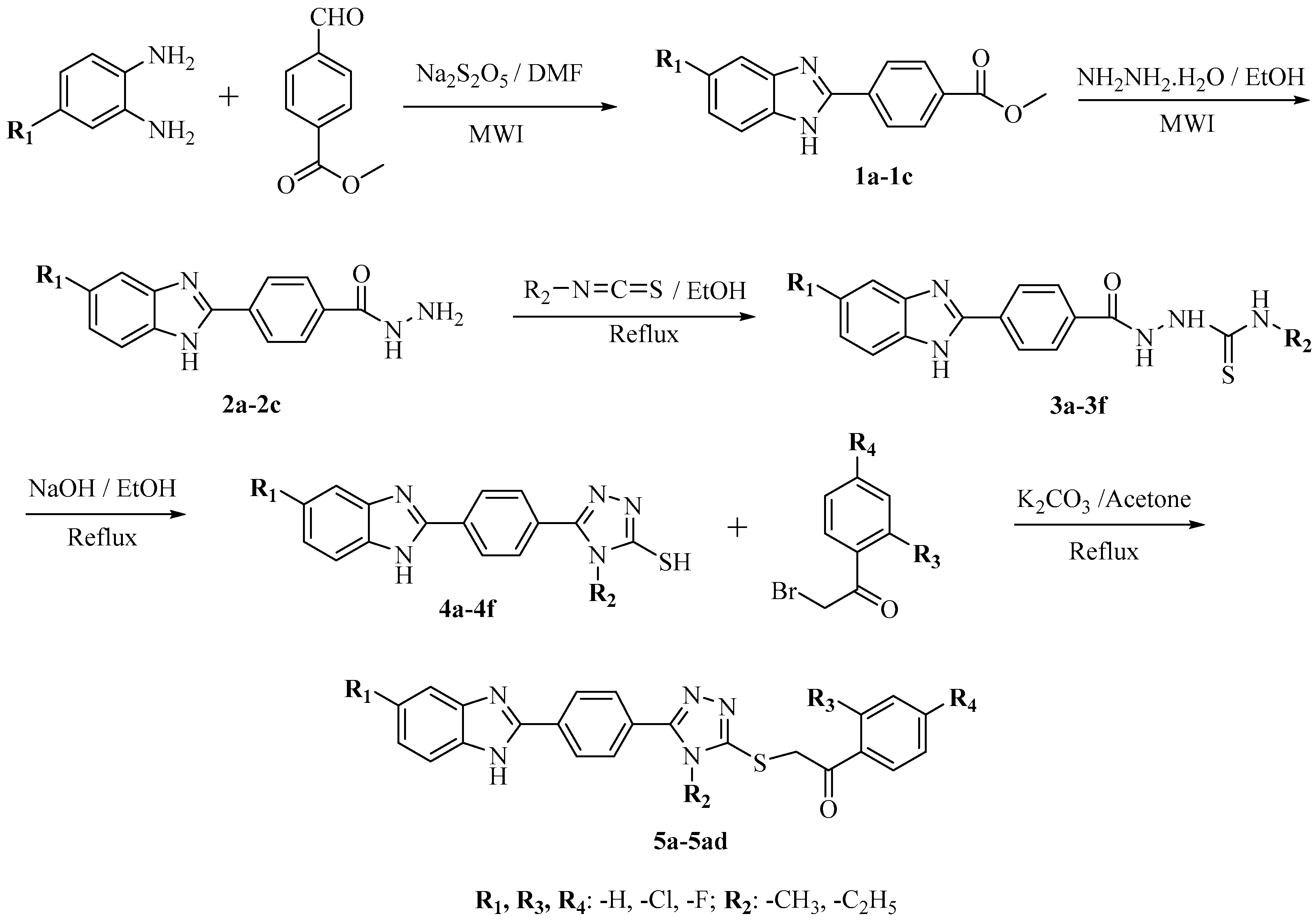
2.1. Chemistry
2.2. Antifungal Activity
2.3. Prediction of ADME Parameters
2.4. Cytotoxicity Evaluation
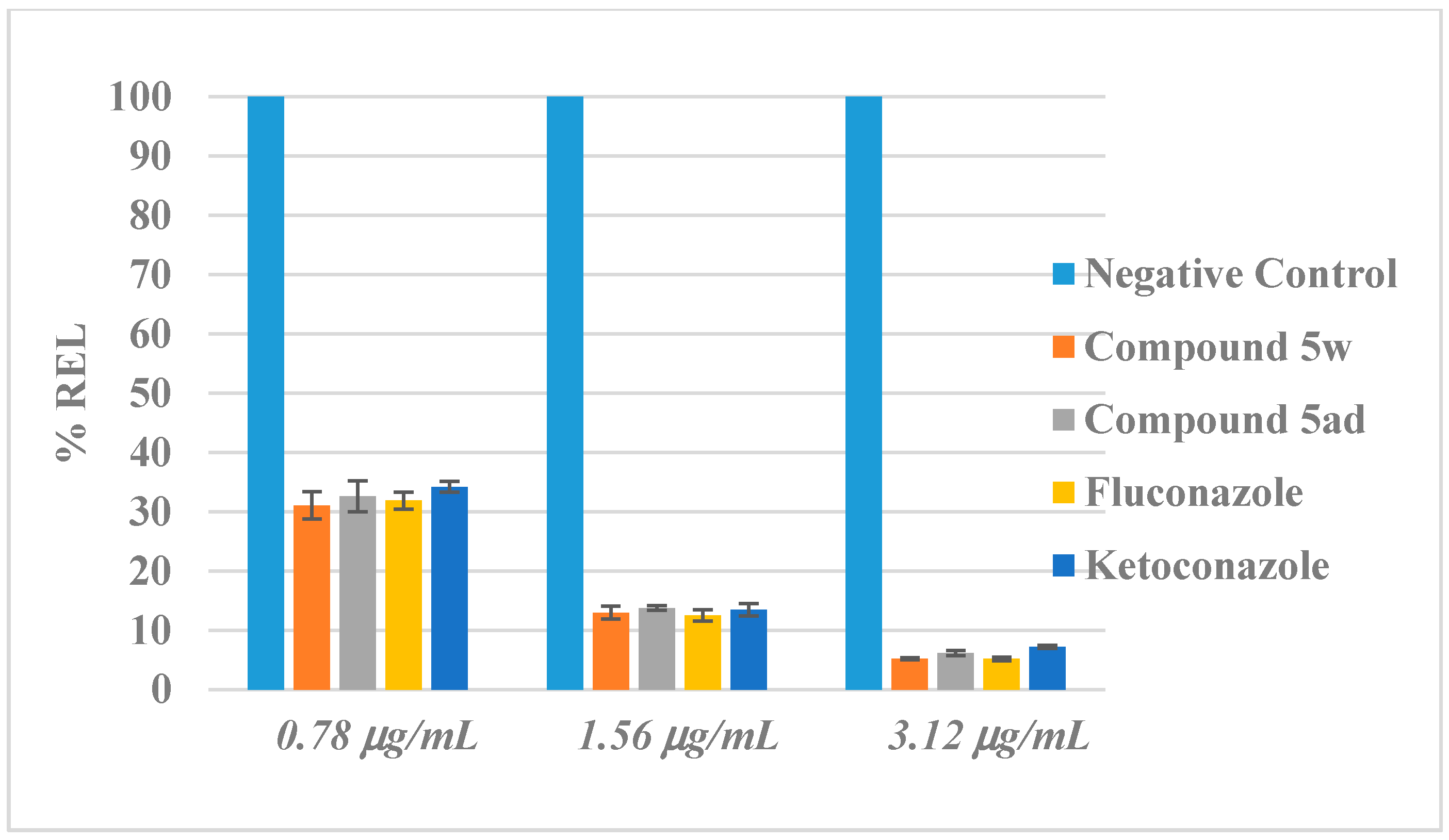
2.5. Inhibition of Ergosterol Biosynthesis
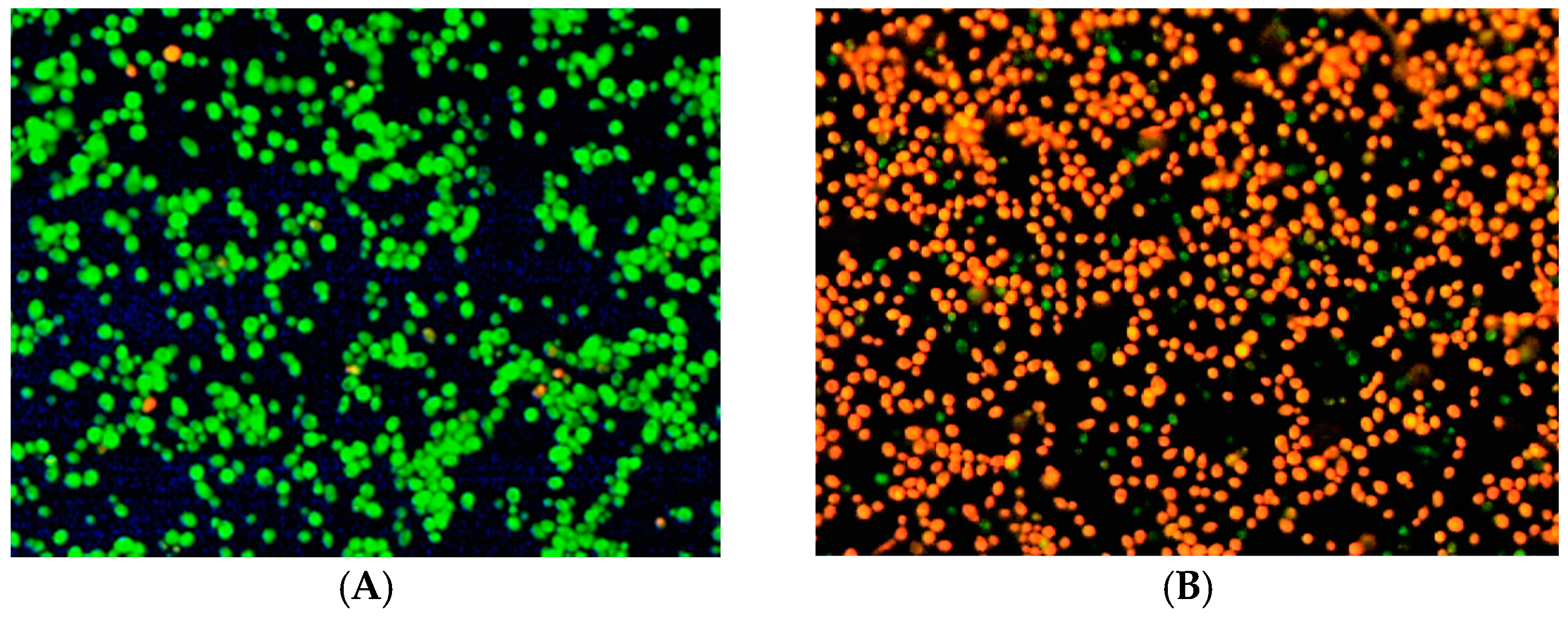
2.6. Fluorescence Microscopy
3. Materials and Methods
3.1. Chemistry
3.1.1. Synthesis of Methyl 4-(5(6)-substituted-1H-benzimidazol-2-yl)benzoate (1a–1c)
3.1.2. Synthesis of 4-(5(6)-Substituted-1H-benzimidazol-2-yl)benzoic Acid Hydrazide (2a–2c)
3.1.3. Synthesis of 2-(4-(5(6)-Substituted-1H-benzimidazol-2-yl)benzoyl)-N’-methyl/ethyl-hydrazine-1-carbothioamide (3a–3f)
3.1.4. Synthesis of 5-(4-((5(6)-Substituted-1H-benzimidazol-2-yl)phenyl)-4-methyl/ethyl-4H-1,2,4-triazole-3-thiol (4a–4f)
3.1.5. General Procedure for Target Compounds (5a–5ad)
3.2. Antimicrobial Assay
3.3. Prediction of ADME Proporties
3.4. Cytotoxicity Assay
3.5. Quantification of Ergosterol Level
3.6. Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Whibley, N.; Gaffen, S.L. Beyond Candida albicans: Mechanisms of immunity to non-albicans Candida species. Cytokine 2015, 76, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Sparber, F.; LeibundGut-Landmann, S. Interleukin 17-mediated host defense against Candida albicans. Pathogens 2015, 4, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cao, Y.; Zhang, J.; Zou, Y.; Chai, X.; Hu, H.; Zhao, Q.; Wu, Q.; Zhang, D.; Jiang, Y.; et al. Design, synthesis and antifungal evaluation of 1-(2-(2,4-difluorophenyl)-2-hydroxy-3-(1H-1,2,4-triazol-1-yl)propyl)-1H-1,2,4-triazol-5(4H)-one. Eur. J. Med. Chem. 2011, 46, 3135–3141. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Kaplancıklı, Z.A.; Turan-Zitouni, G.; Özdemir, A.; İşcan, G.; Akalın, G.; Yıldırım, Ş. Synthesis and anticandidal activity of new triazolothiadiazine derivatives. Eur. J. Med. Chem. 2011, 46, 5562–5566. [Google Scholar] [PubMed]
- Georgopapadakou, N.H.; Walsh, T.J. Human mycoses: Drugs and targets for emerging pathogens. Science 1994, 264, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, R.; Artico, M.; La Regina, G.; Di Pasquali, A.; De Martino, G.; D′Auria, F.D.; Nencioni, L.; Palamara, A.T. Imidazole analogues of fluoxetine, a novel class of anti-Candida agents. J. Med. Chem. 2004, 47, 3924–3926. [Google Scholar] [CrossRef] [PubMed]
- DiDomenico, B. Novel antifungal drugs. Curr. Opin. Microbiol. 1999, 2, 509–515. [Google Scholar] [CrossRef]
- Beck–Sague, C.; Jarvis, W. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J. Infect. Dis. 1993, 167, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Georgopapadakou, N.H. Antifungals: Mechanism of action and resistance, established and novel drugs. Curr. Opin. Microbiol. 1998, 1, 547–557. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, Y.; Dong, G.; Cao, Y.; Miao, Z.; Yao, J.; Zhang, W.; Sheng, C. Novel conformationally restricted triazole derivatives with potent antifungal activity. Eur. J. Med. Chem. 2010, 45, 6020–6026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, Y.; Wang, W.; Wang, S.; Xu, B.; Fan, G.; Dong, G.; Liu, Y.; Yao, J.; Miao, Z.; Zhang, W.; Sheng, C. Discovery of highly potent triazole antifungal derivatives by heterocycle-benzene bioisosteric replacement. Eur. J. Med. Chem. 2013, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, J.; Szulczyk, D.; Koziol, A.E.; Miroslaw, B.; Kedzierska, E.; Fidecka, S.; Busonera, B.; Sanna, G.; Giliberti, G.; La Colla, P.; et al. Disubstituted thiourea derivatives and their activity on CNS: Synthesis and biological evaluation. Eur. J. Med. Chem. 2012, 55, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, A.; Venkatesh, B.C.; Padmavathi, V.; Padmaja, A.; Kondaiah, P.; Krishna, N.S. Synthesis, antimicrobial and cytotoxic activities of sulfone linked bisheterocycles. Eur. J. Med. Chem. 2012, 54, 605–614. [Google Scholar] [CrossRef]
- Zoumpoulakis, P.; Camoutsis, C.H.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents, biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: a review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef] [PubMed]
- Spasov, A.A.; Yozhitsa, I.N.; Bugaeva, L.I.; Anisimova, V.A. Benzimidazole derivatives: Spectrum of pharmacological activity and toxicological properties (a review). Pharm. Chem. J. 1999, 33, 232–243. [Google Scholar] [CrossRef]
- Arjmand, F.; Mohani, B.; Ahmad, S. Synthesis, antibacterial, antifungal activity and interaction of CT-DNA with a new benzimidazole derived Cu(II) complex. Eur. J. Med. Chem. 2005, 40, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Shaker, Y.M.; Omar, M.A.; Mahmoud, K.; Elhallouty, S.M.; El-Senousy, W.M.; Ali, M.M.; Mahmoud, A.E.; Abdel-Halim, A.H.; Soliman, S.M.; El-Divani, H.I. Synthesis, in vitro and in vivo antitumor and antiviral activity of novel 1-substituted benzimidazole derivatives. J. Enzyme Inhib. Med. Chem. 2015, 30, 826–845. [Google Scholar]
- Tonelli, M.; Boido, V.; La Colla, P.; Loddo, R.; Posocco, P.; Paneni, M.S.; Fermeglia, M.; Pricl, S. Pharmacophore modeling, resistant mutant isolation, docking, and MM-PBSA analysis: Combined experimental/computer-assisted approaches to identify new inhibitors of the bovine viral diarrhea virus (BVDV). Bioorg. Med. Chem. 2010, 18, 2304–2316. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Kotadiya, G.M. Microwave-assisted synthesis of benzimidazole bearing 1,3,4-oxadiazole derivatives: Screening for their in vitro antimicrobial activity. Med. Chem. Res. 2014, 23, 4021–4033. [Google Scholar] [CrossRef]
- Desai, N.C.; Shihory, N.; Bhatt, M.; Patel, B.; Karkar, T. Studies on antimicrobial evaluation of some 1-((1-(1H-benzo[d]imidazol-2-yl) ethylidene) amino)-6-((arylidene)amino)-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitriles. Synth. Commun. 2015, 45, 2701–2711. [Google Scholar] [CrossRef]
- Desai, N.C.; Kotadiya, G.M. Synthesis, antimicrobial, and cytotoxic activities of novel benzimidazole derivatives bearing cyanopyridine and 4-thiazolidinone motifs. Med. Chem. Res. 2014, 23, 3823–3835. [Google Scholar] [CrossRef]
- Subhashini, N.J.P.; Boddu, L.; Amanaganti, J. Synthesis, characterization, and antimicrobial activity of new bis-1,2,3-triazol-H-yl-substituted 2-arylbenzimidazoles. Russ. J. Gen. Chem. 2014, 84, 1442–1449. [Google Scholar] [CrossRef]
- Montalvão, S.; Leino, T.O.; Kiuru, P.S.; Lillsunde, K.E.; Yli-Kauhaluoma, J.; Tammela, P. Synthesis and biological evaluation of 2-aminobenzothiazole and benzimidazole analogs based on the clathrodin structure. Arch. Pharm. Chem. Life Sci. 2016, 349, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Parikh, K. Synthesis and evaluation of novel benzimidazole derivatives as antimicrobial agents. Med. Chem. Res. 2014, 23, 1290–1299. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, M.Y.; Wan, F.X.; Qu, Z.Q. Synthesis and biological activity of tri-substituted 1,2,4-triazoles bearing benzimidazole moiety. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 1599–1605. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Yancheva, D.; Anastassova, N.; Anichina, K.; Zvezdanovic, J.; Djordjevic, A.; Markovic, D.; Smelcerovic, A. Synthesis, electronic properties, antioxidant and antibacterial activity of some new benzimidazoles. Bioorg. Med. Chem. 2015, 23, 6317–6326. [Google Scholar] [PubMed]
- Keller, P.; Müller, C.; Engelhardt, I.; Hiller, E.; Lemuth, K.; Eickhoff, H.; Wiesmüller, K.H.; Burger-Kentischer, A.; Bracher, F.; Rupp, S. An antifungal benzimidazole derivative inhibits ergosterol biosynthesis and reveals novel sterols. Antimicrob. Agents Chemother. 2015, 59, 6296–6307. [Google Scholar] [CrossRef] [PubMed]
- Soodamani, V.; Patel, D.; Nayakanti, D.; Josyula, R. An efficient synthesis of novel 2-(5-indolyl)-1H-benzimidazole derivatives and evaluation of their antimicrobial activities. J. Heterocycl. Chem. 2015, 52, 1457–1466. [Google Scholar]
- Jiang, Y.; Han, Q.; Shen, R.; Zang, X.; Wang, B. Synthesis and antimicrobial activity of some new 4H-pyrrolo [1,2-a]benzimidazoles. Chem. Res. Chin. Univ. 2014, 30, 755–758. [Google Scholar] [CrossRef]
- Desai, N.C.; Pandya, D.D.; Kotadiya, M.G.; Desai, P. Synthesis of pyridyl benzimidazoles encompassing 4-thiazolidinone derivatives as potential antimicrobial agents. Lett. Drug Des. Discov. 2013, 10, 942–950. [Google Scholar]
- Vashist, N.; Sambi, S.S.; Kumar, P.; Narasimhan, B. Development of QSAR for antimicrobial activity of substituted benzimidazoles. Drug Res. 2015, 65, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Novelli, F.; Tasso, B.; Vazzana, I.; Sparatore, A.; La Colla, P.; Sanna, G.; Giliberti, G.; Busonera, B.; Farci, P.; et al. Antiviral activity of benzimidazole derivatives. III. Novel anti-CVB-5, anti-RSV and anti-Sb-1 agents. Bioorg. Med. Chem. 2014, 22, 4893–4909. [Google Scholar] [PubMed]
- Kaplancikli, Z.A.; Turan-Zitouni, G.; Revial, G.; Guven, K. Synthesis and study of antibacterial and antifungal activities of novel 2-[[(benzoxazole/benzimidazole-2-yl) sulfanyl] acetylamino] thiazoles. Arch. Pharm. Res. 2004, 27, 1081–1085. [Google Scholar] [PubMed]
- Özdemir, A.; Turan-Zitouni, G.; Asım Kaplancıklı, Z.; Revial, G.; Demirci, F.; İşcan, G. Preparation of some pyrazoline derivatives and evaluation of their antifungal activities. J. Enzyme Inhib. Med. Chem. 2010, 25, 565–571. [Google Scholar] [PubMed]
- Altintop, M.D.; Mohsen, U.A.; Ozkay, Y.; Demirel, R.; Kaplancikli, ZA. Synthesis and antimicrobial activity of benzimidazole-based acetamide derivatives. Turk. J. Pharm. Sci. 2015, 12, 29–38. [Google Scholar]
- Yurttas, L.; Ozkay, Y.; Karaca, H.; Tunali, Y.; Kaplancıkli, ZA. Synthesis and antimicrobial evaluation of some 2,5-disubstituted benzimidazole derivatives. Lett. Drug Des. Discov. 2013, 10, 486–491. [Google Scholar] [CrossRef]
- Ozkay, Y.; Tunalı, Y.; Karaca, H.; Işıkdağ, İ. Antimicrobial activity of a new series of benzimidazole derivatives. Arc. Pharm. Res. 2011, 34, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Ozkay, Y.; Tunalı, Y.; Karaca, H.; Işıkdağ, İ. Antimicrobial activity of a new combination system of benzimidazole and various azoles. Arc. Pharm. 2011, 344, 264–271. [Google Scholar]
- Ozkay, Y.; Tunalı, Y.; Karaca, H.; Işıkdağ, İ. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur. J. Med. Chem. 2010, 45, 3293–3298. [Google Scholar] [PubMed]
- Ozkay, Y.; Tunali, Y.; Karaca, H.; Isikdag, I. Synthesis and antimicrobial activity of some novel benzimidazole hydrazides. Asian J. Chem. 2011, 23, 1503. [Google Scholar]
- Acar-Çevik, U.; Sağlık, B.N.; Ozkay, Y.; Canturk, Z.; Bueno, J.; Demirci, F.; Koparal, A.S. Synthesis of new fluoro-benzimidazole derivatives as an approach towards the discovery of novel Intestinal antiseptic drug candidates. Curr. Pharm. Des. 2017. [Google Scholar] [CrossRef]
- Yadagiri, B.; Lown, J.W. Convenient routes to substituted benzimidazoles and imidazolo[4,5-b]pyridines using nitrobenzene as oxidant. Synth. Commun. 1990, 20, 955–963. [Google Scholar] [CrossRef]
- Verner, E.; Katz, B.A.; Spencer, J.R.; Allen, D.; Hataye, J.; Hruzewicz, W.; Hui, H.C.; Kolesnikov, A.; Li, Y.; Luong, C.; et al. Development of serine protease inhibitors displaying a multicentered short (<2.3 Å) hydrogen bond binding mode: Inhibitors of urokinase-type plasminogen activator and factor Xa. J. Med. Chem. 2001, 44, 2753–2771. [Google Scholar] [PubMed]
- Bhatnagar, I.; George, M.V. Oxidation with metal oxides-II: Oxidation of chalcone phenylhydrazones, pyrazolines, o-aminobenzylidine anils and o-hydroxy benzylidine anils with manganese dioxide. Tetrahedron 1968, 24, 1293–1298. [Google Scholar] [CrossRef]
- Patzold, F.; Zeuner, F.; Heyer, T.H.; Niclas, H. Dehydrogenations using benzofuroxan as oxidant. J. Synth. Commun. 1992, 22, 281–288. [Google Scholar]
- Chikashita, H.; Nishida, S.; Miyasaki, M.; Morita, Y.; Itoh, K. Insitu generation and synthetic application of 2-phenylbenzimidazoline to the selective reduction of carbon carbon double-bonds of electron-deficient olefins. Bull. Chem. Soc. Jpn. 1987, 60, 737–746. [Google Scholar] [CrossRef]
- Beaulieu, P.L.; Hache, B.; Von Moos, E. A practical Oxone (R)-mediated, high-throughput, solution-phase synthesis of benzimidazoles from 1,2-phenylenediamines and aldehydes and its application to preparative scale synthesis. Synthesis 2003, 22, 1683–1692. [Google Scholar] [CrossRef]
- Stephens, F.F.; Bower, J.D. The preparation of benziminazoles and benzoxazoles from Schiff′s bases. Part I. J. Chem. Soc. 1949, 2971–2972. [Google Scholar] [CrossRef]
- Ridley, H.F.; Spickett, R.G.W.; Timmis, G.M. A new synthesis of benzimidazoles and aza-analogs. J. Heterocycl. Chem. 1965, 2, 453–456. [Google Scholar] [CrossRef]
- Algul, O.; Kaessler, A.; Apcin, Y.; Yilmaz, A.; Jose, J. Comparative studies on conventional and microwave synthesis of some benzimidazole, benzothiazole and indole derivatives and testing on inhibition of hyaluronidase. Molecules 2008, 13, 736–748. [Google Scholar] [PubMed]
- Yamashita, T.; Yamada, S.; Yamazaki, Y.; Tanaka, H. New procedure for the synthesis of 2-alkylbenzimidazoles. Synth. Commun. 2009, 39, 2982–2988. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Moreno-Diaz, H.; Aguirre-Crespo, F.; León-Rivera, I.; Villalobos-Molina, R.; Muñoz-Muñiz, O.; Estrada-Soto, S. Design, microwave-assisted synthesis, and spasmolytic activity of 2-(alkyloxyaryl)-1H-benzimidazole derivatives as constrained stilbene bioisosteres. Bioorg. Med. Chem. Lett. 2007, 16, 4169–4173. [Google Scholar] [CrossRef]
- Navarrete-Vázquez, G.; Moreno-Diaz, H.; Estrada-Soto, S.; Torres-Piedra, M.; León-Rivera, I.; Tlahuext, H.; Muñoz-Muñiz, O.; Torres-Gómez, H. Microwave assisted one pot synthesis of 2-(substitutedphenyl)-1H-benzimidazole derivatives. Synth. Commun. 2007, 37, 2815–2825. [Google Scholar]
- El kihel, A.; Essassi, E.M.; Bauchat, P. 1H- and 13C-NMR spectra of condensed benzimidazole and imidazobenzodiazepines. Arab. J. Chem. 2012, 5, 523–526. [Google Scholar] [CrossRef]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; Barros, F.D.M.; Andrade, PM. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]
- Van De Waterbeemd, H.; Gifford, E. ADMET in silico modelling: Towards prediction paradise? Nat. Rev. Drug Discov. 2003, 2, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics, Bratislava, Slovak Republic. Available online: http://www.molinspiration.com/services/properties.html (accessed on 23 January 2017).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 19, 3–26. [Google Scholar] [CrossRef]
- Kramer, J.A.; Sagartz, J.E.; Morris, D.L. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat. Rev. Drug Discov. 2007, 6, 636–649. [Google Scholar] [PubMed]
- International Organization for Standardization. Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity ISO-10993-5, 3rd ed.; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Lupetti, A.; Danesi, R.; Campa, M.; Del Tacca, M.; Kelly, S. Molecular basis of resistance to azole antifungals. Trends Mol. Med. 2002, 8, 76–81. [Google Scholar] [CrossRef]
- Breivik, O.N.; Owades, J.L. Spectrophotometric semi-microdetermination of ergosterol in yeast. Agric. Food Chem. 1957, 5, 360–363. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Rao, R. Beyond ergosterol: Linking pH to antifungal mechanisms. Virulence 2010, 1, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Nobmann, P.; Bourke, P.; Dunne, J.; Henehan, G. In vitro antimicrobial activity and mechanism of action of novel carbohydrate fatty acid derivatives against Staphylococcus aureus and MRSA. J. Appl. Microbiol. 2010, 108, 2152–2161. [Google Scholar] [PubMed]
- EUCAST. Definitive Document EDef 7.1: Method for the Determination of Broth Dilution MICs of Antifungal Agents for Fermentative Yeasts. Clin. Microbiol. Infect. 2008, 14. [Google Scholar] [CrossRef]
- Sağlık, B.N.; Ilgın, S.; Özkay, Y. Synthesis of new donepezil analogues and investigation of their effects on cholinesterase enzymes. Eur. J. Med. Chem. 2016, 124, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Özkay, Ü.D.; Can, Ö.D.; Sağlık, B.N.; Çevik, U.A.; Levent, S.; Özkay, Y.; Atlı, Ö. Design, synthesis, and AChE inhibitory activity of new benzothiazole–piperazines. Bioorg. Med. Chem. Lett. 2016, 26, 5387–5394. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Gheewala, N.; Suthar, A.; Shah, A. In-vitro cytotoxicity activity of Solanum nigrum extract against Hela cell line and Vero cell line. Int. J. Pharm. Pharm. Sci. 2009, 1, 38–46. [Google Scholar]
- Li, Y.; Smith, C.; Wu, H.; Padhee, S.; Manoj, N.; Joseph, C.; Qiao, Q.; Chuanhai, C.; Yin, H.; Cai, J. Lipidated Cyclic AApeptides Display Both Antimicrobial and Antiinflammatory Activity. ACS Chem. Biol. 2014, 9, 211–217. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 5a–5ad are available from the authors. |
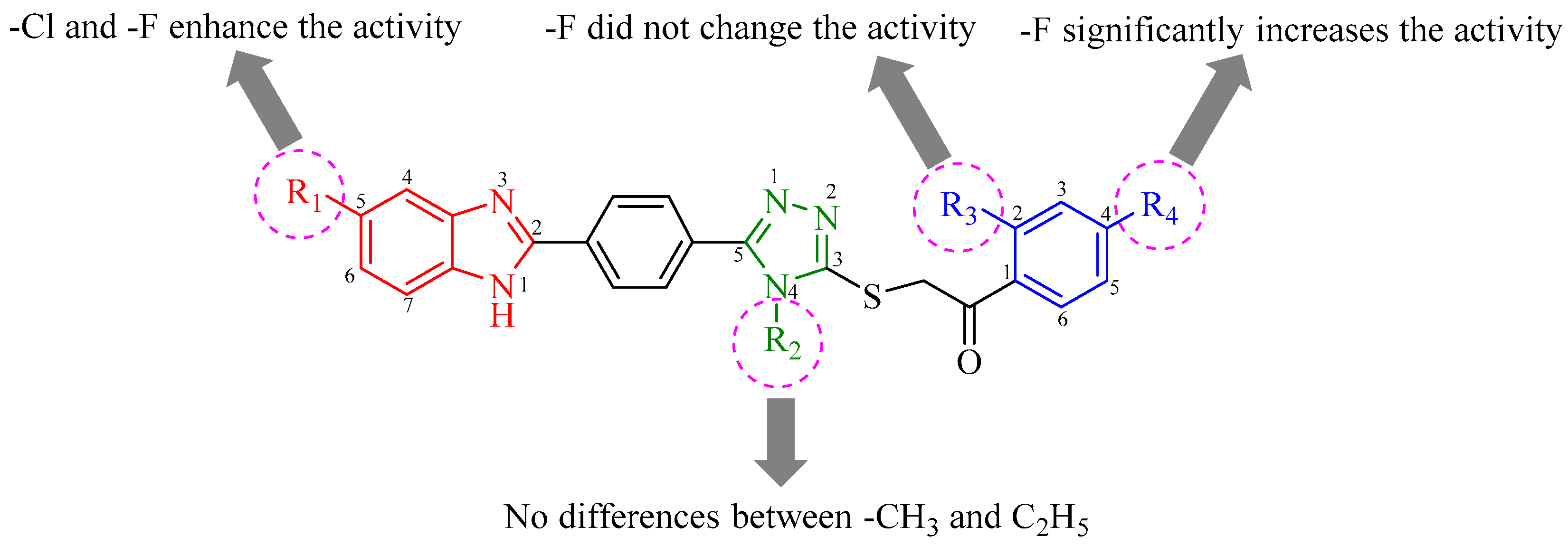
| Compound | R1 | R2 | R3 | R4 | m.p. (°C) | Yield (%) |
|---|---|---|---|---|---|---|
| 5a | -H | -CH3 | -H | -H | 269.5 | 81 |
| 5b | -H | -CH3 | -H | -Cl | 280.6 | 82 |
| 5c | -H | -CH3 | -H | -F | 259.8 | 80 |
| 5d | -H | -CH3 | -Cl | -Cl | 234.7 | 76 |
| 5e | -H | -CH3 | -F | -F | 256.2 | 75 |
| 5f | -H | -C2H5 | -H | -H | 220.9 | 78 |
| 5g | -H | -C2H5 | -H | -Cl | 242.6 | 81 |
| 5h | -H | -C2H5 | -H | -F | 231.3 | 80 |
| 5i | -H | -C2H5 | -Cl | -Cl | 221.7 | 83 |
| 5j | -H | -C2H5 | -F | -F | 238.2 | 86 |
| 5k | -Cl | -CH3 | -H | -H | 260.0 | 87 |
| 5l | -Cl | -CH3 | -H | -Cl | 236.9 | 77 |
| 5m | -Cl | -CH3 | -H | -F | 274.5 | 82 |
| 5n | -Cl | -CH3 | -Cl | -Cl | 274.2 | 77 |
| 5o | -Cl | -CH3 | -F | -F | 264.9 | 85 |
| 5p | -Cl | -C2H5 | -H | -H | 248.5 | 80 |
| 5q | -Cl | -C2H5 | -H | -Cl | 252.2 | 91 |
| 5r | -Cl | -C2H5 | -H | -F | 265.5 | 78 |
| 5s | -Cl | -C2H5 | -Cl | -Cl | 192.0 | 77 |
| 5t | -Cl | -C2H5 | -F | -F | 250.6 | 74 |
| 5u | -F | -CH3 | -H | -H | 277.1 | 81 |
| 5v | -F | -CH3 | -H | -Cl | 252.9 | 84 |
| 5w | -F | -CH3 | -H | -F | 227.4 | 82 |
| 5x | -F | -CH3 | -Cl | -Cl | 228.5 | 89 |
| 5y | -F | -CH3 | -F | -F | 263.2 | 88 |
| 5z | -F | -C2H5 | -H | -H | 253.6 | 90 |
| 5aa | -F | -C2H5 | -H | -Cl | 241.4 | 79 |
| 5ab | -F | -C2H5 | -H | -F | 251.0 | 81 |
| 5ac | -F | -C2H5 | -Cl | -Cl | 223.3 | 87 |
| 5ad | -F | -C2H5 | -F | -F | 234.2 | 76 |
| Compound | C. albicans | C. glabrata | C. krusei | C. parapsilopsis |
|---|---|---|---|---|
| 5a | 100 | 100 | 100 | 100 |
| 5b | 25 | 50 | 50 | 50 |
| 5c | 12.5 | 25 | 25 | 12.5 |
| 5d | 25 | 50 | 50 | 25 |
| 5e | 12.5 | 25 | 25 | 25 |
| 5f | 100 | 100 | 100 | 100 |
| 5g | 25 | 50 | 25 | 50 |
| 5h | 12.5 | 25 | 12.5 | 25 |
| 5i | 25 | 50 | 50 | 50 |
| 5j | 12.5 | 25 | 12.5 | 25 |
| 5k | 12.5 | 25 | 25 | 12.5 |
| 5l | 6.25 | 12.5 | 6.25 | 12.5 |
| 5m | 0.78 | 1.56 | 0.78 | 0.78 |
| 5n | 1.56 | 3.12 | 1.56 | 3.12 |
| 5o | 0.78 | 1.56 | 1.56 | 0.78 |
| 5p | 12.5 | 12.5 | 50 | 25 |
| 5q | 6.25 | 12.5 | 12.5 | 12.5 |
| 5r | 0.78 | 0.78 | 1.56 | 1.56 |
| 5s | 1.56 | 3.12 | 3.12 | 1.56 |
| 5t | 0.78 | 1.56 | 0.78 | 0.78 |
| 5u | 12.5 | 25 | 50 | 50 |
| 5v | 12.5 | 6.25 | 12.5 | 12.5 |
| 5w | 0.78 | 0.78 | 0.78 | 0.78 |
| 5x | 3.12 | 1.56 | 3.12 | 3.12 |
| 5y | 0.78 | 1.56 | 0.78 | 1.56 |
| 5z | 12.5 | 25 | 50 | 50 |
| 5aa | 6.25 | 12.5 | 6.25 | 12.5 |
| 5ab | 0.78 | 1.56 | 1.56 | 0.78 |
| 5ac | 1.56 | 1.56 | 1.56 | 1.56 |
| 5ad | 0.78 | 0.78 | 1.56 | 0.78 |
| Ketoconazole | 0.78 | 1.56 | 1.56 | 1.56 |
| Fluconazole | 0.78 | 1.56 | 1.56 | 0.78 |
| Compound | MW | logP | tPSA | nON | nOHNH | MV | Vio |
|---|---|---|---|---|---|---|---|
| 5m | 477.95 | 6.07 | 76.47 | 6 | 1 | 389.09 | 1 |
| 5n | 528.85 | 7.19 | 76.47 | 6 | 1 | 411.23 | 2 |
| 5o | 495.94 | 6.16 | 76.47 | 6 | 1 | 394.02 | 1 |
| 5r | 491.98 | 6.44 | 76.47 | 6 | 1 | 405.89 | 1 |
| 5s | 542.88 | 7.56 | 76.47 | 6 | 1 | 428.03 | 2 |
| 5t | 509.97 | 6.53 | 76.47 | 6 | 1 | 410.82 | 2 |
| 5w | 461.50 | 5.55 | 76.47 | 6 | 1 | 380.48 | 1 |
| 5x | 512.40 | 6.67 | 76.47 | 6 | 1 | 402.62 | 2 |
| 5y | 479.49 | 5.64 | 76.47 | 6 | 1 | 385.41 | 1 |
| 5ab | 475.52 | 5.93 | 76.47 | 6 | 1 | 397.28 | 1 |
| 5ac | 526.42 | 7.05 | 76.47 | 6 | 1 | 419.42 | 2 |
| 5ad | 493.51 | 6.02 | 76.47 | 6 | 1 | 402.21 | 1 |
| Ketoconazole | 531.44 | 3.77 | 69.08 | 8 | 0 | 452.47 | 1 |
| Fluconazole | 320.30 | 0.05 | 81.66 | 7 | 1 | 266.11 | 0 |
| Compound | IC50 (µg/mL) |
|---|---|
| 5m | 17.38 ± 0.89 |
| 5o | 1.63 ± 0.02 |
| 5r | 13.94 ± 0.69 |
| 5w | 65.28 ± 2.94 |
| 5y | 11.14 ± 0.57 |
| 5ab | 43.13 ± 2.88 |
| 5ad | 119.55 ± 4.39 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaca Gençer, H.; Acar Çevik, U.; Levent, S.; Sağlık, B.N.; Korkut, B.; Özkay, Y.; Ilgın, S.; Öztürk, Y. New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation. Molecules 2017, 22, 507. https://doi.org/10.3390/molecules22040507
Karaca Gençer H, Acar Çevik U, Levent S, Sağlık BN, Korkut B, Özkay Y, Ilgın S, Öztürk Y. New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation. Molecules. 2017; 22(4):507. https://doi.org/10.3390/molecules22040507
Chicago/Turabian StyleKaraca Gençer, Hülya, Ulviye Acar Çevik, Serkan Levent, Begüm Nurpelin Sağlık, Büşra Korkut, Yusuf Özkay, Sinem Ilgın, and Yusuf Öztürk. 2017. "New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation" Molecules 22, no. 4: 507. https://doi.org/10.3390/molecules22040507







